Abstract
Covalent linkage of a bacterial polysaccharide to a protein greatly enhances the carbohydrate's immunogenicity and its binding to solid surfaces in immunoassays. These findings have spurred the development of glycoconjugate vaccines to prevent serious bacterial infections as well as the use of glycoconjugates as coating antigens in bioassays. We evaluated sera from women immunized with unconjugated group B streptococcal (GBS) type III (GBS III) polysaccharide (IIIPS) or with IIIPS covalently linked to tetanus toxoid to assess specificity, sensitivity, and parallelism in dilution curves in two GBS III enzyme-linked immunosorbent assays (ELISAs). One assay used IIIPS mixed with methylated human serum albumin (IIIPS + mHSA) as the coating antigen, and the other used IIIPS covalently linked to HSA (III-HSA). Each coating antigen was associated with a highly specific GBS III bioassay. The sensitivity was higher in the III-HSA ELISA, in which conjugated IIIPS is bound to the plates. Parallelism in titration curves was observed in the III-HSA but not in the IIIPS + mHSA ELISA. The excellent correlation between the concentrations of GBS IIIPS-specific immunoglobulin G (IgG) and the opsonophagocytic activity of these antibodies indicated that the III-HSA assay can predict functionality of vaccine-induced IgG against GBS III disease. The structure of the repeating unit of the capsular polysaccharide of GBS III differs from that of Streptococcus pneumoniae type 14 (Pn14 PS) only by the presence on GBS III of a sialic acid residue at the end of the side chain. The majority of healthy adults responding to GBS III vaccines with a fourfold or greater increase in GBS III-specific IgG antibodies developed antibodies cross-reacting with Pn14 PS (i.e., desialylated GBS IIIPS). The proportion of GBS vaccine responders who developed IgG to the desialylated IIIPS did not depend on whether IIIPS was given in the unconjugated or conjugated form. When present, these vaccine-induced cross-reacting antibodies conferred in vitro antibody-mediated opsonophagocytosis and killing of both GBS III and Pn14, two pathogens that cause invasive disease in young infants.
Group B Streptococcus (GBS) is a leading cause of bacteremia, sepsis, pneumonia, and meningitis in neonates and infants less than 3 months of age (2, 3). Mothers of neonates developing serotype III GBS (GBS III) disease have low concentrations of antibodies to the type III capsular polysaccharide (IIIPS) in their sera at delivery (5). If sufficient amounts of maternal IIIPS-specific antibodies cross the placenta (9, 12), the neonate or young infant will be protected against invasive disease (4, 5). Naturally acquired IIIPS-specific antibodies are predominantly of the immunoglobulin G (IgG) isotype (9, 25), the only isotype passively and actively transported across the placenta to the neonate (30, 34, 36, 41).
The direct correlation between infant immunity to GBS III disease and the presence of maternal IIIPS-specific antibodies was first established with a radioactive-antigen binding assay (RABA) (5). The detection by RABA of low levels of IIIPS-specific immunoglobulin in maternal sera at delivery predicted susceptibility to GBS III disease (5, 9). The RABA quantitates antibodies binding to fluid-phase IIIPS in its native conformation (7, 27). However, this assay has two significant shortcomings: (i) poor sensitivity, or inability to quantitate serum levels of <0.5 to 1.0 μg/ml (9, 25), and (ii) inability to distinguish among immunoglobulin isotypes and subclasses. Therefore, a sensitive and isotype-specific enzyme-linked immunosorbent assay (ELISA) was developed to measure IIIPS-specific IgG in human sera. This assay allows more precise identification of women whose offspring are at significant risk for disease (25; C. J. Baker, V. J. Carey, M. S. Edwards, P. Ferrieri, S. L. Hillier, M. A. Krohn, H.-K. Guttormsen, D. L. Kasper, and R. Platt, submitted for publication).
Recently, to estimate more precisely the quantity of IIIPS-specific IgG needed for protection against early-onset GBS disease in neonates, a case-control study was performed (Baker et al., submitted). Very low levels of IIIPS-specific IgG in maternal sera at delivery correlated significantly with susceptibility to early-onset (age, <7 days) neonatal disease. These study results were derived with an ELISA using IIIPS covalently linked to human serum albumin (HSA) as coating antigen, an assay that quantitates IIIPS-specific IgG at levels of as low as 0.05 μg/ml (25; Baker et al., submitted).
The IIIPS is structurally related to the capsular polysaccharide of type 14 Streptococcus pneumoniae (Pn14 PS); the only difference is the presence of a terminal sialic acid residue in the side chain of the repeating pentasaccharide of IIIPS (27). Both organisms cause serious infections in young infants (3, 35), and for each, type-specific antibodies to the capsular polysaccharide are protective. These structural and immunochemical similarities raised the possibility that type-specific antibodies induced by one of these organisms might protect against disease caused by the other (21, 22, 27). However, naturally acquired Pn14-specific antibodies are not protective against GBS III disease in humans (27). Although unconjugated Pn14 PS vaccine induces IIIPS-specific antibodies in healthy young adults with moderate to high preimmunization levels of such antibodies (>2 μg/ml by RABA) and the induced antibodies opsonize GBS III for killing by human polymorphonuclear leukocytes (PMNs) in vitro (8), these results are not obtained for persons at risk for invasive GBS III disease, i.e., those with low levels of IIIPS-specific antibodies (5, 6, 8).
Unconjugated IIIPS binds poorly to ELISA plates. In contrast, the use of IIIPS conjugated to HSA as a coating antigen substantially increase the binding of the polysaccharide to the solid support and thereby increase the sensitivity of the immunoassay (25). However, there has been controversy regarding the optimal immunoassay for measurement of vaccine-induced and naturally occurring IIIPS-specific IgG (16, 25; D. L. Kasper, M. R. Wessels, H. K. Guttormsen, L. C. Paoletti, M. S. Edwards, and C. J. Baker, Letter, Infect. Immun. 67:4303-4305, 1999). In particular, concern emanates from the following questions. (i) Does use of IIIPS conjugated to HSA as a sensitizing antigen change the epitope(s) of the polysaccharide and thus alter the specificity of the assay (25)? (ii) Does conjugation of IIIPS to tetanus toxoid (TT) to create a conjugate vaccine (III-TT vaccine) for human use create immunogenic neoepitopes (28)? (iii) At what concentration in serum and in what functional capacity does IIIPS vaccine-induced IgG also recognize Pn14 PS (a structurally similar antigen)? To answer these questions, in this study we quantitated the concentrations of IIIPS-specific and Pn14 PS-specific IgG in serum before and after immunization of healthy adults with unconjugated IIIPS and conjugated III-TT vaccines. We measured capsular polysaccharide-specific IgG by ELISA, using three coating antigens: (i) IIIPS mixed with methylated HSA (IIIPS + mHSA), (ii) IIIPS covalently linked to HSA (III-HSA), or (iii) Pn14 PS. The specificities of the IIIPS immunoassays with different coating antigens were tested by inhibition ELISAs. The abilities of various sera from healthy adults to mediate complement-dependent opsonophagocytosis and killing of GBS III and Pn14 was assayed with the use of purified polysaccharides as inhibitors.
MATERIALS AND METHODS
Preparation of GBS III antigens and conjugates.
IIIPS was isolated and purified from strain M781 (43). The degree of purification was assessed by 1H nuclear magnetic resonance (500 MHz). As an ELISA coating antigen, IIIPS + mHSA (kindly supplied by George M. Carlone, Centers for Disease Control and Prevention, Atlanta, Ga.) (1) was compared with III-HSA (Sigma Chemical Company, St. Louis, Mo.) (25). The III-TT conjugate vaccine was prepared by coupling IIIPS to monomeric TT (Amvax, Beltsville, Md.) (28). For III-HSA and III-TT, IIIPS was oxidized to create aldehyde groups on 26 and 50% of the sialic acid residues, respectively. The degree of oxidation of IIIPS was verified by measurement of the newly created aldehydes (43). HSA and TT (separately) were covalently coupled to IIIPS by reductive amination, and the resulting conjugates were purified by gel filtration chromatography. The protein content of the conjugates was estimated by the Folin reaction method of Larson et al. (31) with TT and HSA as standards. The carbohydrate content was assessed by the phenol-sulfuric acid method, with IIIPS as a standard (18).
Purification of Pn14 PS.
Pn14 PS from the American Type Culture Collection (ATCC) (Manassas, Va.) was found to contain substantial quantities of C substance. Thus, 100 mg of ATCC-derived Pn14 PS (lot no. 1299795) was heated at 80°C for 1 h after suspension in 10 ml of 2 N NaOH to degrade unwanted C polysaccharide. After adjustment of the pH to 7, the resulting material was dialyzed against water, lyophilized, and size fractionated on an S-200 HR gel filtration column. Larger-molecular-size carbohydrate-positive fractions (>200,000 Da) detected by the phenol-sulfuric acid assay (18) were pooled, dialyzed against water, and dried before treatment with nucleases and pronases to degrade any contaminating nucleic acids and proteins. The material was again dialyzed against water, lyophilized, and purified on an S-300 HR gel filtration column. Carbohydrate-positive fractions were pooled, reacetylated with acetic anhydride (43), dialyzed, and lyophilized. 1H nuclear magnetic resonance analysis (500 MHz) revealed signals consistent with pure Pn14 PS that had a C polysaccharide content of <1%. The specificity of the Pn14 PS was unchanged after purification, as assayed by inhibition ELISA.
Human sera.
Healthy nonpregnant women, 18 to 40 years of age, who met eligibility criteria and gave written informed consent were enrolled in the study (28). Paired blood samples from 60 women were collected before and 4 weeks after the receipt of unconjugated IIIPS or III-TT vaccine (30 subjects in each group) (28). Blood specimens were processed immediately to preserve endogenous complement, and sera were stored at −80°C until testing. Naturally acquired IIIPS-specific antibodies were defined as those measured in the sera before vaccination. A standard pooled reference serum (streptococcal human reference serum for type III GBS [SHRS III], derived from postimmunization sera from five individuals who had low preimmunization levels of IIIPS-specific IgG (<0.5 μg/ml) and who developed high levels (measured by RABA) after immunization with III-TT, was used as the standard in the ELISAs (25, 28). An additional standard human serum, reference serum 19 (kindly supplied by Ali Fattom, Nabi, Md.) was tested. This serum was obtained from an individual who had high preimmunization serum IIIPS-specific IgG levels (12 μg/ml by ELISA) and who developed higher levels after immunization with tetravalent (types Ia, Ib, II, and III) unconjugated GBS polysaccharide vaccine. The concentration of IIIPS-specific antibodies in the SHRS III serum pool, measured by quantitative precipitin analysis in combination with RABA, was 88 μg/ml, of which 83.5 μg/ml was IgG; for reference serum 19, the IIIPS-specific IgG concentration was 143 μg/ml (15, 25, 29).
RABA.
A previously described RABA was used to quantify IIIPS-specific antibodies in sera (9). SHRS III at dilutions of 1:10, 1:20, 1:40, and 1:80 was tested in each assay to derive a standard curve, and linear regression analysis was used to quantify total IIIPS-specific antibodies. All sera were run in duplicate, and only values with binding of between 20 and 80% were used. Sera with >80% binding underwent further dilutions, and values of <20% corresponded to the lower limit of assay quantitation (1 μg/ml).
ELISA.
An ELISA previously described in detail (25) was used to determine the quantity of IIIPS-specific IgG in sera. In brief, microtiter plates (Immunlon-4 [Dynatech Laboratories, Chantilly, Va.] or Maxisorp [Nunc, Roskilde, Denmark]) were coated with (i) purified III-HSA (5 μg/ml) or (ii) purified IIIPS (5 μg/ml) mixed with mHSA at 0.5 μg/ml (stirred for 5 min prior to coating) (1, 16) in 10 mM phosphate-buffered saline (PBS) (pH 7.4) or 0.1 M carbonate buffer with 0.02% sodium azide (pH 9.6) per well. Plates were sealed, incubated for 6 h at 30°C, and then washed five times with 10 mM Tris buffer with 154 mM NaCl and 0.1% Brij 35 (Sigma). Sera were diluted in 10 mM PBS with 0.05% Brij, 5% fetal bovine serum (NCS; Whittaker Bioproducts, Walkersville, Md.), and 0.02% sodium azide. After sera were added, plates were incubated at 4°C overnight, washed, and reincubated with alkaline phosphatase-conjugated antibody to human IgG (Biosource International, Camarillo, Calif.) in incubation buffer for 2 h at 30°C. Next, 0.1 ml of p-nitrophenyl phosphate in 1 M Tris buffer (pH 9.8) with 0.3 mM MgCl2 was added, and the plates were incubated at 37°C with shaking; after 1 h, the optical density (OD) was determined at 405 and 630 nm. The amount of IIIPS-specific IgG in each serum was ascertained by comparison of the OD of the sample to a standard curve generated with SHRS III. All samples were run in duplicate, and the results were expressed as micrograms per milliliter.
The amount of Pn14 PS-specific IgG in a serum was determined by ELISA as previously described (37). In brief, the wells of Polysorp plates (Nunc) were coated overnight at 37°C with Pn14 PS (10 μg/ml; ATCC) in PBS. After being coated with antigen, plates were washed and blocked with PBS containing 0.5% bovine serum albumin (Sigma) and 0.05% Tween 20. Serum pool 89-SF (a gift from C. Frasch, U.S. Food and Drug Administration, Bethesda, Md.) was used as the standard, with an assigned value of 27.8 μg of Pn14 PS-specific IgG per ml. All samples were preabsorbed for 30 min at room temperature with 10 μg of C polysaccharide (Statens Seruminstitut, Copenhagen, Denmark) and 10 μg of type 22F pneumococcal polysaccharide (supplied by C. Frasch) per 20 μl of serum in a total volume of 1 ml of diluent. Samples were then added to wells, serially diluted, and incubated for 2 h. The wells were washed and incubated with peroxidase-conjugated mouse monoclonal antibody to human IgG (Hybridoma Reagent Lab, Baltimore, Md.), and tetramethyl benzidine was added to permit measurement of the amount of enzyme immobilized to the well. The OD was read at 450 and 600 nm, and the amount of IgG was determined by comparing the OD of the sample with that of the standard by means of a program available from the Centers for Disease Control and Prevention (courtesy of B. Plikaytis).
Inhibition of antibody binding to radioactively labeled IIIPS.
A RABA described previously for detecting antibodies to IIIPS (28) was used to compare the antigenicity in solution of IIIPS as an uncoupled polymer with that of IIIPS conjugated to a protein carrier. The inhibition RABA was performed as follows. A 50-μl volume of 3H-labeled IIIPS (1,000 cpm) was added to 50 μl of SHRS III (28) diluted 1:5 in 0.1 M borate-buffered saline plus 5% bovine serum albumin (pH 8.3). Inhibitors (IIIPS; III-TT, 25% oxidized; III-HSA, 50% oxidized; and Pn14 PS) were individually added to the mixture in serial fivefold dilutions beginning at 1 mg/ml in a volume of 50 μl. The mixtures were incubated at 37°C for 1 h and then at 4°C for 1 h. The proteins were precipitated with ammonium sulfate, and the amount of radioactivity in the supernatant fluids was measured as described previously (28). The percent inhibition was determined as follows: [(counts per minute in the test sample − counts per minute in samples lacking inhibitors)/(counts per minute in samples lacking antibody − counts per minute in samples lacking inhibitors)] × 100.
Inhibition ELISA.
Competitive-inhibition ELISAs of serum binding to plates coated with III-HSA or with IIIPS + mHSA were performed as previously described (25). All sera used in the competitive-inhibition ELISAs were diluted to give A405/630 readings near the middle of the linear portion of the dilution curve (i.e., A405/630 = 1) (26). IIIPS, IIIPS + mHSA, III-HSA, unpurified Pn14 PS, chromatographically purified Pn14 PS, and HSA were used as inhibitors at concentrations ranging from 30 μg/ml to 0.01 ng/ml.
Avidity of GBS IIIPS-specific antibodies.
The avidity of IIIPS-specific antibodies was measured by two methods. In the first, antibody binding to optimally coated ELISA plates (5 μg of III-HSA/ml) was compared with that to suboptimally coated plates (0.05 μg of III-HSA/ml) (17, 20, 42). A405/630 values for the two different coating concentrations were compared for serum dilutions on the linear parts of the titration curves. The avidity index was expressed as the fraction of the antibodies binding to suboptimally coated plates. In the second method, the molar concentration of ammonium thiocyanate (NH4SCN) needed to elute 50% of antibodies bound to III-HSA-coated ELISA plates was assessed (39, 40). In brief, the sera were diluted to give A405/630 readings near the middle of the linear portion of the dilution curve. NH4SCN at concentrations varying from 0 to 4 M in 0.1 M phosphate buffer (pH 6.0) was added and left for 15 min at room temperature before washing. The rest of the assay was the same as the standard III-HSA ELISA (see above).
GBS III opsonophagocytic killing assay.
The ability of sera containing endogenous complement to opsonize GBS III strain M732 for in vitro killing by PMNs from healthy adults was determined by an opsonophagocytosis assay (12). In brief, sera obtained before and 4 weeks after vaccination with unconjugated IIIPS or III-TT conjugate vaccines were tested at a single dilution (1:10). Results were expressed as the log10 reduction in CFU between the time when sera, bacteria, and PMNs were added to the test tube and 40 min later (after incubation at 37°C with gentle rotation).
Inhibition of GBS III opsonophagocytosis and killing.
The opsonophagocytosis assay (43) was modified to assess the functional specificity of postimmunization sera for GBS III strain M781. In brief, human PMNs (75 μl), human serum with low IIIPS-specific IgG as a complement source (12.5 μl), test serum (12.5 μl), washed GBS III grown to an absorbance of 0.3 at 650 nm (12.5 μl), and polysaccharide inhibitor (12.5 μl) were combined in a total of 125 μl of modified Eagle's medium. The final assay concentration of polysaccharide inhibitor (GBS III or Pn14) ranged from 0.01 to 100 μg/ml. The mixture was incubated for 1 h with end-over-end rotation at 37°C. GBS CFU were determined before and after incubation, and the percent CFU reduction was calculated as follows: [1 − (CFU after incubation/CFU before incubation)] × 100. For optimization of this assay, test sera were first titrated in separate opsonophagocytosis assays to determine the highest dilution that resulted in >80% killing of GBS III in the absence of inhibitors.
Pn14 opsonophagocytic killing assay.
The opsonophagocytosis assay for Pn14 has been described previously (44). In brief, Pn14 strain DS2214 was grown in Todd-Hewitt broth with 0.5% yeast extract, aliquoted in 15% glycerol, and kept frozen at −70°C until testing. Cells from a myelocytic line (HL-60) were differentiated into granulocytes via culture for 5 to 6 days in RPMI 1640 with 10% fetal calf serum and 0.8% dimethylformamide (Fisher Scientific). After differentiation, the HL-60 cells were diluted to 106/ml in Hanks' buffer supplemented with 0.1% gelatin and 10% fetal bovine serum. Serum samples were diluted in the same buffer. A 10-μl volume of Pn14 (103 CFU) and 20 μl of test serum were placed in each well of a 96-well microtiter plate. After incubation for 15 min at 37°C, 40 μl of HL-60 suspension and 10 μl of baby rabbit complement (Accurate Chemical & Scientific Corporation, Westbury, N.Y.) were added to each well. The mixture was incubated for 1 h at 37°C with shaking. A 5-μl portion of the reaction mixture was plated onto a Todd-Hewitt broth-0.5% yeast extract agar plate. The plates were incubated overnight in 5% CO2 at 37°C, and colonies were counted. The opsonization titer of a serum was defined as the dilution that resulted in a 50% reduction in CFU from counts in complement controls.
Statistical analysis.
Nonparametric statistics were used with Prism 2.0 software (Graphpad Software, San Diego, Calif.). Spearman rank correlation coefficients (rss) were used to determine correlations between (i) the concentrations of polysaccharide-specific antibodies measured by the different immunoassays and (ii) the concentration of polysaccharide-specific antibodies and the opsonophagocytic killing mediated by these antibodies (log10 reduction in CFU). For antibody concentrations below the level of quantitation for a given assay, a value corresponding to the quantitation limit of the assay was used for plots and statistical comparisons.
RESULTS
The GBS III-HSA and GBS IIIPS + mHSA ELISAs have identical specificity.
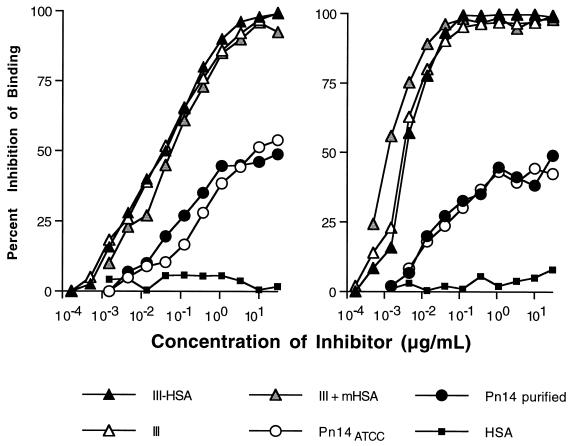
Soluble IIIPS abolished the binding of antibodies in a human serum pool to plates coated with III-HSA (Fig. 1, left panel) or IIIPS + mHSA (Fig. 1, right panel). Binding of IIIPS-specific antibodies in SHRS III to plates coated with III-HSA or IIIPS + mHSA was inhibited similarly by unconjugated IIIPS and by IIIPS conjugated to HSA. These results confirmed the preservation of all epitopes on IIIPS by each coating antigen. Thus, both ELISAs (III-HSA and the IIIPS + mHSA) were highly specific for detection of IIIPS-specific IgG. This equivalence in specificity also was observed when IIIPS + mHSA was used as the inhibitor (Fig. 1). SHRS III contains a Pn14-cross-reactive subpopulation of IIIPS-specific antibodies (25). This subpopulation was detected by each ELISA, whether the coating antigen was IIIPS conjugated to HSA (Fig. 1, left panel) or IIIPS mixed with mHSA (Fig. 1, right panel). Furthermore, the size of this Pn14-cross-reactive antibody subpopulation was the same in the two ELISAs (≈45% of the IIIPS-specific antibodies in SHRS III).
FIG. 1.
Competitive-inhibition IgG ELISAs demonstrate antigenic specificity of a pooled standard human reference serum (SHRS III) in GBS III ELISA using either III-HSA (left panel) or IIIPS + mHSA (right panel) as the coating antigen. The percent inhibition of binding of the IIIPS antibodies in SHRS III was determined by comparison of the A405/630 in the presence and absence of inhibitors as indicated. ATTC, American Type Culture Collection.
The III-HSA ELISA is highly sensitive in detecting GBS III-specific IgG.
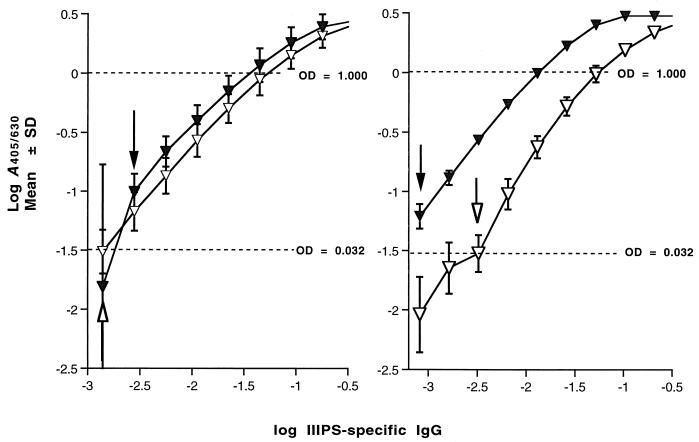
The range of direct proportionality between the observed values and the known concentration of antibodies in the IIIPS-specific IgG standard sera was identified by means of log-log transformations (38). The lowest concentration of IIIPS-specific IgG that could be measured in SHRS III by the III-HSA ELISA was 0.8 ng/ml (Fig. 2, right panel). A sensitivity of 0.8 ng/ml corresponded to a lower limit of quantitation of 80 ng/ml (with a starting test serum dilution of 1:100). The OD values obtained at lower starting dilutions of the test sera did not fall on the linear part of the titration curves and therefore did not improve the sensitivity of the assay. In the IIIPS + mHSA assay, the SHRS III serum pool had a lower limit of quantitation that was almost 1 order of magnitude higher (650 ng/ml) (Fig. 2, right panel). When reference serum 19 from a single individual was used as the standard IIIPS-specific IgG source, the III-HSA assay was less sensitive, with a limit of quantitation by ELISA that was not significantly different regardless of coating antigen used (III-HSA or IIIPS + mHSA) (Fig. 2, left panel).
FIG. 2.
Standard curves from the GBS III ELISA show a relationship between the quantity of specific immunoglobulin and A405/630. (Left panel) Reaction of reference serum 19 with plates coated with III-HSA (filled triangles) or IIIPS + mHSA (open triangles). (Right panel) Reaction of SHRS III with plates coated with III-HSA (filled triangles) or IIIPS + mHSA (open triangles). The arrows indicate the sensitivities of the III-HSA ELISA (filled arrows) and the IIIPS + mHSA ELISA (open arrows). The results represent means ± SDs for six experiments.
The amount of antigen bound to the plates significantly influences the sensitivity of the GBS III ELISAs.
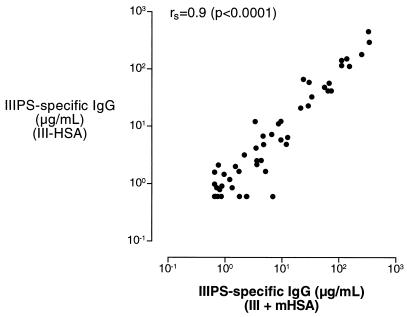
To determine whether the eightfold difference in sensitivity of the two GBS III ELISAs was a result of different concentrations of IIIPS bound to the plates (20, 24), we compared the concentrations of IIIPS-specific IgG in sera from recipients of either unconjugated IIIPS vaccine (n = 30) or III-TT conjugate vaccine (n = 30) as measured by ELISAs with plates that were coated with IIIPS + mHSA or with a suboptimal concentration of III-HSA (2 orders of magnitude lower than the optimal concentration). The amount of IIIPS-specific IgG that bound to plates coated with the suboptimal concentration of III-HSA was in good agreement with that measured with the IIIPS + mHSA assay (Fig. 3). These results suggest that the concentration of IIIPS bound to the plates may be lower when unconjugated IIIPS is used as the coating antigen.
FIG. 3.
Spearman rank correlation coefficient (rs) comparing IIIPS-specific antibodies in sera from healthy adults given GBS vaccines (III-TT, n = 30; IIIPS, n = 30) as measured by ELISAs with a suboptimal (by 2 orders of magnitude) III-HSA coating concentration (y axis) or with III + mHSA (x axis).
For ELISAs with identical specificity, the amount of antigen bound to the plate will be reflected by the concentration of IIIPS in solution that is needed to inhibit binding of 50% of IIIPS-specific antibodies (IC50) for a given serum. Thus, a comparison of the magnitude of the IC50s in the two GBS III ELISAs provides an additional estimate of the antigen concentration on the plates. The IC50 of IIIPS varied considerably with the same IIIPS standard serum (Table 1). The lowest IC50s were observed when unconjugated IIIPS was used as the coating antigen. Differences of 13- and 215-fold in the IC50 of IIIPS were observed when the SHRS III pool was compared to reference serum 19, indicating that a substantially higher concentration of the conjugated form of IIIPS bound to the ELISA plates.
TABLE 1.
IC50s of capsular polysaccharides in the binding of vaccine-induced GBS IIIPS-specific antibodies to IIIPS in the fluid phase (RABA) and in the solid phase (III-HSA and IIIPS + mHSA ELISAs)a
In the III-HSA ELISA, dilution curves for the test sera are parallel to those for the standard human reference serum pool.
Parallelism between the dilution curves for the standard human reference serum and those for the unknown sera is a prerequisite for accurate antibody quantitation when OD values are extrapolated from a standard curve (38). We used linear regression of log-log-transformed data to calculate the slopes defined by diluting sera. In the III-HSA ELISA, the range of observed slopes for the test sera was 0.86 to 1.08, with the GBS III standards (SHRS III and reference serum 19) representing the median value (slopes of 0.95). There was no difference in the slopes of the titration curves for adult sera obtained before and after immunization (with unconjugated IIIPS or III-TT conjugate as the immunogen). In contrast, parallel slopes were not observed when these same pre- and postimmunization sera were tested in the IIIPS + mHSA ELISA (range, 0.77 to 1.21, with a median of 1.07). The slopes for SHRS III and reference serum 19 were 0.95 and 0.93, respectively.
Measured antibody levels are the same whether IIIPS is used in solution (RABA) or supported by a solid surface (ELISA).
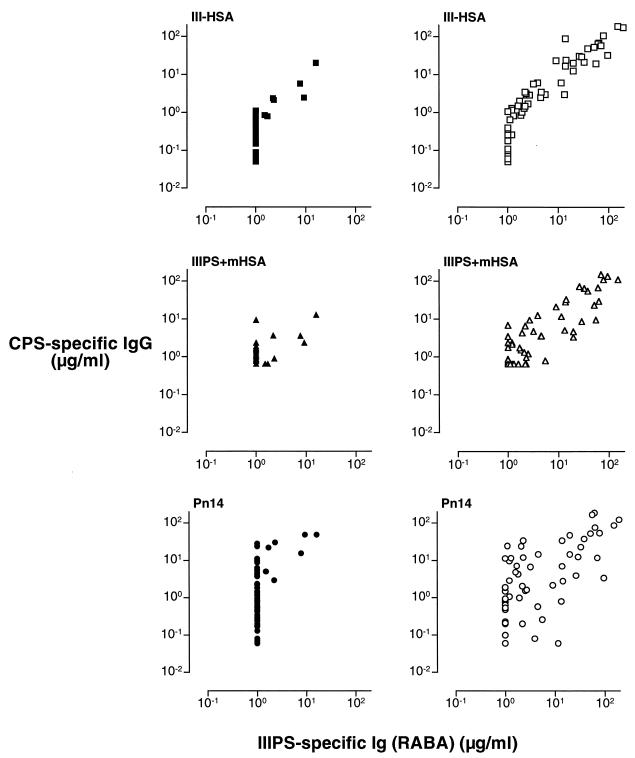
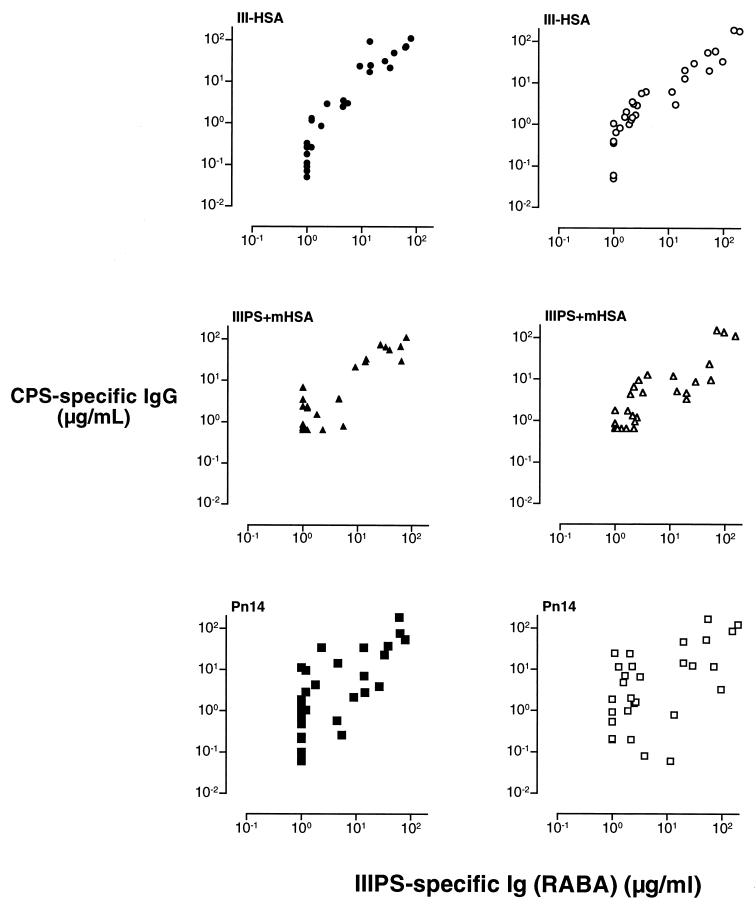
For more than 20 years, the RABA has been the “gold standard” for quantifying IIIPS-specific antibodies (5, 9, 15, 25). This assay has consistently demonstrated a high correlation between low maternal IIIPS-specific antibody levels and GBS III disease in young infants. We observed a significant correlation between the concentrations of vaccine-induced IIIPS-specific antibodies quantified by RABA and those measured either by III-HSA ELISA (rs = 0.96) or by IIIPS + mHSA ELISA (rs = 0.82) (Fig. 4; Table 2). There were no significant differences between the Spearman rank correlation coefficients for IIIPS-specific antibodies induced by the different GBS III vaccines (rs = 0.94 and 0.97 for unconjugated IIIPS and III-TT conjugate, respectively) (Fig. 5; Table 2). The association between the concentrations of naturally acquired IIIPS-specific antibodies (preimmune sera) detected by RABA and the concentrations measured by the two ELISAs could not be reliably estimated because the concentration of specific antibodies in a substantial proportion of the 60 sera tested was below the quantitation limit for both the IIIPS + mHSA ELISA (42 of 60) and the RABA (53 of 60).
FIG. 4.
Comparison of naturally acquired (preimmunization) (left panels) and vaccine-induced (III-TT, n = 30; IIIPS, n = 30) (right panels) IIIPS-specific antibodies in human sera as quantitated by IgG ELISA (y axis) and by RABA (x axis). The coating antigens used in the ELISAs are shown in the upper left corner of each panel. CPS, capsular polysaccharide.
TABLE 2.
Correlation of concentrations of vaccine-induced antibodies binding to IIIPS in solution (RABA) and those binding to native GBS IIIPS and desialylated IIIPS (i.e., Pn14 PS) supported by solid surfaces (ELISAs)
| Vaccinee group | No. of samples tested | Results with indicated ELISA coating antigena
|
||
|---|---|---|---|---|
| III-HSA | IIIPS + mHSA | Pn14 PSb | ||
| IIIPS | 30 | 0.94 (0.88-0.97) | 0.79 (0.59-0.90) | 0.74 (0.52-0.87) |
| III-TT | 30 | 0.97 (0.92-0.98) | 0.87 (0.74-0.94) | 0.55 (0.23-0.77) |
| All | 60 | 0.96 (0.93-0.98) | 0.82 (0.71-0.89) | 0.67 (0.49-0.79) |
Values are given as Spearman rank correlation coefficients between concentrations of GBS IIIPS-specific immunoglobulin in sera quantitated by the RABA and those quantitated by the ELISAs. The values in parentheses are the 95% confidence intervals. All correlations were statistically significant (P < 0.002).
Immunochemically identical to the GBS IIIPS core antigen.
FIG. 5.
Comparison of IIIPS-specific antibodies in human sera elicited by unconjugated IIIPS vaccine (n = 30) (left panels) or by III-TT conjugate vaccine (n = 30) (right panels), as quantitated by ELISA (y axis) and RABA (x axis). The coating antigens used in the ELISAs are shown in the upper left corner of each panel. CPS, capsular polysaccharide.
It has been suggested that immunization with GBS III-TT conjugate vaccine, but not immunization with unconjugated IIIPS, elicits antibodies that react with the desialylated GBS IIIPS, which is identical to Pn14 PS (16). To test this hypothesis, we compared the concentrations of GBS IIIPS- and Pn14 PS-specific IgGs in sera obtained after immunization with unconjugated IIIPS or with III-TT conjugate. Sera from recipients of either GBS vaccine contained antibodies that recognized desialylated IIIPS antigen (Pn14 PS-specific IgG). The concentration of Pn14 PS-specific IgG correlated directly with the levels of IIIPS-specific antibodies binding to native IIIPS in solution (as quantitated by RABA) (rs = 0.67) (Fig. 4; Table 2). There were no significant differences between the Spearman rank correlation coefficient for Pn14 PS-specific IgG elicited by unconjugated IIIPS and that for Pn14 PS-specific IgG elicited by conjugated III-TT vaccine (rs = 0.74 and 0.55, respectively) (Fig. 5; Table 2).
Conjugation of IIIPS to HSA or TT does not alter the fluid-phase epitope specificity of IIIPS.
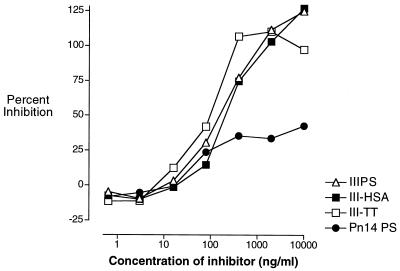
The IIIPS is unencumbered by attachment to a solid phase in the RABA. Thus, we performed RABA inhibitions to determine the epitope specificity of IIIPS after its coupling to proteins. Epitope specificity was not affected by covalent coupling (via controlled reductive amination) of IIIPS to HSA or to TT (Fig. 6). Uncoupled native IIIPS, III-TT, and III-HSA completely inhibited, at similar concentrations, the binding of the III-TT conjugate vaccine-induced human immunoglobulin (SHRS III) to extrinsically labeled soluble IIIPS. Approximately 40% of the GBS IIIPS-specific antibodies in SHRS III were inhibited by Pn14 PS. A similar proportion of IIIPS-specific antibodies was inhibited by Pn14 PS in assays using IIIPS supported by a solid surface (III-HSA and IIIPS + mHSA ELISAs). Thus, the specificities of the GBS III immunoassays tested are identical whether IIIPS is conjugated or unconjugated and whether it is in solution or supported by a solid surface.
FIG. 6.
Competitive-inhibition RABA demonstrating the specificity of GBS IIIPS-specific vaccine-induced antibodies in a standard human reference serum pool (SHRS III). The percent inhibition of binding of SHRS III to IIIPS in solution was determined by comparison of counts per minute in the presence and absence of inhibitor.
Levels of naturally acquired and vaccine-induced IIIPS-specific antibodies correlate with opsonophagocytic killing of GBS III organisms.
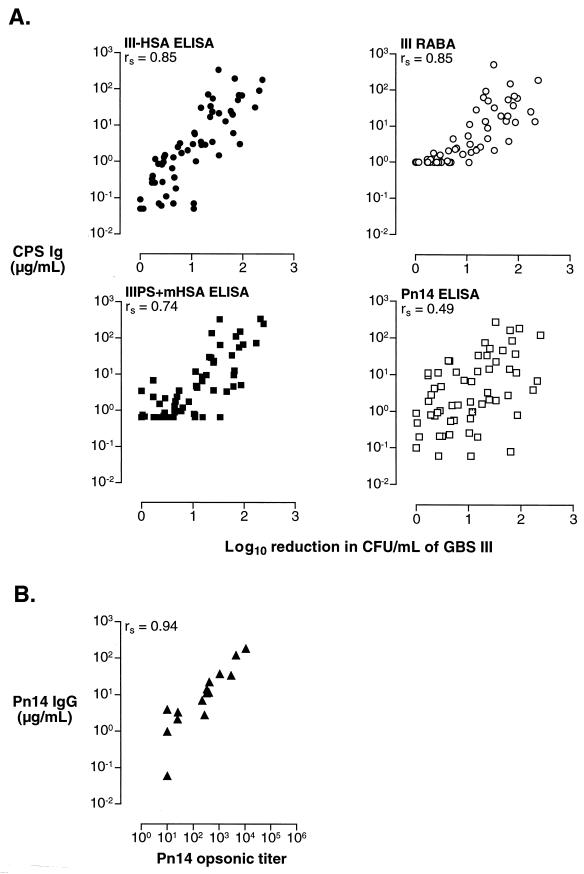
To assess the functional activity of sera from recipients of GBS III vaccines, we tested the ability of these sera to promote opsonophagocytic killing of type III GBS strain M781 (all sera [n = 60]) by human PMNs and of Pn14 strain DS2214 (15 of 60 sera from recipients of unconjugated IIIPS and III-TT conjugate vaccines) by a myelocytic cell line (HL-60) differentiated into granulocytes. There was a direct correlation between the serum IIIPS-specific IgG concentration measured by each immunoassay and opsonophagocytic killing of type III organisms (rs = 0.85 and 0.73 for the III-HSA and IIIPS + mHSA ELISAs, respectively; rs = 0.83 for the RABA) (Fig. 7A). A significant correlation between the concentration of Pn14 PS-specific IgG and the amount of opsonophagocytic killing of type III GBS was also documented (rs = 0.49); however, the correlation between the Pn14 PS-specific IgG and opsonophagocytic killing of Pn14 was stronger (rs = 0.94) (Fig. 7).
FIG. 7.
Correlation of vaccine-induced (III-TT, n = 30; IIIPS, n = 30) IIIPS-specific antibodies in human sera, as measured by ELISA (y axis) and opsonophagocytic killing (x axis) of GBS III (A) or Pn14 (B). Opsonophagocytic killing is the log10 reduction in CFU (A) or the titer resulting in 50% reduction in CFU (B). The coating antigens used in the ELISAs are shown in the upper left corner of each panel. CPS, capsular polysaccharide.
GBS III-specific antibodies exhibit relatively high avidity, whether they are elicited by unconjugated or conjugated vaccine.
The avidity of an antibody strongly influences its binding to an antigen (20, 24, 32). The binding strengths of the GBS vaccine-induced antibodies were estimated by two methods. First, the percentage of IIIPS-specific antibodies binding to suboptimally versus optimally antigen-coated plates was expressed as the avidity index (42). Next, the molar concentration of a chaotropic agent (NH4SCN) required to elute 50% of bound antibodies was determined (39). The median avidity index was 33% (25th to 75th quartile [range], 14 to 78% [2 to ≥100%]; n = 52) for vaccine-induced antibodies and 26% (19 to 36% [7.1 to 254%]; n = 19) for naturally acquired antibodies. The results were the same when avidity was expressed as the molar concentration of NH4SCN needed to elute 50% of the antibodies bound to III-HSA-coated plates, with a median value of 0.74 M (25th to 75th quartile, 0.33 to 1.16 M; range, 0.1 to 3.5 M). There was no significant difference in avidity between naturally acquired and vaccine-induced antibodies or between human antibodies induced by unconjugated IIIPS (n = 24) and those induced by III-TT (n = 28).
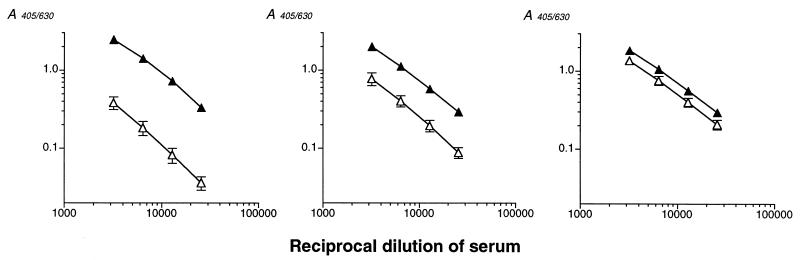
Figure 8 illustrates the differences in titration curves for sera containing low-, intermediate-, and high-avidity IIIPS-specific IgG. SHRS III, a postimmunization serum pool from volunteers with low preimmunization concentrations of IIIPS-specific IgG, had an intermediate avidity index of 34.7 ± 5.7% (mean ± standard deviation [SD] for four experiments), corresponding to the median value for the test samples. In contrast, reference serum 19, obtained from an individual with high preimmunization levels of IIIPS-specific IgG, had a high avidity index of 71.3 ± 10% (mean ± SD for four experiments). The relative affinity for reference serum 19 approached the 75th quartile for all sera tested. The implication is that this serum contains antibodies of higher avidity than those present in sera from the majority of GBS III vaccinees.
FIG. 8.
Dilution curves from GBS III ELISA in which III-HSA was used at an optimal coating concentration (filled triangles) or at a suboptimal coating concentration (2 orders of magnitude lower than optimal) (open triangles). The relationship between the avidity of the antibodies and their binding to ELISA plates is evident. (Left panel) Reaction of a GBS III vaccine-induced serum of low avidity (avidity index, 12%). (Middle panel) Reaction of a GBS III-TT conjugate vaccine-induced serum pool (SHRS III) of intermediate avidity (avidity index, 35%). (Right panel) Reaction of tetravalent GBS polysaccharide vaccine-induced reference serum 19 of high avidity (avidity index, 71%). Results are means ± SDs for four experiments.
A significant proportion of GBS III vaccinees develop antibodies to the desialylated GBS IIIPS.
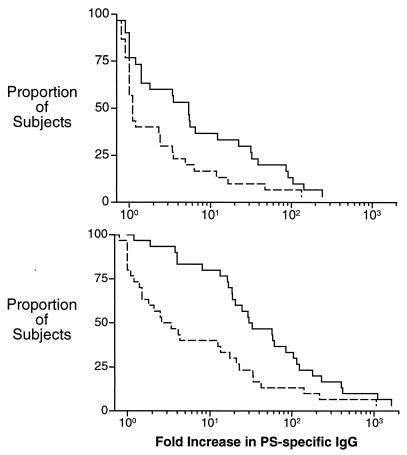
The observed increases in IIIPS- and Pn14 PS-specific IgG after immunization are summarized as a reverse cumulative plot in Fig. 9. A ≥4-fold increase in IIIPS-specific IgG levels in serum was observed for 15 of 30 recipients of unconjugated IIIPS vaccine (Fig. 9, upper panel) and for 26 of 30 recipients of III-TT conjugate vaccine (Fig. 9, lower panel) at 8 weeks after immunization. Thirteen vaccinees (43%) had a <2-fold increase in IIIPS-specific IgG, and two (7%) did not respond to III-TT conjugate vaccine.
FIG. 9.
Reverse cumulative distribution plot of the fold increase in IIIPS-specific IgG in sera 8 weeks after immunization with unconjugated IIIPS (n = 30) (upper panel) or III-TT conjugate vaccine (n = 30) (lower panel). The proportions of vaccine-induced IgG that recognized native IIIPS (solid lines) or Pn14 PS (i.e., desialylated IIIPS) (dotted lines) are indicated.
A significant proportion of GBS III vaccine responders also developed antibodies that recognized the desialylated IIIPS (i.e., Pn14 PS) (Fig. 9). Eleven (73%) of the 15 subjects responding to unconjugated IIIPS vaccine with a ≥4-fold increase in IIIPS-specific IgG also had a ≥2-fold increase in Pn14 PS-specific IgG: five (33%) had 2- to 4-fold increases, and six (40%) had a ≥4-fold increase (Fig. 9, upper panel). In comparison, 4 (15%) of 26 III-TT conjugate vaccine responders had a 2- to 4-fold increase in Pn14 PS-specific IgG, while 13 (50%) had a ≥4-fold increase (Fig. 9, lower panel). Thus, 28 of 41 vaccinees developed IgG specific for the desialylated IIIPS as well as to the native IIIPS antigen. Furthermore, the proportion of vaccine responders who developed Pn14 PS-specific IgG was not dependent on conjugation of the GBS III vaccine or the lack thereof.
Antibodies directed to the desialylated IIIPS (i.e., Pn14 PS) promote opsonophagocytic killing of GBS III and Pn14.
The fact that the repeating unit of Pn14 PS is identical to a part of the repeating unit of IIIPS had raised the question of whether type-specific antibodies induced by one of these organisms might protect against disease caused by the other (21, 22, 27). The observed percent reductions in CFU of GBS III per milliliter in the presence of IIIPS and Pn14 PS are presented in the lower panels of Fig. 10 (IIIPS-induced sera) and 11 (III-TT-induced sera). Our results demonstrate that the subpopulation of GBS III vaccine-induced antibodies that bind to Pn14 PS (Fig. 10 and 11, upper panels) promotes killing of GBS III (Fig. 10 and 11, lower panels). Furthermore, the degree of inhibition of killing of GBS III by Pn14 PS depends on the proportion of IIIPS-specific antibodies that recognize the desialylated IIIPS (i.e., Pn14 PS). In addition, the functional activity of this subpopulation of antibodies does not depend on whether the IIIPS used as the immunogen is unconjugated or conjugated.
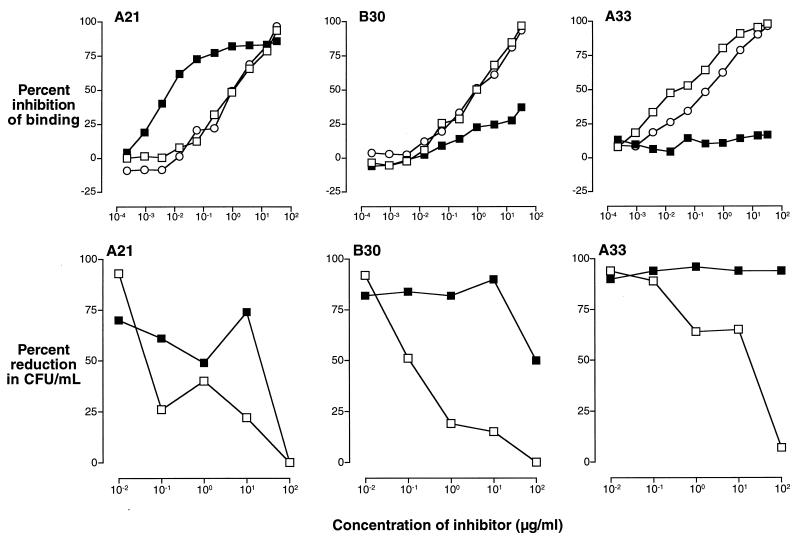
FIG. 10.
Competitive-inhibition ELISAs (upper panels) and the corresponding competitive inhibition of opsonophagocytic killing of GBS III (strain M781) by human PMNs in the presence of endogenous complement (lower panels) demonstrate the functional activity of IIIPS-specific antibodies that cross-react with Pn14 PS. The results shown are for sera from three volunteers immunized with unconjugated IIIPS that represent the observed categories of antibody specificity: left panels, all GBS IIIPS-specific antibodies cross-react with Pn14 PS (A21); middle panels, a subpopulation of GBS IIIPS-specific antibodies cross-react with Pn14 PS (B30); right panels, no GBS IIIPS-specific antibodies cross-react with Pn14 PS (A33). The upper panels show the percent inhibition of binding of IIIPS-induced IgG, as determined by comparison of A405/630 in the presence and absence of inhibitor. The lower panels show the percent reduction in CFU in the presence of inhibitors. The following inhibitors were used: Pn14 PS (filled squares), IIIPS (open squares), and III-HSA (open circles).
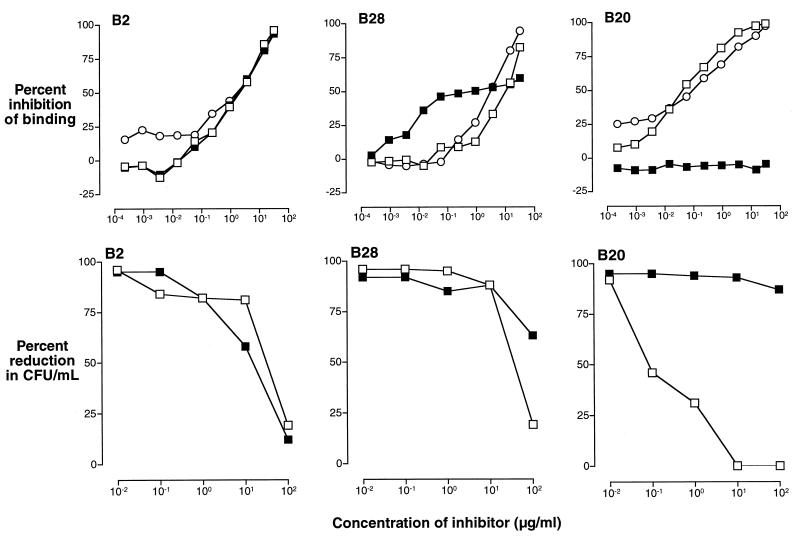
FIG. 11.
Competitive-inhibition ELISAs (upper panels) and the corresponding competitive inhibition of opsonophagocytic killing of GBS III (strain M781) by human PMNs in the presence of endogenous complement (lower panels) demonstrate the functional activity of IIIPS-specific antibodies that cross-react with Pn14 PS. The results shown are for sera from three volunteers immunized with III-TT conjugate vaccine. These sera represent the observed categories of antibody specificity: left panels, all GBS IIIPS-specific antibodies cross-react with Pn14 PS (B2); middle panels, a subpopulation of GBS IIIPS-specific antibodies cross-react with Pn14 PS (B28); right panels, no GBS IIIPS-specific antibodies cross-react with Pn14 PS (B20). The upper panels show the percent inhibition of binding of III-TT-induced IgG, as determined by comparison of A405/630 in the presence and absence of inhibitor. The lower panels show the percent reduction in CFU in the presence of inhibitors. The following inhibitors were used: Pn14 PS (filled squares), IIIPS (open squares), and III-HSA (open circles).
GBS III vaccine-induced antibodies recognizing the desialylated IIIPS represent a subpopulation of IIIPS-specific antibodies.
To determine whether vaccine-induced antibodies that recognize the desialylated IIIPS (i.e., Pn14 PS) represent a subpopulation of IIIPS-specific antibodies or whether they are directed only to Pn14 PS and result from alterations in epitope specificity due to the conjugation of IIIPS, we investigated the effect of purified IIIPS or Pn14 PS upon binding to ELISA plates coated with III-HSA. The results of competitive-inhibition ELISAs for representative sera are shown in Fig. 10 and 11. Three binding patterns are apparent: (i) complete inhibition by both IIIPS and Pn14 PS (upper left panels), (ii) partial inhibition by Pn14 PS and total inhibition by IIIPS (upper middle panels), and (iii) no detectable inhibition by Pn14 PS and total inhibition by IIIPS (upper right panels). The majority of the sera obtained from individuals after immunization with GBS vaccines contained antibodies that recognized purified Pn14 PS.
No evidence exists for neoepitope introduction due to conjugation of IIIPS to TT.
To determine whether conjugation of IIIPS to TT introduces immunogenic neoepitopes, we compared the inhibition of binding of antibodies in sera from IIIPS recipients (Fig. 10) and III-TT conjugate recipients (Fig. 11) to III-HSA-coated plates by native IIIPS and III-HSA. IIIPS and III-HSA similarly inhibited the binding of these sera. Specifically, there was no difference in binding specificity between IIIPS-specific antibodies from volunteers immunized with unconjugated IIIPS and those from volunteers receiving III-TT conjugate vaccine (Fig. 10 and 11, respectively). In short, the two vaccines induced IIIPS-specific IgG of similar specificity.
DISCUSSION
Attention to several parameters is important for accurate quantitation of antibodies by immunoassays. These parameters include specificity, sensitivity, and parallelism of slopes of OD with dilutions of clinical samples and standard reference sera (33, 38). In an effort to validate two ELISAs for quantitation of antibodies to type III GBS polysaccharide, we analyzed sera from 60 healthy young adults before and after immunization with III-TT conjugate vaccine or unconjugated IIIPS vaccine (28). IIIPS covalently conjugated to HSA (25) or IIIPS mixed with methylated HSA (16) was used as the coating antigen. We have previously reported that GBS type-specific polysaccharides conjugated to HSA as coating antigens result in highly sensitive and specific quantitative immunoassays (10, 11, 25, 28). This report extends these findings and provides a comparison of assay parameters important for the two IIIPS-specific IgG ELISAs. Our experiments confirm that use of IIIPS conjugated to HSA as a coating antigen retains the specificity and improves the sensitivity of an ELISA that uses unconjugated IIIPS as a coating antigen (25).
In each assay, all antibodies binding to IIIPS-coated ELISA plates were inhibited by IIIPS in solution. Furthermore, there were no detectable differences in the specificity of the assays with two standard GBS III reference sera. Equivalent specificity of the two ELISAs was further supported by an identical inhibition pattern observed for SHRS III in an immunoassay where IIIPS-specific antibodies bound to native IIIPS in solution (i.e., inhibition RABA).
A subpopulation of ≈45% of all GBS III vaccine-induced antibodies in the pooled GBS III reference serum SHRS III bound to both the desialylated IIIPS (i.e., Pn14 PS) and the native IIIPS. These data define a population of GBS IIIPS-specific antibodies in SHRS III that cross-reacts with Pn14 PS. This finding is in accordance with the structural relatedness of the type-specific polysaccharides of GBS III and Pn14 (27). These two capsular polysaccharides differ only in the presence of a sialic acid residue at the end of the side chain of the repeating pentasaccharide of IIIPS. Thus, the identical antigenicity in the three GBS III immunoassays confirms that the cross-reactive subpopulation of antibodies is not a result of a difference in the specificity of the assays but rather is a result of the presence of antibodies directed to different epitopes on the IIIPS antigen.
Quantitative precipitation has been employed by our group and by others to establish the concentration of polysaccharide-specific antibodies in reference sera used to standardize immunoassays (19, 23, 33). The concentration of IIIPS-specific antibody assigned to SHRS III, i.e., 88 μg/ml, is based on the sum of the values obtained by quantitative precipitation of antibodies and by RABA for nonprecipitating antibodies (25). In contrast, SHRS III has been reported to contain 33 μg of IIIPS-specific antibody per ml when reference serum 19 is used as the GBS III-specific standard serum in the IIIPS + mHSA ELISA (16). There are several possible explanations for this difference.
First, the use of a reference serum containing IIIPS-specific IgG of high avidity may lead to underestimation of serum antibodies of lower avidity. We therefore analyzed the avidity of naturally acquired and vaccine-induced IIIPS-specific IgG by two methods (17, 20, 42). By each method, the avidity of reference serum 19 was higher than that of most sera. Approximately two-thirds of the immune sera tested had lower avidity indices than reference serum 19. A standard curve based on an immune serum with antibodies of higher affinity than those of the subject population may lead to underestimation of the antibody response (33).
Next, unconjugated IIIPS binds poorly to plastic surfaces. Inhibition ELISAs indicated that there was a 13- to 215-fold difference in the amount of IIIPS bound to the plates in the two ELISAs (Table 1). A smaller amount of IIIPS bound to the ELISA plates will accentuate an underestimation of antibodies in test sera, because high-affinity antibodies bind substantially better to suboptimally coated plates than do antibodies of lower affinities. The avidity indices observed for the vaccine-induced test sera varied considerably, with ranges of from 5 to 335 for IIIPS-induced antibodies and from 2 to 151 for III-TT conjugate-induced antibodies. The range of avidity indices was similar for naturally acquired antibodies. Apparent differences in antibody avidity within a subject population will result in substantial differences in the binding of the various antibodies to plates coated with unconjugated polysaccharide.
Finally, the lack of parallelism between the titration slope for reference serum 19 and the slopes for some of the test sera in the IIIPS + mHSA assay may lead to over- or underestimation of IIIPS-specific antibodies. Parallelism between the titration curve for the standard reference serum and that for an unknown is requisite for accurate antibody quantitation when OD values are extrapolated from a standard curve (38). In determining the slopes of titration curves for test sera containing natural and vaccine-induced antibodies, we found a wide range of slopes in the IIIPS + mHSA assay (0.77 to 1.21, with a median of 1.07). In contrast, in the III-HSA assay, parallel slopes for OD were observed with dilutions of the test sera and SHRS III, and the slope of SHRS III represented the median for all sera tested.
In summary, both of the GBS III ELISAs and the RABA are specific for detection of IIIPS-specific antibodies in human sera, and the III-HSA ELISA is the most sensitive. SHRS III represents the study population (both naturally acquired and vaccine-induced antibodies) in terms of the slope of its titration curve and the avidity of its antibodies and therefore is an ideal standard serum for quantitation of IIIPS-specific antibodies.
It has been hypothesized that III-TT glycoconjugate vaccines contain immunogenic neoepitopes (Pn14 PS and possibly other epitopes) and that antibodies to these epitopes do not protect against GBS III disease (16). Protective human immunity to GBS III correlates with the presence of antibodies to the capsular polysaccharide that promote in vitro opsonophagocytosis and killing of GBS III (9, 13, 14, 28; Baker et al., submitted). An association between the concentration of IIIPS-specific antibodies and protection against GBS III disease was first established by RABA (9). To test whether conjugation of IIIPS to TT results in immunogenic nonprotective neoepitope(s), we investigated the association between (i) the concentration of IIIPS-specific antibodies in human sera from healthy adults before and after immunization with GBS III vaccines (by RABA) and the levels of Pn14 PS-specific IgG (i.e., antibodies specific for the desialylated IIIPS) and IIIPS-specific IgG assessed by the polysaccharide-specific ELISAs (Fig. 4 and 5) and (ii) the concentration of IIIPS-specific antibodies and the functional activity of these antibodies.
A strong association was observed between the concentrations of vaccine-induced IIIPS-specific antibodies measured by RABA and those quantitated by the III-HSA and IIIPS + mHSA ELISAs. This association was strongest for the GBS III vaccine-induced antibodies (rs = 0.94 and 0.97 for IIIPS- and III-TT-induced antibodies, respectively) in the III-HSA ELISA. We observed no differences between the antibody levels measured in the immunoassays that could be attributed to the form of the IIIPS used for immunization (unconjugated versus conjugated) (Table 2). Thus, these data do not support the contention that the III-TT conjugate vaccine, produced by controlled conjugation of TT to IIIPS, contains immunogenic neoepitopes.
We also observed a strong association between the concentration of vaccine-induced IIIPS-specific antibodies and their in vitro opsonophagocytic activity (13, 28), indicating that the concentration of IIIPS-specific IgG predicts vaccine-induced protection against GBS disease. In addition, a recent case-control study has revealed that a low maternal concentration of naturally acquired IIIPS-specific IgG in serum at delivery, as detected in the III-HSA assay, protects neonates exposed to GBS III from invasive early-onset disease (Baker et al., submitted). The protective concentration of IIIPS-specific antibodies in serum was below the quantitation limits of both the IIIPS + mHSA ELISA and the RABA.
The structural relatedness of the capsular polysaccharides of GBS III and Pn14, in conjunction with the observed protection against invasive disease in neonates and infants by antibodies elicited to these polysaccharides, raised the possibility that GBS III vaccine-induced antibodies might protect against invasive disease caused by Pn14. The majority (74%) of volunteers who responded to unconjugated IIIPS vaccine with a fourfold or greater increase in IIIPS-specific IgG also had a twofold or greater increase in antibodies to Pn14. A similar cross-reactive Pn14-specific subpopulation of IIIPS-specific antibodies was found in the majority (65%) of human adults responding to the III-TT conjugate vaccine. Therefore, a substantial proportion of adults immunized with GBS III vaccines develop antibodies to desialylated IIIPS. In addition, the presence of a Pn14-cross-reactive antibody subpopulation does not depend on whether IIIPS is administered in an unconjugated or conjugated form.
To further delineate the GBS III-specific antibodies that cross-react with Pn14, we determined the antigenic specificity of vaccine-induced antibodies in the sera of vaccine responders. Inhibition ELISA data demonstrated that the vaccine responders fell into three groups with respect to IIIPS-specific antibodies recognizing Pn14 PS: (i) all vaccine-induced antibodies cross-reacted with Pn14 PS with a relative affinity equal to or greater than that which they exhibited for IIIPS; (ii) a subpopulation of vaccine-induced antibodies recognized Pn14 with a relative affinity that was weaker than, equaled, or was greater than that which they showed for IIIPS; or (iii) none of the vaccine-induced antibodies recognized Pn14 PS. Neither the presence nor the size of the GBS III vaccine-induced cross-reactive Pn14-binding antibody subpopulation depended upon whether IIIPS was given in a conjugated or unconjugated form. Thus, the induction of neoepitopes by conjugation of IIIPS to a carrier protein does not account for this subpopulation.
Despite the similar structures of IIIPS and Pn14 PS, naturally acquired Pn14-specific antibody and antibodies induced by unconjugated Pn14 vaccine in humans at risk for GBS III disease are not opsonic for GBS III (8, 27). In the present report, we provide the first data showing that antibodies induced by either unconjugated IIIPS or III-TT conjugate vaccine are opsonic for both GBS III and Pn14. The level of GBS III vaccine-induced antibodies that recognized Pn14 PS correlated directly with the opsonophagocytic killing of both GBS III and Pn14. Furthermore, if cross-reacting antibodies were present, they were inhibited by purified Pn14 PS in a GBS III opsonophagocytosis assay. The magnitude of this observed reduction in IIIPS-specific antibody-mediated opsonophagocytic killing of GBS III in the presence of Pn14 PS corresponded to the magnitude of the cross-reactive (sub)population of antibodies binding to Pn14. Thus, a significant proportion of humans immunized with IIIPS-based vaccines produce IgG antibodies that bind to the repeating unit of desialylated IIIPS (which is identical to the repeating unit of Pn14 PS) as well as to the complete repeating unit. Not all adults immunized with GBS III vaccines produce antibodies to the cross-reactive epitope. Consequently, GBS III vaccines could not replace Pn14 vaccines for protection against invasive disease due to Pn14. However, when present, these vaccine-induced cross-reacting antibodies do confer antibody-mediated opsonophagocytic killing of Pn14 in vitro. Thus, administration of GBS III vaccine could provide protection against two pathogens that cause invasive disease in young infants.
Acknowledgments
Invaluable technical assistance in the production of vaccines and antigens was provided by April Blodgett, Ken D. Johnson, Julianne Pinel, and Barbara G. Reinap at the Channing Laboratory. Marcia A. Rench and Melissa E. Hickman at Baylor College of Medicine and Angela C. Tramontano and Farah N. Lalani at the Channing Laboratory applied their expertise to the recruitment of subjects, collection of specimens, performance of assays, and analysis of antibody responses.
This work was supported by contract AI-75326, Merit Award AI-23339 (to D.L.K.), and grant AI-85334 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Editor: E. I. Tuomanen
REFERENCES
- 1.Arakere, G., and C. E. Frasch. 1991. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect. Immun. 59:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, C. J. 1997. Group B streptococcal infections. Clin. Perinatol. 24:59-70. [PubMed] [Google Scholar]
- 3.Baker, C. J., and M. S. Edwards. 2001. Group B streptococcal infections, p. 1091-1156. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W.B. Saunders, Philadelphia, Pa.
- 4.Baker, C. J., M. S. Edwards, and D. L. Kasper. 1981. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics 68:544-549. [PubMed] [Google Scholar]
- 5.Baker, C. J., and D. L. Kasper. 1976. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N. Engl. J. Med. 294:753-756. [DOI] [PubMed] [Google Scholar]
- 6.Baker, C. J., and D. L. Kasper. 1977. Immunological investigation of infants with septicemia or meningitis due to group B Streptococcus. J. Infect. Dis. 136(Suppl.):S98-S104. [DOI] [PubMed] [Google Scholar]
- 7.Baker, C. J., D. L. Kasper, and C. E. Davis. 1976. Immunochemical characterization of the “native” type III polysaccharide of group B Streptococcus. J. Exp. Med. 143:258-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker, C. J., D. L. Kasper, M. S. Edwards, and G. Schiffman. 1980. Influence of preimmunization antibody levels on the specificity of the immune response to related polysaccharide antigens. N. Engl. J. Med. 303:173-178. [DOI] [PubMed] [Google Scholar]
- 9.Baker, C. J., D. L. Kasper, I. Tager, A. Paredes, S. Alpert, W. M. McCormack, and D. Goroff. 1977. Quantitative determination of antibody to capsular polysaccharide in infection with type III strains of group B Streptococcus. J. Clin. Investig. 59:810-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker, C. J., L. C. Paoletti, M. A. Rench, H. K. Guttormsen, V. J. Carey, M. E. Hickman, and D. L. Kasper. 2000. Use of capsular polysaccharide-tetanus toxoid conjugate vaccine for type II group B Streptococcus in healthy women. J. Infect. Dis. 182:1129-1138. [DOI] [PubMed] [Google Scholar]
- 11.Baker, C. J., L. C. Paoletti, M. R. Wessels, H. K. Guttormsen, M. A. Rench, M. E. Hickman, and D. L. Kasper. 1999. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J. Infect. Dis. 179:142-150. [DOI] [PubMed] [Google Scholar]
- 12.Baker, C. J., M. A. Rench, M. S. Edwards, R. J. Carpenter, B. M. Hays, and D. L. Kasper. 1988. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N. Engl. J. Med. 319:1180-1185. [DOI] [PubMed] [Google Scholar]
- 13.Baltimore, R. S., D. L. Kasper, C. J. Baker, and D. K. Goroff. 1977. Antigenic specificity of opsonophagocytic antibodies in rabbit antisera to group B streptococci. J. Immunol. 118:673-678. [PubMed] [Google Scholar]
- 14.Baltimore, R. S., D. L. Kasper, and J. Vecchitto. 1979. Mouse protection test for group B Streptococcus type III. J. Infect. Dis. 140:81-88. [DOI] [PubMed] [Google Scholar]
- 15.Basham, L. E., V. Pavliak, X. Li, A. Hawwari, K. L. Kotloff, R. Edelman, and A. Fattom. 1996. A simple, quantitative, reproducible avidin-biotin ELISA for the evaluation of group B streptococcus type-specific antibodies in humans. Vaccine 14:439-445. [DOI] [PubMed] [Google Scholar]
- 16.Bhushan, R., B. F. Anthony, and C. E. Frasch. 1998. Estimation of group B streptococcus type III polysaccharide-specific antibody concentrations in human sera is antigen dependent. Infect. Immun. 66:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruderer, U., M. Deusinger, U. Schurch, and A. B. Lang. 1992. Analyses of affinity distributions within polyclonal populations of antigen-specific antibodies. Evaluation of their accuracy in population detection using monoclonal antibodies. J. Immunol. Methods 151:157-164. [DOI] [PubMed] [Google Scholar]
- 18.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28:350-356. [DOI] [PubMed] [Google Scholar]
- 19.Eisenstein, T. K., B. J. De Cueninck, D. Resavy, G. D. Shockman, R. B. Carey, and R. M. Swenson. 1983. Quantitative determination in human sera of vaccine-induced antibody to type-specific polysaccharides of group B streptococci using an enzyme-linked immunosorbent assay. J. Infect. Dis. 147:847-856. [DOI] [PubMed] [Google Scholar]
- 20.Feldman, R. G., M. E. Hamel, M. A. Breukels, N. F. Concepcion, and B. F. Anthony. 1994. Solid-phase antigen density and avidity of antibodies detected in anti-group B streptococcal type III IgG enzyme immunoassays. J. Immunol. Methods 170:37-45. [DOI] [PubMed] [Google Scholar]
- 21.Fischer, G. W., J. W. Bass, G. H. Lowell, and M. H. Crumrine. 1978. Group B streptococcal and pneumococcal sepsis in newborn infants: the role of pneumococcal antibody. Pediatrics 62:620-621. [PubMed] [Google Scholar]
- 22.Fischer, G. W., G. H. Lowell, M. H. Crumrine, and S. R. Wilson. 1979. Immunoprecipitation and opsonic cross-reaction between type-14 pneumococcus and group-B streptococcus type III. Lancet 1:75-77. [DOI] [PubMed] [Google Scholar]
- 23.Gotschlich, E. C., M. Rey, R. Triau, and K. J. Sparks. 1972. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J. Clin. Investig. 51:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granoff, D. M., P. G. Shackelford, S. J. Holmes, A. H. Lucas. 1993. Variable region expression in the antibody responses of infants vaccinated with Haemophilus influenzae type b polysaccharide-protein conjugates. Description of a new lambda light chain-associated idiotype and the relation between idiotype expression, avidity, and vaccine formulation. J. Clin. Investig. 91:788-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guttormsen, H. K., C. J. Baker, M. S. Edwards, L. C. Paoletti, and D. L. Kasper. 1996. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J. Infect. Dis. 173:142-150. [DOI] [PubMed] [Google Scholar]
- 26.Hornbeck, P. 1994. Enzyme-linked immunosorbent assays, p. 2.1.2-2.1.22. In J. Coligan, A. Kruisbeek, D. Margulies, E. Shevach, and W. Strober (ed.), Current protocols in immunology, vol. 1. John Wiley and Sons, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 27.Kasper, D. L., C. J. Baker, R. S. Baltimore, J. H. Crabb, G. Schiffman, and H. J. Jennings. 1979. Immunodeterminant specificity of human immunity to type III group B streptococcus. J. Exp. Med. 149:327-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasper, D. L., L. C. Paoletti, M. R. Wessels, H. K. Guttormsen, V. J. Carey, H. J. Jennings, and C. J. Baker. 1996. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J. Clin. Investig. 98:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotloff, K. L., A. Fattom, L. Basham, A. Hawwari, S. Harkonen, and R. Edelman. 1996. Safety and immunogenicity of a tetravalent group B streptococcal polysaccharide vaccine in healthy adults. Vaccine 14:446-450. [DOI] [PubMed] [Google Scholar]
- 30.Kristoffersen, E. K., and R. Matre. 1996. Co-localization of the neonatal Fc gamma receptor and IgG in human placental term syncytiotrophoblasts. Eur. J. Immunol. 26:1668-1671. [DOI] [PubMed] [Google Scholar]
- 31.Larson, E., B. Howlett, and A. Jagendorf. 1986. Artificial reductant enhancement of the Lowry method for protein determination. Anal. Biochem. 155:243-248. [DOI] [PubMed] [Google Scholar]
- 32.Lehtonen, O. P., and M. K. Viljanen. 1980. Antigen density in ELISA; effect on avidity dependency. J. Immunol. Methods 36:63-70. [DOI] [PubMed] [Google Scholar]
- 33.Madore, D. V., N. M. Strong, and S. A. Quataert. 1999. Validation and standardization of serologic methods for evaluation of clinical immune responses to vaccines, p. 43-75. In L. C. Paoletti and P. M. McInnes (ed.), Vaccines, from concept to clinic: a guide to the development and clinical testing of vaccines for human use. CRC Press LLC, Boca Raton, Fla.
- 34.Matre, R., O. Tonder, and C. Endresen. 1975. Fc receptors in human placenta. Scand. J. Immunol. 4:741-745. [DOI] [PubMed] [Google Scholar]
- 35.Musher, D. M. 2001. Pneumococcal infections, p. 882-889. In E. Braunwald, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrisons's principles of internal medicine, 15th ed. The McGraw-Hill Companies, Inc., New York, N.Y.
- 36.Pitcher-Wilmott, R. W., P. Hindocha, and C. B. Wood. 1980. The placental transfer of IgG subclasses in human pregnancy. Clin. Exp. Immunol. 41:303-308. [PMC free article] [PubMed] [Google Scholar]
- 37.Plikaytis, B. D., D. Goldblatt, C. E. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Kayhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 38:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plikaytis, B. D., P. F. Holder, L. B. Pais, S. E. Maslanka, L. L. Gheesling, and G. M. Carlone. 1994. Determination of parallelism and nonparallelism in bioassay dilution curves. J. Clin. Microbiol. 32:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polanec, J., I. Seppala, S. Rousseau, and K. Hedman. 1994. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J. Clin. Lab. Anal. 8:16-21. [DOI] [PubMed] [Google Scholar]
- 40.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 41.Story, C. M., J. E. Mikulska, and N. E. Simister. 1994. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J. Exp. Med. 180:2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wessels, M. R., L. C. Paoletti, D. L. Kasper, J. L. DiFabio, F. Michon, K. Holme, and H. J. Jennings. 1990. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J. Clin. Investig. 86:1428-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu, X., B. Gray, S. Chang, J. I. Ward, K. M. Edwards, and M. H. Nahm. 1999. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 180:1569-1576. [DOI] [PubMed] [Google Scholar]