Abstract
Campylobacter jejuni 81-176 pgl mutants impaired in general protein glycosylation showed reduced ability to adhere to and invade INT407 cells and to colonize intestinal tracts of mice.
There is an increasing awareness of the existence of prokaryotic glycoproteins (36), often in complex surface structures such as pili (7, 8, 28, 39), S layers (37), and flagella (6, 10, 11, 12, 19, 23, 44). Among glycosylated flagellins, those of Campylobacter spp. are the best characterized (11, 16, 42). The nature and extent of flagellin glycosylation have been determined for strain 81-176, one of the best-characterized strains of Campylobacter jejuni (2, 3, 5, 21, 29, 41, 45, 46) and one which has been documented to cause diarrheal disease in two volunteer feeding studies 5; D. T. Tribble, unpublished data). Flagellin from 81-176 contains 19 sites of O-linked glycosylation to the monosaccharide pseudaminic acid (5,7-diacetamido-3,5,7,9-tetradeoxy-l-glycero-l-manno-nonulosonic acid) and analogs of pseudaminic acid (42). Additionally, C. jejuni 81-176 has been shown to contain a general protein glycosylation (pgl) system affecting many other soluble and membrane-associated proteins (41). The only reported phenotype of pgl mutants has been the loss of immunogenicity of multiple proteins as detected by Western blot analyses using polyclonal, hyperimmune rabbit antisera, changes that were identical to those seen following chemical deglycosylation of the same protein preparations (42). However, neither the identity of the proteins glycosylated by the pgl system nor the chemical nature of the attached carbohydrate(s) has been reported. This study describes additional phenotypes of 81-176 pglB and pglE mutants. The predicted protein encoded by pglB shows significant similarity to domains of an oligosaccharide transferase of Saccharomyces cerevisiae (48) and an ortholog in Methanobacterium spp. (38). PglE shows highest similarity to a putative aminotransferase involved in lipopolysaccharide synthesis in Bacteroides fragilis (9). The protein also shows homology to proteins involved in glycosylation of pilin in Neisseria spp. (20, 31) and flagellin in Caulobacter crescentus (23) and Aeromonas caviae (13, 32).
Growth comparisons.
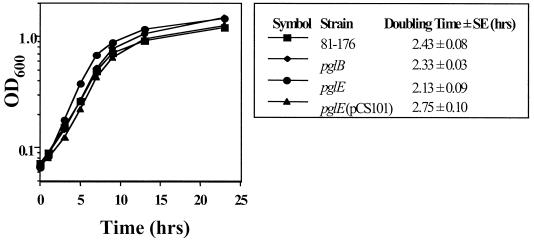
Cell morphology, as determined by transmission electron microscopy, was similar for 81-176 and pglB and pglE mutants (results not shown). Bacterial growth curves (Fig. 1) indicated that both mutants had slightly faster doubling times relative to 81-176. However, only the pglE mutant demonstrated a statistically significant increase in growth rate (P < 0.05) compared to the wild type by paired t-test analysis. Complementation of the pglE mutation in trans with plasmid pCS101, an Escherichia coli-Campylobacter shuttle vector containing an intact copy of pglE and its putative promoter (41), restored the wild-type doubling time.
FIG. 1.
Growth curve of 81-176 and pgl mutants. Bacterial cultures were adjusted to an OD600 of 0.1 and grown in MH broth under microaerophilic conditions with shaking at 37°C. The relationship of OD600 to viable count was equivalent for all strains examined. The mean doubling time of each strain from two or three experiments is shown. The growth curve shown is an example of one experiment.
Since numerous soluble and membrane proteins appear to be glycosylated by the pgl system, it was possible that the mutants would display increased sensitivity to growth inhibitors. The sensitivity of wild-type 81-176 and the pglE mutant to a variety of agents was determined by the method of Yethon et al. (47). Cultures were adjusted to an optical density at 600 nm (OD600) of 0.1 in Mueller-Hinton (MH) broth supplemented with inhibitors. Growth was compared following incubation at 37°C under microaerophilic conditions with overnight shaking for 14 h. Growth was considered positive if the OD600 was greater than 0.2 (47). No differences between the wild type and the pglE mutant were observed for growth in 0.05 mg of sodium dodecyl sulfate per ml (40, 47), 0.1 and 0.2% (wt/vol) sodium deoxycholate (34), or 0.0625 and 0.125 M NaCl (1, 33) (data not shown). In addition, no differences between the wild type and either pgl mutant were observed for growth in MH broth at pH 7.2 versus MH broth adjusted to pH 5.0 or 6.0 (data not shown).
Adherence to and invasion of INT407 cells.
Motility has been shown to be required for C. jejuni adherence to and invasion of intestinal epithelial cells. Since pglB and pglE mutants show wild-type levels of motility (41), adherence and invasion assays using a human intestinal epithelial cell line (INT407) were done as previously described (27, 46, 47). The pglB mutant adhered at 38% and invaded at 4.4% of the level of the wild-type strain, while the pglE mutant adhered at 59% and invaded at 9.2% of wild-type levels (Table 1). When the pglE mutant was complemented in trans with pCS101, the strain adhered and invaded at levels comparable to those of the wild type.
TABLE 1.
C. jejuni adherence to and invasion of INT407 cellsa
| Strain | % (mean ± SE)
|
|
|---|---|---|
| Adherence | Invasion | |
| 81-176 | 5.91 ± 0.85 | 2.93 ± 0.19 |
| 81-176 pglB | 2.26 ± 0.74b | 0.13 ± 0.06b |
| 81-176 pglE | 3.49 ± 0.97 | 0.27 ± 0.12b |
| 81-176 pglE(pCS101) | 6.32 ± 0.89 | 1.85 ± 0.41 |
Approximately 3 × 106 bacteria were added to a layer of approximately 4 × 105 cells (multiplicity of infection of 8) and incubated at 37°C. For determination of adherence, after 2 h of incubation with bacteria, INT407 cells were washed four times in Hanks' balanced salt solution with strong agitation for 2 min prior to lysing of the monolayer with 0.01% Triton X-100. For determination of invasion, the monolayer was incubated with 100 mg of gentamicin per ml in minimal essential medium (Gibco) for an additional 2 h prior to lysis with Triton X-100. Bacteria were enumerated by plate count, and the data are the percentages of the inoculum which adhered to or invaded INT407 cells in four or five independent experiments.
P < 0.05 by paired t-test analyses.
Mouse colonization.
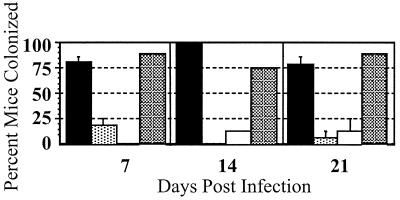
Experiments reported herein were conducted according to the principles set forth in reference 26a. Hsd:ICR mice were fed 6 × 109 to 9 × 109 organisms and monitored for colonization for 3 weeks as described previously (46). Four freshly passed fecal pellets per animal were homogenized in phosphate-buffered saline (Sigma) daily and plated on C. jejuni selective agar (Remel). An animal was considered to be no longer colonized by C. jejuni after three consecutive negative cultures. As shown in Fig. 2, mice were colonized with wild-type 81-176 for 21 days. Both pglB and pglE strains demonstrated a significant reduction in percent colonization (P < 0.001, using paired t-test analysis) as early as day 7 postinfection, and colonization remained significantly low through 21 days (Fig. 2). The presence of pCS101 in the pglE mutant in trans restored wild-type levels of colonization at all time points examined.
FIG. 2.
Colonization of Hsd:ICR mice by C. jejuni. Each group contained 12 to 16 mice in two separate experiments except the group fed pglE(pCS101), which contained eight mice in a single experiment. Data are means ± standard errors. Black bar, 81-176; lightly stippled bar, pglB strain; white bar, pglE strain; heavily stippled bar, pglE strain carrying pCS101. The pglB and pglE mutants showed a statistically significant reduction in colonization (P < 0.001) compared to the wild type at all time points.
Conclusions.
Despite an increasing awareness of the presence of glycoproteins in bacteria, little is understood about the biological significance of these modifications. Recent studies in bacteria have suggested that carbohydrate modifications on proteins can play a role in adhesion (4, 22, 24, 26), protection against proteolytic cleavage (18), solubility (25), antigenic variation (11, 15, 17), and protective immunity (16, 35). The glycosyl modifications on campylobacter flagellin are immunogenic and surface exposed in the flagellar filament (17, 30), suggesting that they may protect the flagellin protein from the immune system. The glycosyl modifications synthesized by the pgl genes on other campylobacter proteins have also been shown to be highly immunogenic (41). The observation that mutations in either pglB or pglE in 81-176 resulted in a significant reduction in adherence to and invasion of INT407 cells in vitro, and a reduced ability to colonize the intestinal tract of mice suggests a role for the general protein glycosylation system in virulence. Adherence to and invasion of C. jejuni 81-176 have been shown to be multifactorial, requiring motility (45, 46), the capsular polysaccharide (2), particular ganglioside mimicries in the lipooligosaccharide (14), a plasmid-encoded type IV secretion system (3), protein adhesins (29), and potentially other undetermined factors. It remains to be determined which glycoprotein(s) is responsible for the observed changes in virulence in the pgl mutants. Given the extent of general protein glycosylation in C. jejuni (41), the responsible proteins could be either soluble glycoproteins affecting key pathogenic processes or surface exposed glycoproteins that, like their eukaryotic counterparts (43), play a direct role in cellular interactions.
Acknowledgments
We thank Robert Williams for electron microscope analysis.
This work was supported by Naval Medical Research and Development Command Work no. 61102A3M161102BS13 AK.111. and Interagency Agreement FDA 224-93-2444.
Editor: J. T. Barbieri
REFERENCES
- 1.Abram, D. D., and N. N. Potter. 1984. Survival of Campylobacter jejuni at different temperatures in broth, beef, chicken and cod supplemented with sodium chloride. J. Food Prot. 47:795-800. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. [DOI] [PubMed] [Google Scholar]
- 3.Bacon, D. J., R. A. Alm, D. H. Burr, L. Hu, D. J. Kopecko, C. P. Ewing, T. J. Trust, and P. Guerry. 2000. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 68:4384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz, I., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA-I adhesin. Mol. Microbiol. 40:1403-1413. [DOI] [PubMed] [Google Scholar]
- 5.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 6.Brimer, C. D., and T. C. Montie. 1998. Cloning and comparison of fliC genes and identification of glycosylation in the flagellin of Pseudomonas aeruginosa a-type strains. J. Bacteriol. 178:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castric, P. 1995. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology 141:1247-1254. [DOI] [PubMed] [Google Scholar]
- 8.Castric, P., F. J. Cassels, and R. W. Carlson. 2001. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J. Biol. Chem. 276:26479-26485. [DOI] [PubMed] [Google Scholar]
- 9.Comstock, L. E., M. J. Coyne, A. O. Tzianabos, and D. L. Kasper. 1999. Interstrain variation of the polysaccharide B biosynthesis locus of Bacteroides fragilis: characterization of the region from strain 638R. J. Bacteriol. 181:6192-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deakin, W. J., V. E. Parker, E. L. Wright, K. J. Ashcroft, G. J. Loake, and C. H. Shaw. 1999. Agrobacterium tumefaciens possesses a fourth flagellin gene located in a large gene cluster concerned with flagellar structure, assembly and motility. Microbiology 145:1397-1407. [DOI] [PubMed] [Google Scholar]
- 11.Doig, P., N. Kinsella, P. Guerry, and T. J. Trust. 1996. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol. Microbiol. 19:379-387. [DOI] [PubMed] [Google Scholar]
- 12.Ge, Y., C. Li, L. Corum, C. A. Slaughter, and N. W. Charon. 1998. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J. Bacteriol. 180:2418-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gryllos, I., J. G. Shaw, R. Gavin, S. Merino, and J. M. Tomas. 2001. Role of flm locus in mesophilic Aeromonas species adherence. Infect. Immun. 69:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerry, P., R. Alm, C. Szymanski, and T. J. Trust. 2000. Structure, function, and antigenicity of Campylobacter flagella, p. 405-421. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 16.Guerry, P., P. Doig, R. A. Alm, D. H. Burr, N. Kinsella, and T. J. Trust. 1996. Identification and characterization of genes required for post-translational modification of Campylobacter coli VC167 flagellin. Mol. Microbiol. 19:369-378. [DOI] [PubMed] [Google Scholar]
- 17.Harris, L. A., S. M. Logan, P. Guerry, and T. J. Trust. 1987. Antigenic variation of Campylobacter flagellin. J. Bacteriol. 169:5066-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann, J. L., P. O'Gaora, A. Gallagher, J. E. Thole, and D. B. Young. 1996. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 15:3547-3554. [PMC free article] [PubMed] [Google Scholar]
- 19.Josenhans, C., R. L. Ferrero, A. Labigne, and S. Suerbaum. 1999. Cloning and allelic exchange mutagenesis of two flagellin genes of Helicobacter felis. Mol. Microbiol. 33:350-362. [DOI] [PubMed] [Google Scholar]
- 20.Kahler, C. M., L. E. Martin, Y.-L. Tzeng, Y. K. Miller, K. Sharkey, D. S. Stephens, and J. K. Davies. 2001. Polymorphisms in pilin glycosylation locus of Neisseria meningitidis expressing class II pili. Infect. Immun. 69:3597-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korlath, J. A., M. T. Osterholm, L. A. Judy, J. C. Forfang, R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 22.Kuo, C.-C., N. Takahashi, A. F. Swanson, Y. Ozeki, and S.-I. Hakomori. 1996. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Investig. 98:2813-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leclerc, G., S. P. Wang, and B. Ely. 1998. A new class of Caulobacter crescentus flagellar genes. J. Bacteriol. 180:5010-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marceau, M., and X. Nassif. 1999. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J. Bacteriol. 181:656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miron, J., and C. W. Forsberg. 1999. Characterisation of cellulose-binding proteins that are involved in the adhesion mechanism of Fibrobacter intestinalis DR7. Appl. Microbiol. Biotechnol. 51:491-497. [DOI] [PubMed] [Google Scholar]
- 26a.National Research Council. 1986. Guide for the care and use of laboratory animals. DHHS publication (NIH) 86-23. Institute of Laboratory Animal Resources, National Research Council, Bethesda, Md.
- 27.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 90:6884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parge, H. E., K. T. Forest, M. J. Hickey, D. A. Christensen, E. D. Getzoff, and J. A. Trainer. 1995. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature 378:32-38. [DOI] [PubMed] [Google Scholar]
- 29.Pei, Z., C. Burucoa, B. Grignon, S. Baqar, X.-Z. Huang, D. J. Kopecko, A. L. Bourgeois, J.-L. Fauchere, and M. J. Blaser. 1998. Mutation in peb1A of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect. Immun. 66:938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Power, M., P. Guerry, W. D. McCubbin, C. M. Kay, and T. J. Trust. 1994. Structural and antigenic characteristics of Campylobacter coli FlaA flagellin. J. Bacteriol. 176:3303-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power, P. M., L. F. Roddam, M. Dieckelmann, Y. N. Srikhanta, Y. C. Tan, A. W. Berrington, and M. P. Jennings. 2000. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology 146:967-979. [DOI] [PubMed] [Google Scholar]
- 32.Rabaan, A. A., I. Gryllos, J. M. Tomas, and J. G. Shaw. 2001. Motility and the polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infect. Immun. 69:4257-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reezal, A., B. McNeil, and J. G. Anderson. 1998. Effect of low-osmolality nutrient media on growth and culturability of Campylobacter species. Appl. Environ. Microbiol. 64:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera-Amill, V., B. J. Kim, J. Keshu, and M. E. Konkel. 2001. Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J. Infect. Dis. 183:1607-1616. [DOI] [PubMed] [Google Scholar]
- 35.Romain, F., C. Horn, P. Pescher, A. Namane, M. Riviere, G. Puzo, O. Barzu, and G. Marchal. 1999. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect. Immun. 67:5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaffer, C., M. Graninger, and P. Messner. 2001. Prokaryotic glycosylation. Proteomics 1:248-261. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer, C., and P. Messner. 2001. Glycobiology of surface layer proteins. Biochimie 83:591-599. [DOI] [PubMed] [Google Scholar]
- 38.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, H. Lee, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, J. N. Reeve, et al. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum H: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stimson, E., M. Virji, K. Makepeace, A. Dell, H. R. Morris, G. Payne, J. R. Saunders, M. P. Jennings, S. Barker, M. Panico, et al. 1995. Meningococcal pilin: a glycoprotein substituent substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 17:1201-1214. [DOI] [PubMed] [Google Scholar]
- 40.Sukupolvi, S., M. Vaara, I. M. Helander, P. Viljanen, and P. H. Makela. 1984. New Salmonella typhimurium mutants with altered outer membrane permeability. J. Bacteriol. 159:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szymanski, C. M., R. Yao, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 32:1022-1030. [DOI] [PubMed] [Google Scholar]
- 42.Thibault, P., S. M. Logan, J. F. Kelly, J.-R. Brisson, C. P. Ewing, T. J. Trust, and P. Guerry. 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276:34862-34870. [DOI] [PubMed] [Google Scholar]
- 43.Varki, A. 1993. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3:97-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyss, C. 1998. Flagellins, but not endoflagellar sheath proteins, of Treponema pallidum and of pathogen-related oral spirochetes are glycosylated. Infect. Immun. 66:5751-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao, R., D. H. Burr, P. Doig, T. J. Trust, H. Niu, and P. Guerry. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14:883-893. [DOI] [PubMed] [Google Scholar]
- 46.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 23:1021-1031. [DOI] [PubMed] [Google Scholar]
- 47.Yethon, J. A., D. E. Heinrichs, M. A. Monteiro, M. B. Perry, and C. Whitfield. 1998. Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J. Biol. Chem. 273:26310-26316. [DOI] [PubMed] [Google Scholar]
- 48.Zufferey, R., R. Knauer, P. Burda, I. Stagljar, S. te Heesen, L. Lehle, and M. Aebi. 1995. STT3, a highly conserved protein required for yeast oligosaccharyl transferase activity in vivo. EMBO J. 14:4949-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]