Abstract
Attenuated Salmonella strains are an attractive live vector for delivery of a foreign antigen to the human immune system. However, the problem with this vector lies with plasmid segregation and the low level of expression of the foreign gene in vivo when constitutive expression is employed, leading to a diminished immune response. We have established inducible expressions of foreign genes in the Salmonella enterica serovar Typhi CVD908 vaccine strain using the tetracycline response regulatory promoter. To set up this system, a tetracycline repressor (tetR) was integrated into a defined ΔaroC locus of the chromosome via suicide plasmid pJG12/tetR-neo. To remove the neo gene conferring kanamycin resistance from the locus, a cre expression vector under the control of the tetracycline response promoter was transformed into the clone; expression of the Cre recombinase excised the neo gene and generated the end strain CVD908-tetR. Expression of the luciferase reporter gene in this strain is dependent on the presence of tetracycline in the medium and can be regulated up to 4,773-fold. Moreover, the tightly controlled expression of major merozoite surface protein 1 (MSP1) and parts of Plasmodium falciparum was achieved, and the product yield was increased when the inducible expression system was employed. Inoculation of bacteria harboring plasmid pZE11/MSP142 in mice produced the protein in liver and spleen controlled by the inducer. The persistence of the plasmid-carrying bacteria in mice was determined. Peak colonization of both liver and spleen was detected on the third day postinoculation and was followed by a decline in growth curves. After 14 days postinfection, the majority of the bacteria (>90%) recovered from the liver and spleen of the mice retained the plasmid when expression was induced; this clearly indicated that stability of the expression vector in vivo was improved by inducible expression. Establishment of the regulatory system in the vaccine strain may broaden the range of its use by enhancing plasmid stability and expression levels in vivo. Moreover, the availability of the vaccine strain inducibly expressing the entire MSP1 provides possibilities for examining its immunogenicity, particularly the cellular response in animal models.
Numerous antigens that may constitute effective components of vaccines have become accessible through current advances in molecular and cell biology. Despite this progress, a major problem in the development of subunit vaccines is concerned with the manner in which antigens must be presented to the immune system in order to elicit a protective immune response. In this context, the use of highly attenuated strains of Salmonella enterica serovar Typhi as a basis for development of a recombinant bacterial live vaccine has been an interesting and attractive proposal (3, 11, 13-15, 22) and has resulted in the development of strains potentially suitable for human use. The oral administration and route of infection of salmonellas produce an efficient colonization in Peyer's patches and spleen; this appears particularly promising, as the secretory and humoral as well as cellular immune responses can be elicited. Indeed, a number of reports describe successful immunization with recombinant salmonellas in various animal models.
In the search for a malarial vaccine, a recombinant bacterial system would be particularly valuable due to the potential provided by low-cost production and simplicity of administration. Indeed, the various heterologous antigens examined in salmonellas include the circumsporozoite proteins of Plasmodium falciparum, which have been tested for their immunogenicity in animal models as well as in humans (14).
The Salmonella enterica serovar Typhi strain CVD908-htrA, also considered for immunization in humans, carries three deletions which affect the ΔaroC, ΔaroD, and htrA loci. Due to these deletions, it is highly attenuated yet retains sufficient immunogenicity (12, 33, 36, 48, 49). Potential problems with the use of the carrier lie in plasmid segregation in vivo, where antibiotic selective pressure is usually not available. By 24 h postinoculation, the majority of the carrier strain cells recovered from the tissues of immunized animals have lost the plasmid, resulting in loss of expression of the foreign antigen (5). Inducible systems that allow tightly controlled expression of the protein in the host should circumvent this problem in principle.
This strategy was successfully applied using attenuated Salmonella strains with the Escherichia coli nitrite reductase promoter NirB induced under anaerobiosis, to directly stabilize in vivo expression of fragment C of tetanus toxin (7, 13). Mice immunized with a single oral dose of salmonellas harboring the construct developed high titers of anti-fragment C antibodies and were protected against tetanus challenge; however, very weak immunity was induced in mice immunized with salmonellas in which the fragment was expressed under the control of the constitutive tac promoter (7). More recently, a promoter activated in vivo within macrophages was used for the expression of heterologous proteins in S. enterica serovar Typhimurium. This approach enhances the immunogenicity of the antigen in animal models (9, 13, 31). Nevertheless, these in vivo-activated promoters do not allow very tight control of expression, which again will prevent the inclusion of some antigens.
The merozoite surface protein 1 (MSP1) of Plasmodium falciparum is a 190- to 200-kDa glycoprotein synthesized during schizogony. This protein is proteolytically processed into a number of smaller fragments, termed MSP183, MSP133, MSP136, and MSP142. MSP142 undergoes a second processing event to generates MSP130 and MSP119 (21). The latter contains two disulfide-bonded epidermal growth factor-like domains and is anchored into the merozoite membrane by a glycosylphosphatidylinositol moiety, the only region of MSP1 which is carried into the host erythrocyte still associated with the merozoite surface (4).
For a number of reasons, this antigen has been considered an attractive candidate vaccine in the control of malaria. The most convincing evidence for this view comes from Aotus monkey trials which showed that immunization of Aotus monkeys with native MSP1 material can provide partial to complete protection against parasite challenge (10, 18, 35, 41). Similar immunization experiments in rodents also generated high levels of protection (20). Moreover, monoclonal antibodies and antisera specific for the antigen can also inhibit parasite growth both in vitro and in vivo (17, 26, 30). The MSP1 gene, however, is highly unstable in E. coli due to its unusually high A+T content, which renders cloning of the full-length gene impossible (39). This obstacle has been resolved by gene synthesis with reduced A+T contents using human codon usage (34). Transfection in various systems, such as E. coli and mammalian cells, with this gene can produce the entire recombinant MSP1 protein.
During the last decade, many efforts have been made to identify those domains of the merozoite surface protein which confer protective immunity. Growing evidence showed that the cysteine-rich carboxyl terminus may mediate protection. Immunization of monkeys with MSP142 provided protection against challenge with the parasite. Moreover, antibodies to this fragment inhibited parasite growth in vitro. However, no convincing data are available which allow us to exclude any other regions outside the C terminus for protective immunity.
Serum antibodies from donors with malaria which can associate with merozoites can inhibit parasite multiplication in vitro and prevent merozoite dispersal. These antibodies recognized the fragment in the middle (i.e., MSP131) besides the C-terminal fragments (29). In addition, the best protection was achieved by immunization with full-length native MSP1 in rodents and Aotus monkeys (20, 41). Therefore, attempts should be made to examine the potential of all processed fragments as components of the vaccine.
Here, we describe the development of an S. enterica serovar Typhi CVD908 strain that allows the controlled transcription of a target gene via anhydrotetracycline (aTc). In this system, the E. coli Tet repressor (TetR) shuts off transcription in the absence of tetracycline; in the presence of a very low concentration of aTc (0.1 μg/ml), the expression of the gene is induced 4,773-fold. By varying the aTc concentration, expression can also be adapted to intermediate levels. The resulting S. enterica serovar Typhi CVD908-TetR produced TetR constitutively and tightly regulated not only the luciferase indicator gene but also a gene encoding the 190-kDa merozoite surface antigen protein 1 (MSP1) and parts of the malaria parasite P. falciparum. Moreover, expression of the malaria antigen was detected in both liver and spleen of mice in an aTc-dependent fashion.
It would be interesting to determine whether the combination of the gene encoding this important antigen with the regulated S. enterica serovar Typhi delivery system is capable of efficiently eliciting protective immune responses.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
A double aro mutant of Salmonella enterica serovar Typhi CVD908 strain and two suicide plasmids, pJG9 and pJG12, were kindly provided by M. M. Levin and J. E. Galen (unpublished data). Escherichia coli DH5αZ1 was obtained by moving the tandem tetR, lacI, and spr genes into the attB locus of E. coli DH5α (28), and plasmids pZS4 (28), pREP4 (45), and pZE11 (28) were kindly provided by H. Bujard.
The plasmid pBluescript/cre with a cre cDNA gene in its multiple cloning site is used in our lab. E. coli strains were grown in Luria-Bertani (LB) broth. S. enterica serovar Typhi CVD908 strains, and their derivatives were grown in LB-DHB broth (LB broth with 0.0001% [wt/vol] 2,3-dihydroxybenzoate) and in Aro broth for expression studies (13).
DNA manipulation.
Restriction endonuclease digestions, plasmid isolation, agarose gel electrophoresis, restriction fragment isolation, ligation, transformation with calcium chloride, restriction fragment blunting, chromosomal DNA extraction, alkaline phosphatase treatment, and PCR product purification were carried out according to published standard protocols (38) or following kit manual recommendations. PCR amplification parameters were as follows: predenaturation for 2.5 min at 94°C for 1 cycle; denaturation for 20 s at 94°C, annealing for 30 s at 53°C, and extension for 1.5 min at 72°C for 28 cycles; and complete extension for 7 min at 72°C for 1 cycle.
Oligonucleotide primers.
All oligonucleotide primers used in this study are listed in Table 1. Primers Neo1 and Neo2, forward and reverse primers for amplification of the neo gene from plasmid pREP4, respectively, contain the 34-bp loxP sequence in the 5′ terminus. The products amplified by these primers are flanked on both sides by two loxP sequences in the same direction. The primer pairs Tet1/Tet2 and Cre1/Cre2 were designed to amplify the tetR gene and cre cDNA gene from their respective plasmids, pZS4 and pBluescript/cre. A modified luciferase gene (28) was amplified by Luci1/Luci2. Primers N32 and N33 hybridize to the 5′-terminal region of aroC, encoding the first seven amino acids, and to the 3′-terminal region, encoding the last six amino acids and stop codon, respectively.
TABLE 1.
Oligonucleotides
| Oligonucleotide | Sequencea | Description |
|---|---|---|
| N32 | 5′-ATGGCAGGAAACACAATTGGA-3′ | 5′ primer for aroC |
| N33 | 5′-CCAGCGTGGAATCTCTGTCTT-3′ | 3′ primer for aroC |
| Tet1 | 5′-GAagatctGGCCCTTTCGTCTTCACCTC-3′ | 5′ primer for tetR |
| Tet2 | 5′-CTAgctagcTAGCTCCTGAAAATCTCGCC-3′ | 3′ primer for tetR |
| Neo1 | 5′-CTAgctagcATAACTTCGTATAGCATACATTATACGAAGTTATCTTCACGCTGCCGCAAGCAC-3′ | 5′ primer for neo |
| Neo2 | 5′-CTAgctagcATAACTTCGTATAGCATACATTATACGAAGTTATGACCAAAGCGGCCATCGTGC-3′ | 3′ primer for neo |
| Cre1 | 5′-GGggtaccATGTCCAATTTACTGACCG-3′ | 5′ primer for cre |
| Cre2 | 5′-AActgcagCTAATCGCCATCTTCCAGC-3′ | 3′ primer for cre |
| Luci1 | 5′-GGggtaccATGGAAGACGCCAAAAACAT-3′ | 5′ primer for luci |
| Luci2 | 5′-AActgcagTTACAATTTGGACTTTCCGC-3′ | 3′ primer for luci |
Endonuclease sites are in lowercase letters, and the loxP sequence is underlined.
Construction of plasmids. (i) Construction of plasmid pJG12/tetR-neo.
Plasmid pJG12 contains a temperature-sensitive replicon, the chloramphenicol acetyltransferase gene (cat), and a counterselectable sacB gene encoding the levansucrase enzyme that is lethal to gram-negative bacteria, such as salmonellas grown in the presence of sucrose. The construction of pJG12/tetR-neo was carried out in two steps. First, the tetR gene driven by the pN25 promoter was amplified by primers Tet1 and Tet2 from pZS4. The amplified products were digested with BglII and NheI and cloned into the multiple cloning site of pBluescript for DNA sequence confirmation. Error-free tetR gene was cut from pBluescript clones with the same two restriction enzymes and replaced the aph gene in the ΔaroC fragment on pJG12 to generate the pJG12/tetR plasmid. To obtain the neo gene flanked on both sides by two loxP sequences in the same direction, primer pair Neo1/Neo2 was used. The products amplified from pREP4 were inserted into the unique NheI site of pJG12/tetR. This plasmid was termed pJG12/tetR-neo. Restriction enzyme analysis showed that the tetR and neo genes were oriented in a head-to-head manner.
(ii) Construction of plasmid pJG9/cre.
The cDNA gene encoding Cre recombinase, amplified from pBluescript/cre with primers Cre1/Cre2, was digested with KpnI and PstI and ligated to pBluescript once more for DNA sequence confirmation. The error-free cre cDNA gene was inserted into plasmid pZE11 between the KpnI and PstI sites to construct pZE11/cre. On this plasmid, the original ATG start codon was replaced by that of the cre gene at the same position. The PLtetO-1-cre-T1 cassette was cut from pZE11/cre with XhoI and StyI, treated with Klenow fragment, and then inserted into the SmaI site of pJG9, the parental plasmid of pJG12 without the aph and ΔaroC cassette, producing pJG9/cre. The orientation of the PLtetO-1-cre-T1 cassette was identified by endonuclease analysis.
(iii) Construction of expression plasmid.
The cDNA genes encoding firefly luciferase were amplified using primers Luci1/Luci2. Error-free luci cDNA gene products were inserted into pZE11 between the KpnI and PstI sites to produce pZE11/luci. The ATG start codon of pZE11 was replaced by that of the luci cDNA gene at the same position. The entire msp1 gene of Plasmodium falciparum and parts thereof were synthesized as described previously (34). Since there were no appropriate sites in pZE11 for cloning the msp1 gene, the plasmid was reconstructed to create restriction sites for BamHI and ClaI that were compatible with the synthetic msp1 gene flanked by the same sites. The msp1 gene as well as parts thereof, msp142 and msp131, were cloned into the multiple cloning site of pZE11. The resulting plasmids were termed pZE11/msp1, pZE11/msp142, and pZE11/msp131, respectively. pZE11 is an episomal vector carrying the PltetO-1 promoter, which directs transcription of the genes of interest controlled by aTc and the gene conferring resistance to ampicillin.
Electroporation.
Overnight cultures were diluted 1:100 with LB-DHB broth and incubated for 3 h at 37°C with shaking. Cells were spun down for 10 min at 6,000 rpm and washed three times with 1 culture volume of ice-cold distilled water before being resuspended in ice-cold distilled water with a final volume of 1/100 of the original culture volume. Plasmid DNA was added to 40 μl of this cell suspension and settled to the bottom of a 0.2-cm cuvette. Electroporation was performed in a gene pulser (Bio-Rad) with the parameters set at 2.5 kV, 200 Ω, and 25 μF. Immediately after the pulsing, the cells were gently resuspended in Aro broth and incubated for 1 h without antibiotic before plating on Aro agar with the appropriate antibiotic (13).
Insertion of tetR-neo cassette into the ΔaroC locus of CVD908.
The insertion experiment was performed according to the protocol of M. M. Levin (unpublished data). pJG12/tetR-neo was transferred into CVD908 by electroporation. The bacterial cells were grown on Aro agar containing kanamycin (45 μg/ml) at 37°C for 16 h. Several colonies were pooled and streaked on another kanamycin plate. After incubation at 44°C for about 48 h, one colony was inoculated into LB-DHB broth supplemented with 10% sucrose and incubated at 30°C for 16 h with vigorous shaking. Kanamycin-resistant and chloramphenicol-sensitive colonies were screened, and PCR identification was carried out. The positive colony was termed strain CVD908-tetR-neo.
Excision of neo gene from the chromosome.
Plasmid pJG9/cre was electroporated into the CVD908-tetR-neo strains (unpublished data). Overnight cultures of CVD908-tetR-neo harboring pJG9/cre were diluted 1:100 in Aro broth containing 20 μg/ml chloramphenicol followed by incubation at 30°C for 4 h with shaking. Anhydrotetracycline was added to the cultures for a final concentration of 100 ng/ml, followed by incubation at 37°C for another 4 h to induce the expression of Cre recombinase and to allow the site-specific recombination mediated by the Cre/LoxP system. Several hundred colonies were screened for kanamycin sensitivity and chloramphenicol resistance. After PCR identification, one positive colony was inoculated into LB-DHB broth containing 10% sucrose and grown at 30°C for 16 h with shaking to cure the pJG9/cre. One colony sensitive to both kanamycin and chloramphenicol was termed CVD908-tetR and was used in subsequent expression experiments.
Western blot.
Overnight cultures of CVD908-tetR harboring different expression plasmids were diluted 1:100 in Aro broth and grown at 37°C for a 3-h period. After supplementation with aTc for a final concentration of 100 ng/ml, the cultures were grown continuously for another 4 h. Cultured cells were harvested by centrifugation at 12,000 rpm for 1 min and resuspended in distilled water. The resuspended cells were mixed 1:1 with twice-concentrated loading buffer and then boiled for 15 min. The loading buffer was either with mercaptoethanol or without mercaptoethanol when monoclonal antibody MAb5.2 was applied.
The samples were fractionated on sodium dodecyl sulfate (SDS) gels according to standard protocols (38). After the protein gels were transferred onto the nitrocellulose membrane, the transferred proteins were probed with either polyclonal (rabbit anti-MSP131 serum) or monoclonal (MAb5.2) antibodies, followed with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG) or anti-mouse IgG, respectively. The blots were developed with BCIP (5-bromo-4-chloro-3-indolylphosphate) and NBT (nitroblue tetrazolium) mixtures as the substrate for alkaline phosphatase.
Luciferase determination.
CVD908-tetR strains harboring pZE11/luci were induced by aTc as described above, and 0.5-ml cultures were spun down for 1 min at 12,000 rpm. The pellet was resuspended in 50 μl of lysis buffer (1 mM EDTA, 1 mg of lysozyme per ml) and incubated at room temperature for at least 15 min. Then 300 μl of distilled water and 300 μl of buffer I (100 mM KH2PO4, 1 mM dithiothreitol [DTT], pH 7.8) were added, followed by centrifugation at 12,000 rpm for 10 min. Then 10-μl aliquots of the supernatant were mixed with 100 μl of luciferase assay system (Promega) just prior to detection, and luciferase activity was measured (10 s; delay, 0 s) in a Lumat LB 9507. Activities were normalized and expressed as relative light units (RLU) per number of viable cells (28).
Detection of expression of MSP142 in vivo.
The bacterial strain CVD908-tetR harboring pZE11/msp142 from an overnight culture were diluted in LB-DHB medium at 1:500 and incubated at 37°C for 16 h. Then 2.5 ml of the culture was inoculated in 50 ml of LB medium and cultured for another 5 h with shaking. The culture was diluted to yield a cell concentration of 107/0.2 ml.
Female BALB/c mice (18 to 20 g) received a single 0.2-ml dose of the culture intraperitoneally. At 24 h postinoculation, the mice in induction groups were injected via the tail vein with various concentrations of aTc. The liver and spleen of the mice were examined to determine expression of MSP142 by an immunohistochemical method using fluorescently labeled monoclonal antibody MAb5.2.
RESULTS
Cre/LoxP-mediated integration of the tetR cassette into the chromosome.
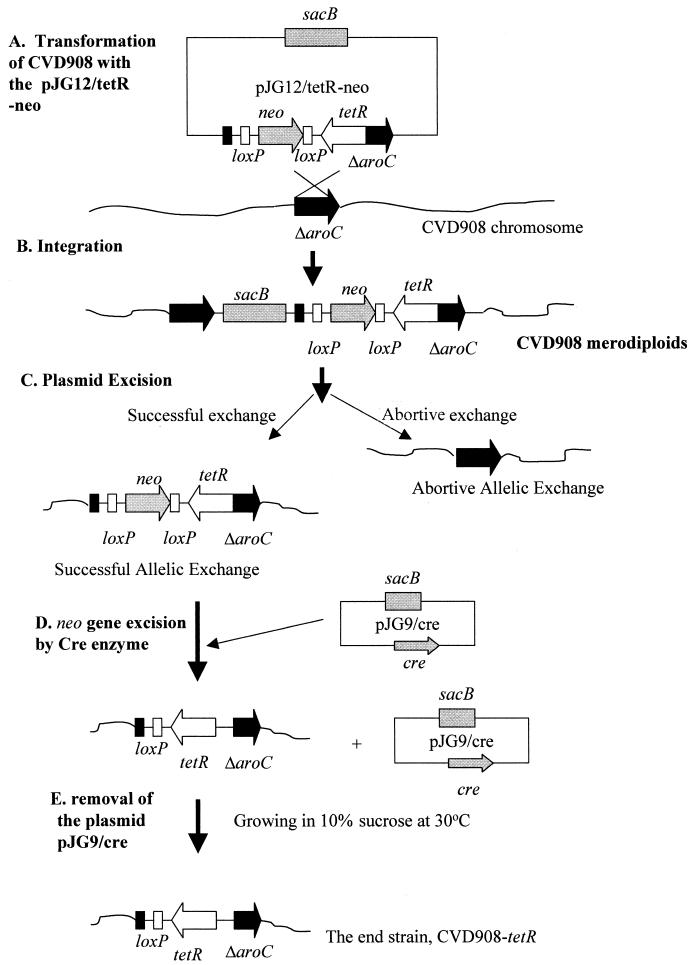
To establish the tetracycline regulatory system in this strain, it was necessary to integrate the tetR cassette into the chromosome of the strain. Since S. enterica serovar Typhi CVD908 is an attenuated vaccine strain, it is important to integrate foreign genes into the defined locus of the chromosome to prevent reverse mutation. Moreover, any resistant selective marker should be eliminated from the endstrain because of intended human use. Thus, the mutant aroC (ΔaroC) gene was chosen as the target for integration of tetR cassette. We used a temperature-sensitive suicide vector, pJG12, for the integration. This vector contains a temperature-sensitive mutation in repA that is essential for the function of the pSC101 origin of replication. This vector also contains a counterselectable marker, the sacB gene, to encode an enzyme that hydrolyzes sucrose and becomes lethal in gram-negative bacteria such as salmonellas grown in the presence of sucrose.
In this experiment, the Cre/LoxP system was employed to integrate the tetR cassette into the chromosome as outlined in Fig. 1. Cre/LoxP requires only the phage-encoded recombinase, Cre, and two copies of its 34-bp target site, loxP. In this experiment, plasmid pJG12/tetR-neo (Fig. 1A) was constructed as described in Materials and Methods, and the integration of the plasmid was accomplished by using the following three steps.
FIG. 1.
Cre/LoxP-mediated mobilization of the tetR cassette into the ΔaroC locus of the chromosome of strain CVD908.
(i) Integration of plasmid pJG12/tetR-neo into the ΔaroC locus.
The plasmid pJG12/tetR-neo was electroporated into strain CVD908 (Fig. 1A), and transformants were selected by Aro agar plates containing 45 μg of kanamycin per ml for merodiploid intermediates (Fig. 1B). One isolated colony was inoculated into LB-DHB medium containing 10% sucrose and incubated at 30°C with vigorous aeration for 16 h. These conditions allowed excision of the plasmid from the chromosome by homologous recombination (Fig. 1C) to generate two kinds of strains with successful as well as abortive allelic exchanges.
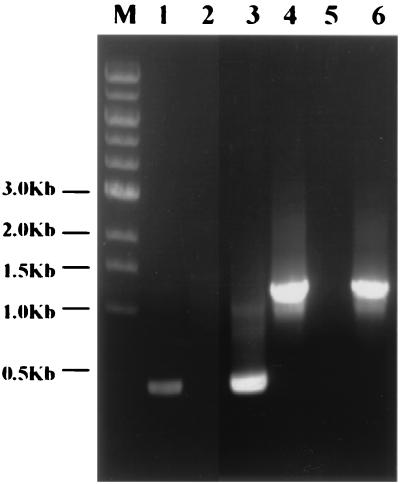
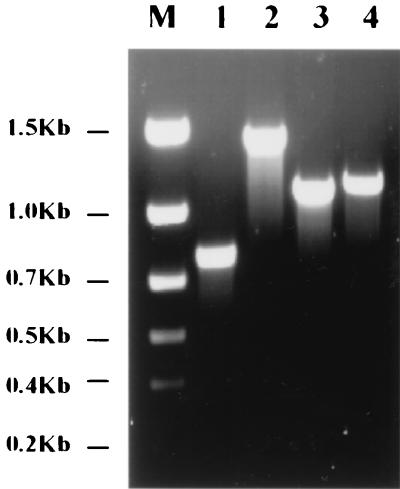
The strain with the successful exchange (designated CVD908 tetR-neo) was recovered from plates containing 45 μg of kanamycin per ml. Figure 2 shows the results of the PCR identification, indicating the mobilization of the tetR-neo-loxP fragment into the ΔaroC locus. A total of 0.4 kb of the ΔaroC gene was amplified by PCR from CVD908 using N32/N33 (Fig. 2, lane 1), whereas no resulting product was observed when Tet2/N33 was used (Fig. 2, lane 2).
FIG. 2.
PCR identification of integrated CVD908-tetR-neo-loxP. Lanes: M, DNA markers; 1, CVD908 (N32/N33); 2, CVD908 (Tet2/N33); 3, CVD908 merodiploid (N32/N33); 4, CVD908 merodiploid (Tet2/N33); 5, CVD908-tetR-neo-loxP (N32/N33); 6, CVD908-tetR-neo-loxP (Tet2/N33).
For the merodiploid clone, 0.4- as well as 1.2-kb fragments were generated when using N32/N33 (Fig. 2, lane 3) and Tet2/N33 (Fig. 2, lane 4), respectively. However, the kanamycin-resistant clone with a successful exchange produced only 1.2-kb fragments when the N32/N33 (Fig. 2, lane 5) and Tet2/N33 (Fig. 2, lane 6) primers were employed, indicating that the tetR-neo-loxP fragment had been mobilized into the ΔaroC locus. We noted that both the merodiploid clone and the clone with successful exchange failed to produce the 2.7-kb fragments of tetR-neo-loxP when using the N32/N33 primers. This failure may have resulted due to the conditions and Taq enzyme employed here, which may not allow the generation of such long fragments.
(ii) Excision of the neo expression cassette from the chromosome.
Suicide vector pJG9/cre (Fig. 1D), which inducibly expresses the Cre recombinase, was constructed. The plasmid was introduced into the clone described above by electroporation. Expression of the cre gene efficiently excised the loxP-flanked neo gene from the chromosome. Clones with the neo gene excised were found to be sensitive to kanamycin and recovered by reverse selection, i.e., selecting the clones that can grow on the plate without kanamycin but not with 45 μg of kanamycin per ml.
Figure 3 illustrates the outcome of the PCR identification of the selected clone that is sensitive to kanamycin. Expected sizes of the tetR gene and aroC::tetR fragments are 0.7 and 1.4 kb, respectively. Our results demonstrated that the two PCR products were amplified from the clone (Fig. 3, lanes 1 and 2). Moreover, the expected sizes of the other two fragments also produced in the clone using primers Tet1/N32 and Tet2/N33, respectively (Fig. 3, lanes 3 and 4), indicated that the neo gene had been removed from the locus of the chromosome and that only the tetR cassette remained in the locus.
FIG. 3.
PCR identification of strain CVD908-tetR. Lanes: M, DNA markers; 1, Tet1/Tet2; 2, N32/N33; 3, Tet1/N32; 4, Tet2/N33.
(iii) Removing the suicide vector pJG9/cre from the selected clone.
Since the clone from previous step still contained the pJG9/cre suicide plasmid, its removal was necessary in order to generate the end strain intended for human use. For this purpose, the clone was grown in a medium containing 10% sucrose. The sacB gene in the plasmid encodes the levansucrase enzyme that is lethal to the bacteria in the presence of sucrose. Thus, bacteria containing the plasmid were destroyed, while the clones of interest without the plasmid were recovered from the medium and found to be sensitive to chloramphenicol. One of these clones was termed CVD908-tetR.
Quantitation of controlled expression of luciferase by TetR in CVD908-tetR.
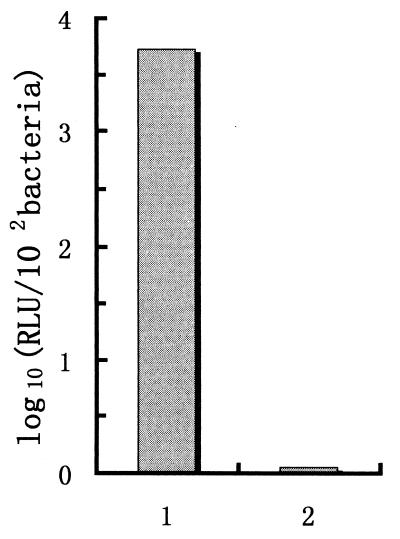
Plasmid pZE11 contains a tet-controlled promoter, and insertion of the luciferase gene into this plasmid generated expression plasmid pZE11/luci. CVD908-tetR was transformed by electroporation with pZE11/luci. The transformants were assayed for luciferase activity. As shown in Fig. 4, in the absence of aTc, luciferase activity of 1.1 × 10−2 RLU/cell was detected, versus 5,250 × 10−2 RLU/cell in the presence of 0.1 μg of aTc per ml. These data clearly indicated that the expression of luciferase is completely dependent upon the presence of aTc and can be regulated up to three orders of magnitude (4,773-fold activation factors).
FIG. 4.
Detection of luciferase activity in strain CVD908-tetR. Bars: 1, RLU in the presence of aTc; 2, RLU in the absence of aTc. The luciferase activity was defined as the RLU in the strain and is represented as the number of RLU per 102 cells.
Inducible expression of MSP1 and parts thereof by aTc.
The modification of the 5′- and 3′-end sequence of Plasmodium falciparum synthetic msp1 as well as parts thereof allowed their insertion into the pZE11 vector. The resulting plasmids, pZE11/msp1, pZE11/msp133, and pZE11/msp142, respectively, were electroporated into the CVD908-tetR strains. The transformants were evaluated for their expression in a tetracycline-dependent fashion.
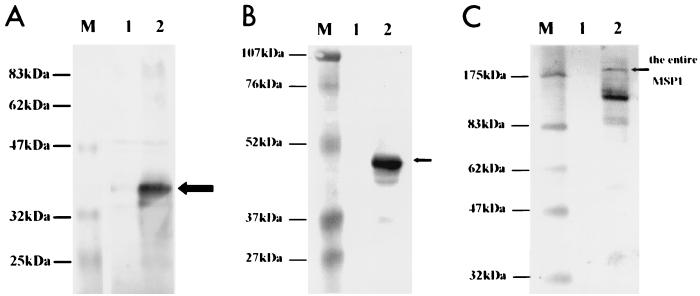
Figure 5 indicates that the expression of MSP1, MSP133 and MSP142 was dependent on the presence of the inducer. Transcription of the genes was completely shut off in the absence of aTc, whereas full expression of the proteins was achieved in the presence of aTc. In the expression of the entire MSP1, several degraded MSP1-derived proteins were detected (Fig. 5C). Monoclonal antibody MAb5.2 recognizes a conformational epitope at the C-terminal region of MSP1. Interestingly, it interacted with both MSP1 and MSP142 produced in the vaccine strain, implying that at least this conformational epitope closely resembled the native one.
FIG. 5.
Tetracycline-controlled expression of the entire MSP1 and parts thereof in strain CVD908-tetR detected by Western blot analysis in the presence of aTc (lane 2) and in the absence of aTc (lane 1). (A) MSP131 and rabbit anti-MSP131 serum. (B) MSP142 and MAb5.2. (C) MSP1 and MAb5.2.
In order to compare the yield of inducible and constitutive expressions of MSP142 in the vaccine strain, strain CVD908 containing the msp142 gene was constructed for this purpose. The outcome showed that the product in inducible expression constituted 8.3% of total proteins in the range from 27 to 76 kDa, determined by scanning densitometry of the Coomassie-stained gel, versus 3.9% in constitutive expression, indicating that tetracycline-controlled expression enhanced the expression level of the protein in the vaccine strain.
To investigate inducible expression in vivo, 12 female BALB/c mice were inoculated intraperitoneally with 107 CFU of the viable CVD908-tetR strain expressing MSP142 in a single dose. The mice were divided into four groups (three in each) and given different concentrated amounts of the aTc: 0, 25, 50, and 150 μg. The inducer was given via tail vein at 24-h intervals over a 3-day period. The liver and spleen of the mice were assayed for expression of MSP142 by an immunohistochemical method using fluorescently labeled MAb5.2, which recognizes MSP142. The data revealed that expression of MSP142 was detected in liver and spleen of all mice injected with the various doses of aTc, whereas no interaction was observed in the mice not given aTc. The liver sections of the mice showed strong staining with the fluorescent antibody in the blood vessels, whereas the interaction in the spleen sections was noted within the cells.
Growth curve and plasmid stability of CVD908-tetR-neo strain in mice.
The CVD908-tetR-neo strain contains the neo gene, which confers resistance to kanamycin, while the strain harboring pZE21/MSP142 plasmid is resistant to both kanamycin and ampicillin. Using the resistance markers, we determined the persistence of the plasmid-carrying strain in the liver and spleen of mice under induced and noninduced conditions.
Female BALB/c mice were injected intraperitoneally with 107 CFU of viable CVD908-tetR-neo harboring pZE21/MSP142. On the following day, expression was induced in the mice that intravenously received 50 μg of aTc. After a 7-day period, the induction was repeated. The mice were sacrificed on days 3, 7, 14, 21, and 28 postinfection. The livers and spleens of the mice were collected and homogenized. The homogenates were plated on LB agar containing both kanamycin and ampicillin or only kanamycin for incubation.
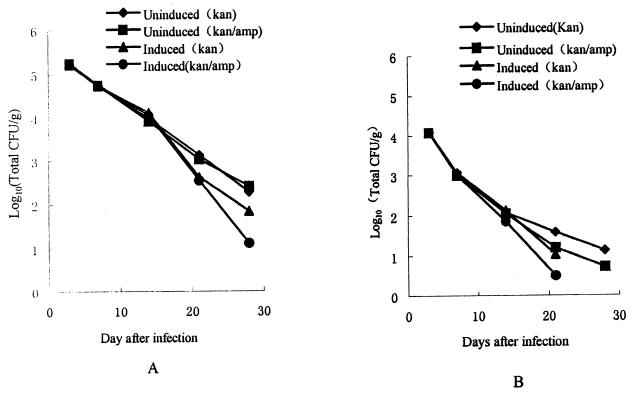
Figure 6 indicates the persistence of the organisms in the liver (Fig. 6A) and the spleen (Fig. 6B) of the mice within both the induction and noninduction groups. The peak colonization of both liver and spleen was on day 3 postinfection, but the bacterial counts within the liver were higher than those in spleen. After 3 days, the growth curves continued to decline. Over the first 14 days postinfection, the persistence of the organisms recovered from mice in both the induction and noninduction groups showed no significant difference. In addition, more than 90% of the bacteria recovered from both groups showed resistance to both antibiotics, indicating that the organisms did retain the plasmid.
FIG. 6.
CFU of S. enterica serovar Typhi CVD908-tetR harboring plasmid pZE11/MSP142 in mice. (A) Spleen. (B) Liver. kan, kanamycin; amp, ampicillin.
DISCUSSION
The use of the attenuated Salmonella strain as a vaccine carrier for delivery of foreign antigens has been studied extensively (40, 42). However, the potential problem concerning the use of the carrier is plasmid instability in vivo, where antibiotic selective pressure is usually unavailable (5). A number of approaches have been attempted to stabilize the expression of foreign antigens, including the asd+ vector/Δasd host lethal system (32), the integration of a foreign gene onto the Salmonella chromosome (14, 44), and in vivo-activated inducible systems (9, 19, 31).
Three in vivo-inducible promoters, p nirB, p pagC, and p katG, were tested for their ability to direct in vivo expressions of foreign genes in the Salmonella vaccine strain and their immunogenicity (9). Use of the p pagC promoter produced a high level of expression of the foreign gene in vivo as well as significantly inducing high levels of antigen-specific antibodies (19).
Here, we have established a tetracycline-inducible expression system in the S. enterica serovar Typhi CVD908 vaccine strain. This system can regulate expression of a foreign gene over a broad range spanning more than three orders of magnitude and can vary the concentration of the expressed antigen via varying concentrations of the stimuli (28). Moreover, regulatory expression of foreign antigens in vitro as well as in vivo was achieved, and the yield of MSP142 in this system was increased in comparison to the constitutive expression of the protein. Turning expression of foreign antigens on and off in vivo may provide a possible solution to overcome the instability of plasmids in vivo due to overexpression of a foreign gene.
Our data showed that after 14 days postinfection, more than 90% of the bacteria recovered from the liver as well as the spleen of mice retained the plasmid, in contrast to a similar study which reported loss of plasmid from more than 99% of bacteria after only 24 h (5). The obvious implication is that the stability of the plasmid in vivo improved in the tetracycline-controlled expression system. We evaluated the antibody response to MSP142 in mice immunized with the vaccine strain inducibly expressing MSP142, but specific antibodies were not detectable. However, when we immunized mice with the CVD908-tetR strain inducibly expressing a chimeric gene consisting of thrombospondin-related anonymous protein and circumsporozoite protein of Plasmodium falciparum, specific antibodies were induced in mice of the induced group in contrast to no specific antibodies in mice of the noninduced group and in the vector control group (unpublished data).
It is not surprising that there was a poor antibody response in immunized mice, since the Salmonella-delivered antigen favored induction of a cellular immune response. Sadoff et al. reported that immunization of mice with an attenuated Salmonella vaccine strain expressing circumsporozoite protein provided protection against lethal challenges even though antibodies for circumsporozoite protein remained undetectable in these immunized mice (37).
The rationale behind the choice of the tetracycline regulatory system is that the stimuli can be used in humans and can be readily absorbed and broadly distributed to different tissues in the body with no toxicity at effective concentrations. The tetracycline concentration used in such induction experiments is far below therapeutic dose, and so it is highly unlikely that the emergence of antibiotic resistance or any other side effect known for tetracycline will be encountered. Moreover, the tetracycline-controlled expression system is presently being developed for gene therapy (16).
The proposed in vivo inducible vaccine in humans would be both desirable and effective in oral administration for the following reasons. The orally administered live attenuated Salmonella recombinant vaccine translocates to intracellular locations. After a 24-h period, tetracycline is easily administered to induce the expression of the gene of interest, with follow-up administration of the antibiotic after 1 week to further boost the desired immune response
For consideration for human use, the end Salmonella vaccine strain should not contain any resistant selectable marker. In addition, random integration of foreign genes into the chromosome of the Salmonella vaccine strain should be avoided. To set up the regulatory expression system in the vaccine strain, it is essential to integrate the tetR cassette into a defined locus of the chromosome. aroC is one of the genes that was deleted for attenuating the strain (22, 27). Thus, the ΔaroC locus was used as the target for integration of the tetR cassette. Plasmid pJG12 contains the ΔaroC sequence, and insertion of a foreign gene into the sequence allows integration into the locus via homologous recombination.
In our initial experiment, the Cre/LoxP system was not employed, and the integration was performed directly using a pJG12 suicide system, but this was unsuccessful. In this experiment, we combined the use of two steps for mobilization of the tetR cassette into the locus as previously described (unpublished data). Briefly, the first step involved integration of the aph (aminoglycoside 3′-phosphotransferase) gene encoding resistance to kanamycin into the locus. In this step, plasmid pJG12 containing aph was introduced into strain CVD908 by electroporation, and the aph gene was integrated into the locus via homologous recombination. The resulting clones with the integration were recovered from the plate containing 25 μg of kanamycin per ml and designated strain CVD908-aph. The second step replaced the aph gene with the tetR gene, generating strain CVD908-tetR.
Initially, in this step, plasmid pJG12/tetR was constructed. As described earlier, the S. enterica serovar Typhi CVD908-aph strain was transformed with plasmid pJG12/tetR by electroporation. Our PCR data confirmed that the plasmid was integrated into the locus (merodiploids). However, the merodiploids were not stable, and the plasmid was excised when the bacteria were incubated at 30°C, a permissive temperature for the origin of replication, resulting in two types of allelic exchange, successful allelic exchange (CVD908-tetR) and abortive allelic exchange (CVD908-ΔaroC).
Since the frequency of successful allelic exchange was very low at 10−5 and no antibiotic resistance marker was evident in the clones with successful allelic exchange, isolating these colonies became very difficult. We grew the CVD908 merodiploids at 30°C for plasmid excision and then prepared the chromosomal DNA from the bacterial population for PCR identification of the tetR gene. The tetR gene (0.7 kb) and tetR gene plus part of the ΔaroC gene (1.0 kb) were indeed produced with the Tet1/Tet2 and N33/Tet2 primers, respectively; this indicated that clones with successful allelic exchange were present in the population. However, we screened about 10,000 clones via Southern blot and PCR to identify the clones with a successful exchange, but it was not a successful effort (unpublished data).
As an alternative strategy, we used the Cre/LoxP system (24, 43, 50) in combination with the pJG suicide vector for the integration. In this experiment, as described previously, we started by integrating the tetR-neo-loxP fusion gene into the ΔaroC locus of the chromosome. Clones with the integration were easily obtained via the neo resistance selective marker. The neo gene was excised from the fusion gene by transformation of the clone with suicide plasmid pJG9/cre. We observed that expression of the Cre recombinase excised the neo gene in an efficient manner. Finally, plasmid pJG9/cre was cured from the bacteria by growth in conditions lethal to the bacteria containing the plasmid. This three-step integration system functioned very efficiently in developing the vaccine strain. It provided an effective means to integrate any other gene, such as those for heterologous antigens, onto the chromosome of the vaccine strain.
Immunization of mice with salmonellas expressing the circumsporozoite protein of Plasmodium berghei resulted in protection against sporozoite challenge in the absence of serum antibody response (37). Subsequent studies showed that specific CD8+ cytotoxic T lymphocytes were responsible for this protection (1). This suggests that heterologous antigens delivered by salmonellas can associate with major histocompatibility class I molecules in antigen-presenting cells (6).
We constructed S. enterica serovar Typhi CVD908 inducibly expressing the entire MSP1 and parts thereof for P. falciparum by tetracycline. There is evidence that MSP1 is expressed in hepatocytes of the liver stage (2, 46, 47). Although protective humoral immunity of MSP1 against blood stage parasites has been well documented (8, 23, 25), no attempt has been made to test its cellular immunity against the liver stage, particularly cytotoxic T lymphocytes, which may play an important role in destroying infected hepatocytes.
The expression of the entire MSP1 in the CVD908-tetR vaccine strain may provide possibilities for investigation of the potential protective humoral immunity against the blood stage parasite as well as overall cellular immunity, particularly specific cytotoxic T lymphocytes against infected hepatocytes.
Acknowledgments
We thank Hermann Bujard for valuable discussions as well as providing the regulated plasmid and Myron M. Levine and James Galen for providing the Salmonella strain and plasmids. Jingling Du is acknowledged for technical assistance.
This investigation received financial support from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) and the National Natural Science Foundation of China.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aggarwal, A., S. Kumar, R. Jaffe, D. Hone, M. Gross, and J. Sadoff. 1990. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J. Exp. Med. 172:1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley, S. B., J. W. Barnwell, M. D. Bates, W. E. Collins, and M. R. Hollingdale. 1987. Plasmodium vivax: exoerythrocytic schizonts recognized by monoclonal antibodies against blood-stage schizonts. Exp. Parasitol. 64:188-194. [DOI] [PubMed] [Google Scholar]
- 3.Barry, E. M., O. G. Gomez-Duarte, S. Chatfield, R. Rappuoli, M. Dizza, G. A. Losonsky, J. E. Galen, and M. M. Levine. 1996. Expression and immunogenicity of pertussis toxin S1 subunit-tetanus toxin fragment C fusions in Salmonella typhi vaccine strain CVD 908. Infect. Immun. 64:4172-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas, L., and J. D. Clements. 1993. Stability, immunogenicity and expression of foreign antigens in bacterial vaccine vectors. Vaccine 11:126-135. [DOI] [PubMed] [Google Scholar]
- 6.Catic, A., G. Dietrich, I. Gentschev, W. Goebel, S. H. Kaufmann, and J. Hess. 1999. Introduction of protein or DNA delivered via recombinant Salmonella typhimurium into the major histocompatibility complex class I presentation pathway of macrophages. Microbes Infect. 1:113-121. [DOI] [PubMed] [Google Scholar]
- 7.Chatfield, S. N., I. G. Charies, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, J. A. 1994. Merozoite surface antigen-1 of Plasmodium. Parasitol. Today 9:50-54. [DOI] [PubMed] [Google Scholar]
- 9.Dunstan, S. J., C. P. Simmons, and R. A. Strugnell. 1999. Use of in vivo-regulated promoters to deliver antigens from attenuated Salmonella enterica var. Typhimurium. Infect. Immun. 67:5133-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etlinger, H. M., P. Caspers, H. Matile, H. J. Schoenfeld, D. Stubeber, and B. Takacs. 1991. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect. Immun. 59:3498-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galen, J. E., O. G. Gomez-Duarte, G. A. Losonsky, J. L. Halpern, C. S. Lauderbaugh, S. Kaintuck, M. K. Reymann, and M. M. Levine. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15:700-708. [DOI] [PubMed] [Google Scholar]
- 12.Galen, J. E., J. Nair, J. Y. Wang, S. S. Wasserman, M. K. Tanner, M. B. Sztein, and M. M. Levine. 1999. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect. Immun. 67:6424-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Duarte, O. G., J. E. Galen, S. N. Chatfield, R. Rappuoli, L. Eidels, and M. M. Levine. 1995. Expression of fragment C of tetanus toxin fused to a carboxyl-terminal fragment of diphtheria toxin in Salmonella typhi CVD 908 vaccine strain. Vaccine 13:1596-1602. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez, C., D. Hone, F. R. Noriega, C. O. Tacket, J. R. Davis, G. Losonsky, J. P. Nataro, S. Hoffman, A. Malik, E. Nardin, M. B. Sztein, D. G. Heppner, T. R. Fouts, A. Isibasi, and M. M. Levine. 1994. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity in humans. J. Infect. Dis. 169:927-931. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez, C. R., F. R. Noriega, S. Huerta, A. Santiago, M. Vega, J. Paniagua, V. Ortiz-Navarrete, A. Isibasi, and A. A. Levine. 1998. Immunogenicity of a Salmonella typhi CVD 908 candidate vaccine strain expressing the major surface protein gp63 of Leishmania mexicana mexicana. Vaccine 16:1043-1052. [DOI] [PubMed] [Google Scholar]
- 16.Gossen, M., S. Freundlieb, G. Bender, G. Muller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766-1769. [DOI] [PubMed] [Google Scholar]
- 17.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall, R., J. E. Hyde, M. Goman, D. L. Simmons, I. A. Hope, M. Mackay, and J. G. Scaife. 1984. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. Nature 311:379-382. [DOI] [PubMed] [Google Scholar]
- 19.Hohmann, E. L., C. A. Oletta, W. P. Loomis, and S. I. Miller. 1995. Macrophage-inducible expression of a model antigen in Salmonella typhimurium enhances immunogenicity. Proc. Natl. Acad. Sci. USA 92:2904-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holder, A. A., and R. R. Freeman. 1981. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 294:361-364. [DOI] [PubMed] [Google Scholar]
- 21.Holder, A. A., J. S. Sandhu, Y. Hillman, L. S. Davey, S. C. Nicholls, H. Cooper, and M. J. Lockyer. 1987. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology 94(Pt. 2):199-208. [DOI] [PubMed] [Google Scholar]
- 22.Hone, D. M., A. M. Harris, S. Chatfield, G. Dougan, and M. M. Levine. 1991. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine 9:810-816. [DOI] [PubMed] [Google Scholar]
- 23.Hui, G. S. N., and C. N. Hashimoto. 1998. Pathways for potentiation of immunogenicity during adjuvant-assisted immunizations with Plasmodium falciparum major merozoite surface protein 1. Infect. Immun. 66:5329-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilby, N. J., M. R. Snaith, and J. A. H. Murray. 1993. Site-specific recombinases: tools for genome engineering. Trends Genet. 9:413-421. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. G. Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locher, C. P., L. Q. Tam, S. P. Chang, J. S. McBride, and W. A. Siddiqui. 1996. Plasmodium falciparum: gp195 tripeptide repeat-specific monoclonal antibody inhibits parasite growth in vitro. Exp. Parasitol. 84:74-83. [DOI] [PubMed] [Google Scholar]
- 27.Lowe, D. C., T. C. Savidge, D. Pickard, L. Eckmann, M. F. Kagnoff, G. Dougan, and S. N. Chatfield. 1999. Characterization of candidate live oral Salmonella typhi vaccine strains harboring defined mutations in aroA, aroC, and htrA. Infect. Immun. 67:700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz, R., and H. Bujard. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyon, J. A., J. M. Carter, A. W. Thomas, and J. D. Chulay. 1997. Merozoite surface protein-1 epitopes recognized by antibodies that inhibit Plasmodium falciparum merozoite dispersal. Mol. Biochem. Parasitol. 90:223-234. [DOI] [PubMed] [Google Scholar]
- 30.Majarian, W. R., T. M. Daly, W. P. Weidanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131-3137. [PubMed] [Google Scholar]
- 31.Medina, E., P. Paglia, M. Rohde, M. P. Colombo, and C. A. Guzman. 2000. Modulation of host immune responses stimulated by Salmonella vaccine carrier strains by using different promoters to drive the expression of the recombinant antigen. Eur. J. Immunol. 30:768-777. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama, K., S. M. Kelly, and R. Curtiss III. 1998. Construction of an asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Bio/Technology 6:693-697. [Google Scholar]
- 33.Orr, N., J. E. Galen, and M. M. Levine. 1999. Expression and immunogenicity of a mutant diphtheria toxin molecule, CRM(197), and its fragments in Salmonella typhi vaccine strain CVD 908-htrA. Infect. Immun. 67:4290-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan, W., E. Ravot, R. Tolle, R. Frank, R. Mosbach, I. Turbachova, and H. Bujard. 1999. Vaccine candidate MSP-1 from Plasmodium falciparum: a redesigned 4917 bp polynucleotide enables synthesis and isolation of full-length protein from Escherichia coli and mammalian cells. Nucleic Acids Res. 27:1094-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrin, L. H., B. Merkli, M. Loche, C. Chizzolini, J. Smart, and R. Richle. 1984. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J. Exp. Med. 160:441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickett, T. E., M. F. Pasetti, J. E. Galen, M. B. Sztein, and M. M. Levine. 2000. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect. Immun. 68:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadoff, J. C., W. R. Ballou, L. S. Baron, W. R. Majarian, R. N. Brey, W. T. Hockmeyer, J. F. Young, S. J. Cryz, J. Ou, G. H. Lowell, and J. D. Chulay. 1988. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science 240:336-338. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sandhu, J. S., and J. F. Kennedy. 1994. Expression of the merozoite surface protein gp195 in vaccinia virus. Vaccine 12:56-64. [DOI] [PubMed] [Google Scholar]
- 40.Shata, M. T., L. Stevceva, S. Agwale, G. K. Lewis, and D. M. Hone. 2000. Recent advances with recombinant bacterial vaccine vectors. Mol. Med. Today 6:66-71. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui, W. A., L. Q. Tam, K. J. Kramer, G. S. N. Hui, S. E. Case, K. M. Yamaga, S. P. Chang, E. B. T. Chan, and S. C. Kan. 1987. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 84:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirard, J., C., F. Niedergang, and J. P. Kraehenbuhl. 1999. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol. Rev. 171:5-26. [DOI] [PubMed] [Google Scholar]
- 43.Sternberg, N., B. Sauer, R. Hoess, and K. Abremski. 1986. Bacteriophage P1 cre gene and its regulatory region. Evidence for multiple promoters and for regulation by DNA methylation. J. Mol. Biol. 187:197-212. [DOI] [PubMed] [Google Scholar]
- 44.Strugnell, R. A., D. Maskell, N. Fairweather, D. Pickard, A. Cockayne, C. Penn, and G. Dougan. 1990. Stable expression of foreign antigens from the chromosome of Salmonella typhimurium vaccine strains. Gene 88:57-63. [DOI] [PubMed] [Google Scholar]
- 45.Stuber, D., H. Matile, and G. Garotta. 1990. System for high level production in E. coli and rapid purification of recombinant proteins: application to epitope mapping, preparation of antibodies and structure-function analysis, p. 121-152. In I. Lefkovits and B. Pernis (ed.), Immunological methods, vol. IV. Academic Press, Orlando, Fla. [Google Scholar]
- 46.Suhrbier, A., A. A. Holder, M. F. Wiser, J. Nicholas, and R. E. Sinder. 1989. Expression of the precursor of the major merozoite surface antigens during the hepatic stage of malaria. Am. J. Trop. Med. Hyg. 40:351-355. [DOI] [PubMed] [Google Scholar]
- 47.Szarfman, A., D. Walliker, J. S. McBride, J. A. Lyon, I. A. Quakyi, and R. Carter. 1988. Allelic forms of gp195, a major blood-stage antigen of Plasmodium falciparum, are expressed in liver stages. J. Exp. Med. 167:231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tacket, C. O., M. B. Sztein, G. A. Losonsky, S. S. Wasserman, J. P. Nataro, R. Edelman, D. Pickard, G. Dougan, S. N. Chatfield, and M. M. Levine. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 65:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tacket, C. O., M. B. Sztein, S. S. Wasserman, G. Losonsky, K. L. Kotloff, T. L. Wyant, J. P. Nataro, R. Edelman, J. Perry, P. Bedford, D. Brown, S. Chatfield, G. Dougan, and M. M. Levine. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 68:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon, Y. G., J. H. Cho, and S. C. Kim. 1998. Cre/loxP-mediated excision and amplification of large segments of the Escherichia coli genome. Genet. Anal. 14:89-95. [DOI] [PubMed] [Google Scholar]