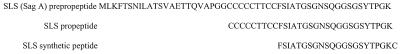Abstract
Virtually all group A streptococci (GAS) produce streptolysin S (SLS), a cytolytic toxin that is responsible for the beta-hemolysis surrounding colonies of the organisms grown on blood agar. SLS is an important virulence determinant of GAS, and recent studies have identified a nine-gene locus that is responsible for synthesis and transport of the toxin. SLS is not immunogenic; thus, no neutralizing antibodies are evoked during the course of natural infection. In the present study, we show that a synthetic peptide containing amino acid residues 10 to 30 of the putative SLS (SagA) propeptide [SLS(10-30)] coupled to keyhole limpet hemocyanin evoked antibodies in rabbits that completely neutralized the hemolytic activity of the toxin in vitro. Inhibition of hemolysis was reversed by preincubation of the immune serum with soluble, unconjugated peptide, indicating the specificity of the antibodies. In addition, antibodies that were affinity purified over an SLS(10-30) peptide column completely inhibited SLS-mediated hemolysis. The SLS(10-30) antisera did not opsonize group A streptococci; however, when combined with type-specific M protein antisera, the SLS antibodies significantly enhanced phagocytosis mediated by M protein antibodies. Thus, we have shown for the first time that it is possible to raise neutralizing antibodies against one of the most potent bacterial cytolytic toxins known. Our data also provide convincing evidence that the sagA gene actually encodes the SLS peptide of GAS. The synthetic peptide may prove to be an important component of vaccines designed to prevent GAS infections.
Group A streptococci (GAS) cause a wide variety of clinical syndromes, ranging from uncomplicated infections of the pharynx and skin to life-threatening necrotizing fasciitis and streptococcal toxic shock syndrome (19). Among the many known or suspected virulence determinants produced by GAS are two cytolytic toxins, streptolysin S (SLS) and streptolysin O (SLO). SLO is a well-characterized, oxygen-labile virulence determinant that lyses eukaryotic cells after binding to membrane cholesterol (12, 13). SLO is immunogenic in humans, and the anti-SLO (ASO) titer is widely used as an indicator of recent streptococcal infection. Until recently, the characterization of SLS had eluded many investigators. This oxygen-stable toxin is responsible for the beta-hemolysis surrounding colonies of GAS grown on blood agar plates (1, 14). In addition to red blood cells, SLS lyses a wide variety of eukaryotic cells, including myocardial cells, kidney cells, platelets, lymphocytes, and neutrophils (11, 17, 21). Early studies showed that SLS is an unstable polypeptide with a molecular mass of ∼2.8 kDa (3) that is bound to carrier molecules, such as serum albumin, RNA core, or lipoteichoic acid (14, 17, 20). On the basis of its molecular weight, SLS has been described as the most potent bacterial hemolysin (21). Injection of rabbits with purified preparations of SLS resulted in rapid death preceded by intravascular hemolysis and changes in the electrocardiogram (21). Unlike SLO, SLS is not immunogenic. This may be the result of the toxicity of SLS for lymphocytes or its small size or possibly because it is always bound to a carrier, making potential epitopes cryptic.
Recent studies have provided considerable data regarding the genetic basis for SLS production and its role in the pathogenesis of GAS infections. Betschel et al. (4) produced SLS-deficient mutants of GAS that showed reduced virulence in a mouse model of soft tissue infections. The Tn916 insertion site was localized upstream from an open reading frame encoding a peptide of 53 amino acids, which they designated sagA (4). A subsequent report described a nine-gene cluster (sagA-sagI) that was responsible for synthesis and transport of active SLS (16). Expression of SLS is repressed by the two-component regulatory system CsrR-CsrS, which also down-regulates expression of hyaluronic acid capsule and pyrogenic exotoxin B (10).
Because of the potent cytolytic effects of SLS and its role in the pathogenesis of GAS infections, it was of interest to determine whether antibodies could be raised against a nontoxic peptide of SagA that would neutralize the native toxin. Therefore, we used the structure of SagA (4, 16) to select a 20-amino-acid C-terminal peptide for synthesis. We show that rabbit antisera raised against the synthetic peptide coupled to keyhole limpet hemocyanin (KLH) specifically and completely neutralized the hemolytic activity of SLS produced by GAS.
MATERIALS AND METHODS
Synthesis and coupling of SLS(10-30)C.
The sequence of SagA was obtained from previous publications (4, 16) and from the University of Oklahoma streptococcal genome database (www.genome.ou.edu/strep.html). The N terminus of the propeptide of SagA (Fig. 1) contains seven cysteine and two threonine residues, which we surmised may be involved in its cytolytic activity. Thus, we synthesized a peptide (Research Genetics, Inc., Huntsville, Ala.) copying amino acids 10 to 30 of the putative propeptide (Fig. 1). The C-terminal cysteine was added to facilitate coupling to KLH by using a bifunctional cross-linker (7). The conjugated peptide was purified as previously described (7).
FIG. 1.
Amino acid sequences of the SLS prepropeptide and propeptide and the synthetic peptide that was used to raise anti-SLS sera.
Immunization of rabbits.
Three New Zealand White rabbits were each immunized with 300 μg of SLS(10-30)-KLH that had been emulsified in complete Freund adjuvant (7). Booster injections of the same dose in saline were given at 4 and 8 weeks. Serum was obtained before the first injection and at 2-week intervals thereafter.
Assays for antibody-mediated inhibition of SLS-induced hemolysis.
Type 24 GAS (Vaughn strain) were grown to late log phase in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) containing 1% yeast extract. Culture supernatant was collected after centrifugation and stored in aliquots at −80°C. Bacterial cell-associated SLS activity was detected by using type 24 streptococci that were grown to mid-log phase and then pelleted, washed, and resuspended in phosphate-buffered saline (PBS) to an optical density at 530 nm of 1.0.
Inhibition of SLS-induced hemolysis was assayed by mixing 0.5 ml of culture supernatant diluted 1:2 in PBS, 0.5 ml of either preimmune or immune rabbit serum diluted 1:2 in PBS, and 1.0 ml of a 2% suspension of washed sheep red blood cells (SRBC) in PBS. The reaction mixtures were incubated at 37°C for 45 min and centrifuged (1,000 × g). The A540 was measured to determine the release of hemoglobin. Cell-associated SLS activity was similarly detected by using 1 ml of freshly grown, washed bacteria instead of diluted culture supernatant. The amount of SLS added to the reaction mixture resulted in approximately 80% of the total hemolysis as determined by lysis in water. Based on previous studies using semiquantitative assays (14), this indicates that the reaction mixtures contained approximately 1 to 2 hemolytic units of SLS.
In some experiments, cholesterol (500 μg/ml) or trypan blue (13 μg/ml; Sigma Chemical Co., St. Louis, Mo.) was added to the reaction mixtures to specifically inhibit the lytic activity of SLO or SLS, respectively (4). Peptide inhibition experiments were performed by preincubating the rabbit antisera with various concentrations of SLS(10-30) at 37°C for 45 min prior to adding the serum to the reaction mixture.
Affinity purification of SLS(10-30) antibodies.
Antipeptide antibodies were affinity purified from immune rabbit serum over a column containing SLS(10-30) covalently linked to Affi-Gel 10 (Bio-Rad, Inc., Hercules, Calif.) as previously described (7). Control antibodies were purified from rabbit antiserum raised against a synthetic peptide of type 2 M protein using a column containing SM2(1-35)C (9). Total protein concentrations were determined, and both samples were adjusted to contain 1.2 mg of antibody per ml.
ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were performed on preimmune and immune rabbit sera by using SLS(10-30) as the solid-phase antigen as previously described (15).
Inhibition of SLS activity on blood agar plates.
Type 24 streptococci were streaked onto blood agar that contained 10% preimmune or immune serum against SLS(10-30). Beta-hemolysis was observed after overnight growth at 37°C.
Opsonization and phagocytosis assays.
In vitro phagocytosis assays (2) were performed to determine the effect of anti-SLS(10-30) sera on M protein antibody-mediated opsonization. The test mixtures consisted of 0.05 ml of a standard suspension of type 24 streptococci grown to mid-log phase, 0.05 ml of various dilutions of anti-SLS(10-30) or control sera, 0.05 ml of M protein antiserum, and 0.35 ml of whole, heparinized (10 U/ml), nonimmune human blood. For these assays, the number of CFU per leukocyte was approximately 10. The opsonic M protein antiserum used in these studies was raised in rabbits against a recombinant fusion protein that contained the amino-terminal 12 amino acids of the mature type 24 M protein and the B subunit of E. coli heat-labile toxin (8). Additionally, a nonopsonic rabbit antiserum against the M protein-negative mutant of type 24 streptococci (ΩM24) was used as a negative control.
The tubes containing the reaction mixtures were rotated end over end for 60 min at 37°C. Smears were then made on glass slides and stained with Wright's stain (Sigma Diagnostics, St. Louis, Mo.). The assays were performed in triplicate, and opsonization was quantitated by counting 50 consecutive neutrophils and calculating the percentage with associated streptococci (percent opsonization). Statistical analyses were performed by using the Student t test with MultiStat 1.1 software (Biosoft, Inc., Ferguson, Mo.).
RESULTS
Immunogenicity of SLS(10-30)-KLH.
Preimmune and immune sera from all three rabbits were assayed for the presence of antibodies against the SLS(10-30) peptide by ELISA. The preimmune sera did not contain detectable levels of antipeptide antibodies, while the immune sera obtained after the second injection (weeks 6 to 13) all had ELISA titers ranging from 12,800 to 51,200.
Inhibition of SLS activity by rabbit antisera against SLS(10-30).
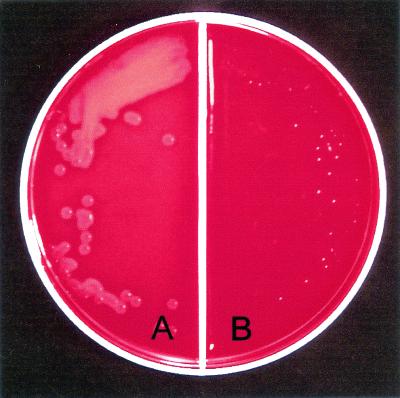
In initial experiments, the immune rabbit sera were screened for SLS-inhibiting activity by mixing either preimmune or immune serum in blood agar plates and observing cultured type 24 streptococci for zones of beta-hemolysis. The immune serum (Fig. 2B) significantly inhibited beta-hemolysis, while the preimmune serum had no effect (Fig. 2A).
FIG. 2.
Inhibition of SLS activity by anti-SLS(10-30) serum. The blood agar contained 10% preimmune serum (A) or immune antiserum (B). Type 24 GAS were streaked onto each side and incubated at 37°C overnight.
In subsequent experiments, we performed quantitative assays of hemoglobin release from SRBC to determine the specificity and sensitivity of the antibody-mediated inhibition of SLS activity (Table 1). Preincubation of growth supernatant with rabbit immune serum against SLS(10-30) completely inhibited hemolysis of SRBC (Table 1, experiment 1). In a separate experiment, complete inhibition of hemolysis by the immune serum was also observed when the reaction mixture contained cholesterol, which specifically inhibits SLO-mediated hemolysis but has no effect on the activity of SLS (Table 1, experiment 2). The bacterial cell-associated hemolytic activity was similarly inhibited in the presence of immune serum, but not in the presence of preimmune serum, and the addition of cholesterol had no effect on the level of inhibition (Table 1, experiment 3). These results indicate that the neutralizing activity of the immune serum was specific for SLS that was either present in the growth supernatant or cell associated. In addition, preincubation of the growth supernatant with trypan blue resulted in complete inhibition of hemolysis (data not shown), indicating that all of the hemolytic activity observed with this preparation was actually mediated by SLS.
TABLE 1.
Inhibition of SLS hemolytic activity by rabbit antiserum against SLS(10-30)
| Expt, SLS source | Reaction mixturea content
|
A540 | % Inhibition of hemolysis | |
|---|---|---|---|---|
| Test serum | Cholesterol concn (mg/ml) | |||
| Expt 1, supernatant | Preimmune | None | 1.64 | |
| 9-wk immune | None | 0.05 | 97.0 | |
| Expt 2, supernatant | Preimmune | None | 2.66 | |
| 9-wk immune | None | 0.07 | 97.4 | |
| Preimmune | 0.5 | 2.70 | ||
| 9-wk immune | 0.5 | 0.07 | 97.4 | |
| None (THB)b | None | 2.66 | ||
| Expt 3, bacterial cells | Preimmune | None | 1.97 | |
| 9-wk immune | None | 0.01 | 99.5 | |
| Preimmune | 0.5 | 2.30 | ||
| 9-wk immune | 0.5 | 0.01 | 99.6 | |
Reaction mixtures contained 0.5 ml of serum diluted 1:2 before use, 1 ml of a 2% washed suspension of SRBC, and 0.5 ml of either growth supernatant diluted 1:2 with PBS or bacterial cell pellet diluted with PBS to an optical density of 1.0.
THB, Todd-Hewitt broth.
In subsequent studies, we performed serial dilutions of anti-SLS(10-30) to determine the potency of the neutralizing antibodies (Table 2). Dilution of the immune serum to 1:8 in the reaction mixture resulted in 97% inhibition of hemolysis, while dilution to 1:16 produced approximately 50% inhibition. No inhibitory activity was seen at a final dilution of 1:32 (Table 2).
TABLE 2.
Titration of anti-SLS activity in rabbit antiserum raised against SLS (10-30)
| Serum | Final serum dilution | A540 | % Inhibition of hemolysis |
|---|---|---|---|
| Preimmune | 1:8 | 1.64 | |
| 9 wk | 1:8 | 0.05 | 97 |
| Preimmune | 1:16 | 1.85 | |
| 9 wk | 1:16 | 0.76 | 59 |
| Preimmune | 1:32 | 1.82 | |
| 9 wk | 1:32 | 1.85 | 0 |
Specificity of synthetic peptide antibodies.
To ensure that the SLS-neutralizing antibodies in the immune serum were actually directed against the SLS(10-30) peptide, we performed peptide inhibition assays (Table 3). Preincubation of the immune serum with SLS(10-30) at either 250 or 50 μg/ml reversed the neutralizing activity of the immune serum to levels approaching that observed with preimmune serum. Preincubation of fresh Todd-Hewitt broth with SLS(10-30) at 250 μg/ml resulted in no hemolysis of SRBC (data not shown), indicating that the peptide itself did not possess hemolytic activity.
TABLE 3.
Specificity of anti-SLS(10-30) activity, determined by peptide inhibition assays
| Serum | Concn (μg/ml) of added SLS(10-30)a | A540 | % Total hemolysis |
|---|---|---|---|
| Preimmune | None | 1.47 | 100 |
| 250 | 1.45 | 99 | |
| Immune (9 wk) | None | 0.06 | 4 |
| 250 | 1.35 | 92 | |
| 50 | 1.17 | 80 |
Serum was diluted 1:2 in PBS and preincubated with the synthetic peptide at 37°C for 45 min prior to addition to the reaction mixture.
Additional evidence for the specificity of the SLS-neutralizing activity in immune serum was obtained by using antibodies that were affinity purified over a column containing SLS(10-30) (Table 4). Control antibodies were purified from rabbit serum raised against a synthetic peptide of type 2 M protein over a column containing SM2(1-35)C. The purified antibody preparations were adjusted to contain 1.2 mg of total protein per ml, and ELISA titers were determined by using the respective peptides as the solid-phase antigens. The titer of the affinity-purified SLS(10-30) antibodies was 12,800, and the titer of the purified SM2 antibodies was 12,800, both of which are comparable to the respective titers of the immune sera (data not shown). The affinity-purified SLS(10-30) antibodies neutralized 95% of the SLS-mediated hemolysis, while the control SM2 antibodies had no effect (Table 4). These results show directly that antibodies against SLS(10-30) are responsible for inhibiting SRBC lysis induced by SLS.
TABLE 4.
Inhibition of SLS-mediated hemolysis by affinity-purified antibodies against SLS(10-30)
| Antibody (concn [mg/ml]) | A540 | % Inhibition of hemolysisa |
|---|---|---|
| Preimmune serum | 1.59 | |
| 14-wk immune serum | 0.03 | 98 |
| Affinity-purified anti-SLS(10-30)b (1.2) | 0.08 | 95 |
| Affinity-purified anti-S-M2(1-35)Cc (1.2) | 1.80 | 0 |
Reaction mixtures contained growth supernatant from type 24 streptococci diluted 1:4, 2% SRBC, and 0.5 mg of cholesterol per ml to inhibit SLO activity.
Specific antibodies were eluted from an affinity column containing the synthetic peptide SLS(10-30)
Control antibodies were purified from rabbit serum raised against a synthetic peptide of type 2 M protein, S-M2(1-35)C.
Effect of SLS antiserum on M protein antibody-mediated opsonization of GAS.
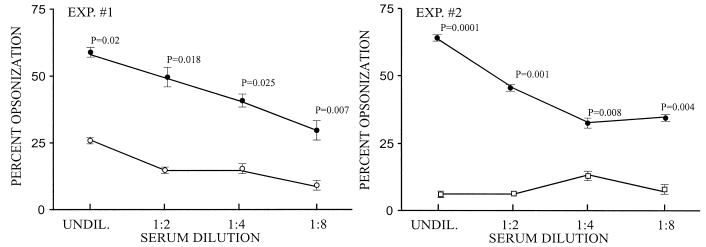
Experiments were performed to determine whether neutralizing antibodies against SLS can enhance the phagocytosis of GAS mediated by M protein antibodies. In vitro phagocytosis assays were performed with nonimmune human blood and type 24 streptococci. In initial experiments, the addition of SLS(10-30) antisera did not promote phagocytosis above the levels observed with preimmune sera (data not shown). In subsequent experiments, twofold dilutions of anti-SLS(10-30) serum or control sera were mixed with a constant 1:2 dilution of type-specific M24 antiserum and then added to the reaction mixture containing type 24 streptococci and whole human blood (Fig. 3). Compared to the preimmune serum, the SLS(10-30) antiserum significantly enhanced the level of M24 antibody-mediated phagocytosis at each dilution tested (Fig. 3, experiment 1). The effect on phagocytosis declined as the SLS(10-30) antiserum dilution increased.
FIG. 3.
Neutralizing antibodies against SLS potentiate M protein antibody-mediated opsonization and phagocytosis of GAS. Phagocytosis assays were performed with type 24 GAS and nonimmune human blood. All of the reaction mixtures contained a constant amount of type-specific M24 antibody (1:2 dilution) plus serial dilutions of either anti-SLS(10-30) (•) or control (○, □) serum. In experiment (EXP.) 1, the control serum was preimmune SLS(10-30) serum (○), and in experiment 2, the control serum was rabbit antiserum against the M protein-negative mutant of type 24 streptococci, ΩM24 (□). The assays were performed in triplicate, and P values were derived with Student's t test. UNDIL., undiluted.
One potential explanation for the enhanced levels of phagocytosis is that some of the SLS(10-30) antibodies bound to cell-associated SLS and promoted opsonization in concert with M antibodies. To control for this possibility, we used an antiserum raised in rabbits against an M-negative mutant of type 24 streptococci (ΩM24) (Fig. 3, experiment 2). The titer of this nonopsonic antiserum against whole type 24 streptococci, as determined by ELISA, was equivalent to the titer of the type-specific M antiserum against the M24-LT-B fusion protein. Again, the SLS(10-30) antiserum, compared to the ΩM24 antiserum, enhanced phagocytosis promoted by M24 antibodies and the differences were significant at each dilution of anti-SLS(10-30) tested (Fig. 3B). Taken together, these data suggest that SLS-neutralizing antibodies may potentiate opsonization and phagocytosis of GAS that is mediated by M protein antibodies.
DISCUSSION
SLS has been described as the most potent of bacterial cytolytic toxins (21). The potency of the toxin, the broad range of host cells that it lyses, and the fact that it is always bound to carrier molecules are thought to be the primary reasons that it is not immunogenic. We have shown for the first time that neutralizing antibodies can be evoked by a nontoxic synthetic peptide of SLS (SagA). This observation may have significant implications for our understanding of the role of SLS in the pathogenesis of group A streptococcal infections and also in the development of vaccines designed to prevent these infections and their complications.
The characterization of SLS has been an elusive subject of intense interest for many years (21). Earlier investigations focused on the chemical composition of SLS purified from culture supernatants of GAS (1, 3) and the toxicity of SLS in vitro and in vivo (11, 17, 21). Only recently has there been considerable progress in understanding the genetic and structural basis for the role of SLS in the pathogenesis of streptococcal infections. SLS-negative Tn916 insertion mutants were shown to be much less virulent than the wild-type strains in a mouse model of soft tissue infection (4). In a more recent study, Nizet et al. (16) identified a nine-gene operon in GAS that is sufficient to confer the SLS beta-hemolytic phenotype on Lactococcus lactis when expressed from a plasmid. Structural analyses of the translated proteins from the sag operon revealed that SagA resembles the family of bacteriocins, with a 23-amino-acid leader peptide and a putative enzyme cleavage site following GG, which would result in a 30-amino-acid propeptide. In addition, those authors predicted that other genes in the sag operon may encode an enzyme responsible for posttranslational modification of the propeptide and integral membrane proteins that could possibly be involved in secretion of the toxin (16).
The structural similarities between the SagA propeptide and bacteriocins indicate that posttranslational modifications may lead to the formation of a cyclic structure at the N terminus of SagA resulting from thioester bonds between serine, threonine, or glycine residues and neighboring cysteine residues (16, 18). In the present study, we hypothesized that the C-terminal 20-amino-acid peptide of SagA, which does not contain cysteine residues, would be devoid of toxicity and therefore may be immunogenic. When coupled to KLH, the SLS(10-30) peptide evoked antibodies that completely neutralized the hemolytic activity of native SLS in bacterial supernatants and on the bacterial cell surface.
The SLS-neutralizing activity of the synthetic peptide antisera indicates that the antibodies were evoked by epitopes whose conformation was maintained in the native toxin, even after the proposed posttranslational modifications (16, 18). In addition, the antibodies reacted with the native toxin despite its association with carrier molecules. It is thought that the SLS-carrier complex protects GAS from the toxicity of the free peptide and that the carrier then delivers the toxin to host cell membranes (21). It is interesting to speculate that the synthetic peptide antibodies may function as high-affinity carriers capable of displacing the native SLS from natural carriers, such as serum albumin or lipoteichoic acid, yet the antibodies are not able to deliver the toxin to target cells, perhaps because of high-affinity binding of the toxin to the antigen-combining site. This hypothesis is partially supported by our finding that SLS could not be detected in Western blots of GAS culture supernatants (unpublished data), suggesting that SLS bound to carrier molecules was not available for antibody binding when on a solid support. The binding of neutralizing antibodies may require the delivery of SLS from the carrier to antibodies in an equilibrium reaction that can only occur in a fluid phase.
The ability of SLS antibodies to potentiate M protein antibody-mediated phagocytosis is consistent with a previous report by Ofek et al. showing that sublethal amounts of SLS inhibit phagocytosis (17). Those authors speculated that SLS depolarized the membrane of phagocytic cells, which resulted in secondary metabolic effects on phagocytosis. In the present study, we clearly demonstrated that SLS antibodies potentiated the phagocytosis of GAS that were opsonized by M protein antibodies. A review of the blood smears by light microscopy did not reveal any quantitative or qualitative differences in the polymorphonuclear leukocytes in the presence of preimmune sera or immune sera against SLS (unpublished observation). Our results suggest that neutralizing antibodies against SLS can block the antiphagocytic effect of the toxin (17), thereby promoting more efficient M protein antibody-mediated opsonization and phagocytosis. It is not known whether the SLS antibodies exert this effect by neutralizing the secreted or cell-bound SLS, since the hemolytic activity of both was blocked by the antiserum.
In summary, we have shown for the first time that neutralizing antibodies can be raised against a synthetic fragment of group A streptococcal SLS. The antibodies specifically and completely inhibited SLS-mediated hemolysis of SRBC and potentiated opsonization and phagocytosis of GAS mediated by type-specific M protein antibodies. Our results also provide convincing evidence that the sagA gene encodes the SLS peptide of GAS. Because of the potency of SLS as a cytolytic toxin for many target cells and its known role in the virulence of GAS, further studies will focus on the potential role of SLS-neutralizing antibodies in preventing infections or in modifying the outcomes of these infections. In addition, SLS peptides may be important components of M protein-based vaccines designed to prevent the most common and serious group A streptococcal infections (6).
Acknowledgments
This work was supported by U.S. Public Health Service grant AI-10085 (J.B.D.), by Merit Review grants from the Department of Veterans Affairs (J.B.D., D.L.H., and H.S.C.), and by research funds provided by ID Biomedical Corporation, Bothell, Wash.
We thank Deborah Bueltemann for expert secretarial assistance in preparing the manuscript.
Editor: E. I. Tuomanen
Footnotes
This article is dedicated to Gene H. Stollerman, whose wise counsel and encouragement, especially related to the importance of SLS as a virulence determinant, are greatly appreciated.
REFERENCES
- 1.Alouf, J. E., and C. Loridan. 1988. Production, purification, and assay of streptolysin S. Methods Enzymol. 165:59-64. [DOI] [PubMed] [Google Scholar]
- 2.Beachey, E. H., G. H. Stollerman, E. Y. Chiang, T. M. Chiang, J. M. Seyer, and A. H. Kang. 1977. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of the type 24 M antigen. J. Exp. Med. 145:1469-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheimer, A. W. 1967. Physical behavior of streptolysin S. J. Bacteriol. 93:2024-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betschel, S. D., S. M. Borgia, N. L. Barg, D. E. Low, and J. C. S. De Azavedo. 1998. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect. Immun. 66:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney, H. S., M. S. Bronze, J. B. Dale, and D. L. Hasty. 1994. Analysis of the role of M24 protein in streptococcal adhesion and colonization by use of omega-interposon mutagenesis. Infect. Immun. 62:4868-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale, J. B. 1999. Multivalent group A streptococcal vaccines. Infect. Dis. Clin. N. Am. 13:227-243. [DOI] [PubMed] [Google Scholar]
- 7.Dale, J. B., and E. H. Beachey. 1986. Localization of protective epitopes of the amino terminus of type 5 streptococcal M protein. J. Exp. Med. 163:1191-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale, J. B., E. C. Chiang, J. W. Lederer, and M. S. Bronze. 1993. Protective immunogenicity of a recombinant hybrid protein containing a fragment of type 24 streptococcal M protein and the B subunit of Escherichia coli labile toxin, p. 409-412. In: H. Ginsberg, F. Brown, R. Chanock, and R. Lerner (ed.), Vaccines 93: modern approaches to new vaccines. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 9.Dale, J. B., M. Simmons, E. C. Chiang, and E. Y. Chiang. 1996. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 14:944-948. [DOI] [PubMed] [Google Scholar]
- 10.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hryniewicz, W., and J. Pryjma. 1977. Effect of streptolysin S on human and mouse T and B lymphocytes. Infect. Immun. 16:730-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehoe, M. A., L. Miller, J. A. Walker, and G. J. Boulnois. 1987. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging, thiol-activated toxins. Infect. Immun. 55:3228-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loridan, C., and J. E. Alouf. 1986. Purification of RNA-core induced streptolysin S, and isolation and haemolytic characteristics of the carrier-free toxin. J. Gen. Microbiol. 132:307-315. [DOI] [PubMed] [Google Scholar]
- 15.McLellan, D. G., E. Y. Chiang, H. S. Courtney, D. L. Hasty, S. C. Wei, M. C. Hu, M. A. Walls, J. J. Bloom, and J. B. Dale. 2001. Spa contributes to the virulence of type 18 group A streptococci. Infect. Immun. 69:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizet, V., B. Beall, D. J. Bast, V. Datta, L. Kilburn, D. E. Low, and J. C. De Azavedo. 2000. Genetic locus for streptolysin S production by group A streptococcus. Infect. Immun. 68:4245-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ofek, I., S. Bergner-Rabinowitz, and I. Ginsburg. 1972. Oxygen-stable hemolysins of group A streptococci. VIII. Leukotoxic and antiphagocytic effects of streptolysins S and O. Infect. Immun. 6:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahl, H. G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 19.Stevens, D. L. 1999. The flesh-eating bacterium: what's next? J. Infect. Dis. 179:S366-S374. [DOI] [PubMed] [Google Scholar]
- 20.Theodore, T. S., and G. B. Calandra. 1981. Streptolysin S activation by lipoteichoic acid. Infect. Immun. 33:326-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wannamaker, L. W. 1983. Streptococcal toxins. Rev. Infect. Dis. 5:S723-S732. [DOI] [PubMed] [Google Scholar]