Abstract
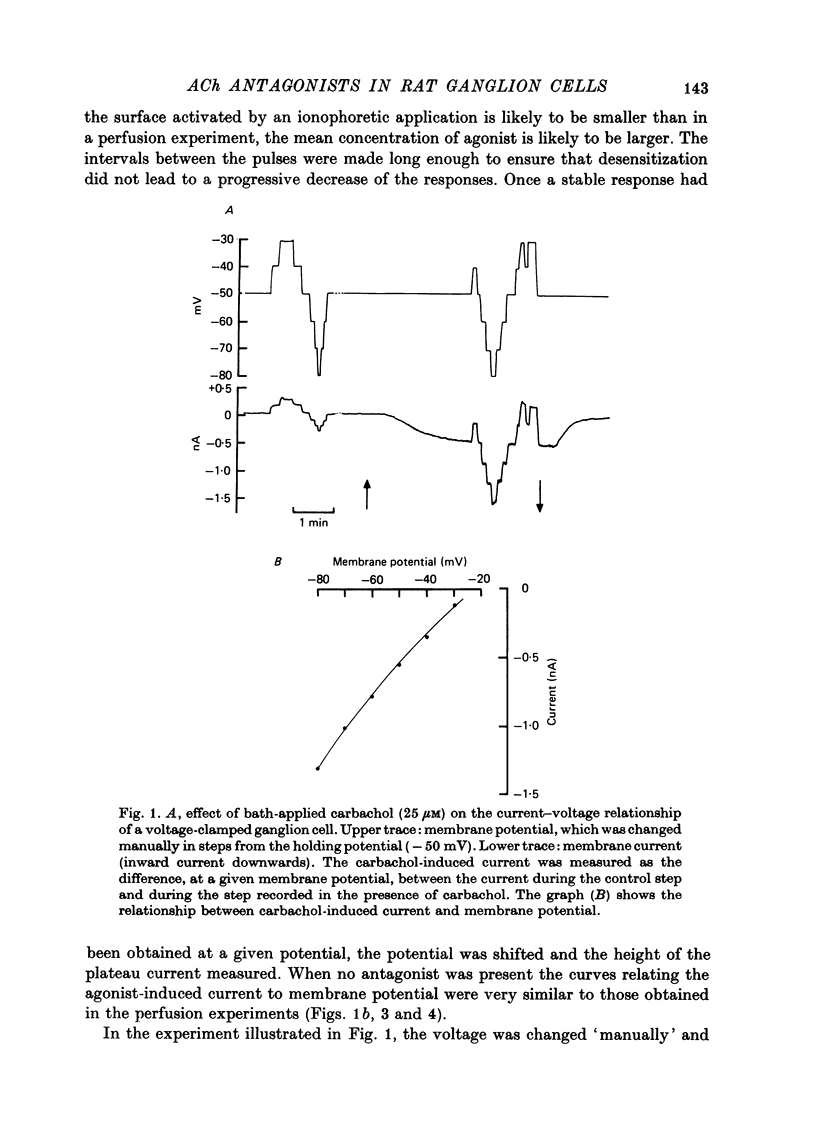
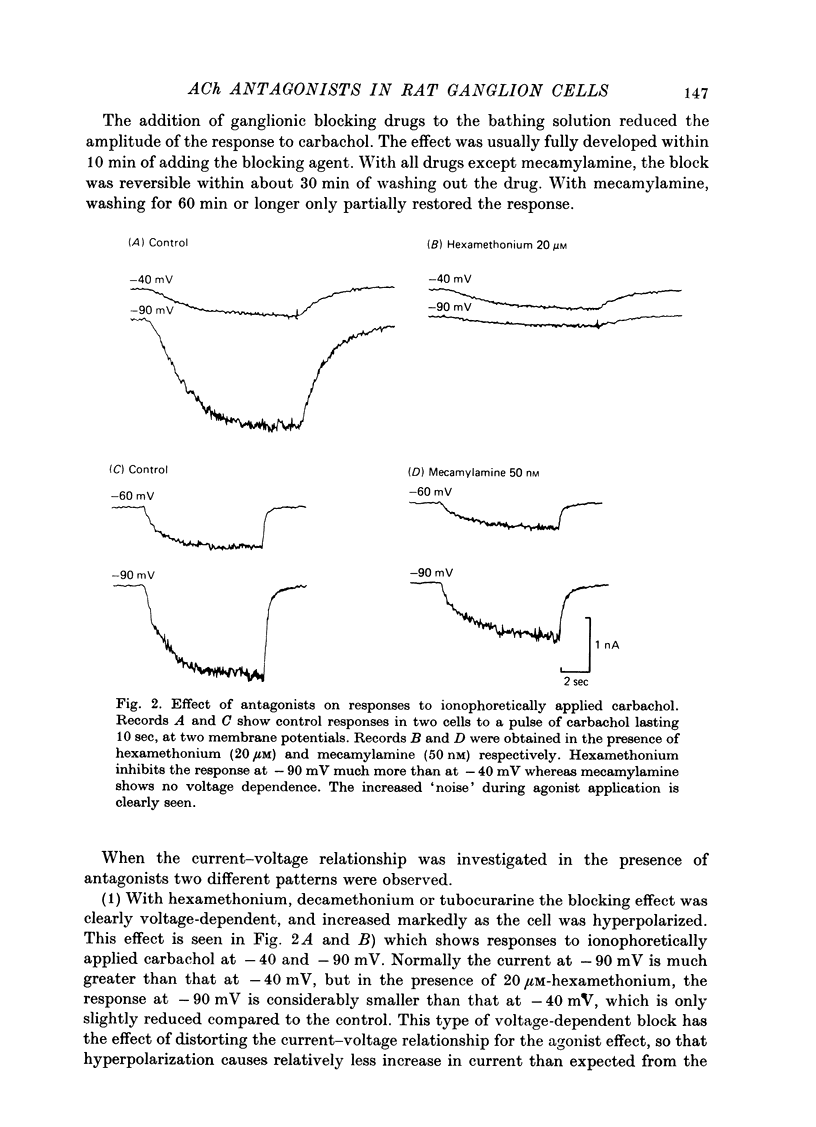
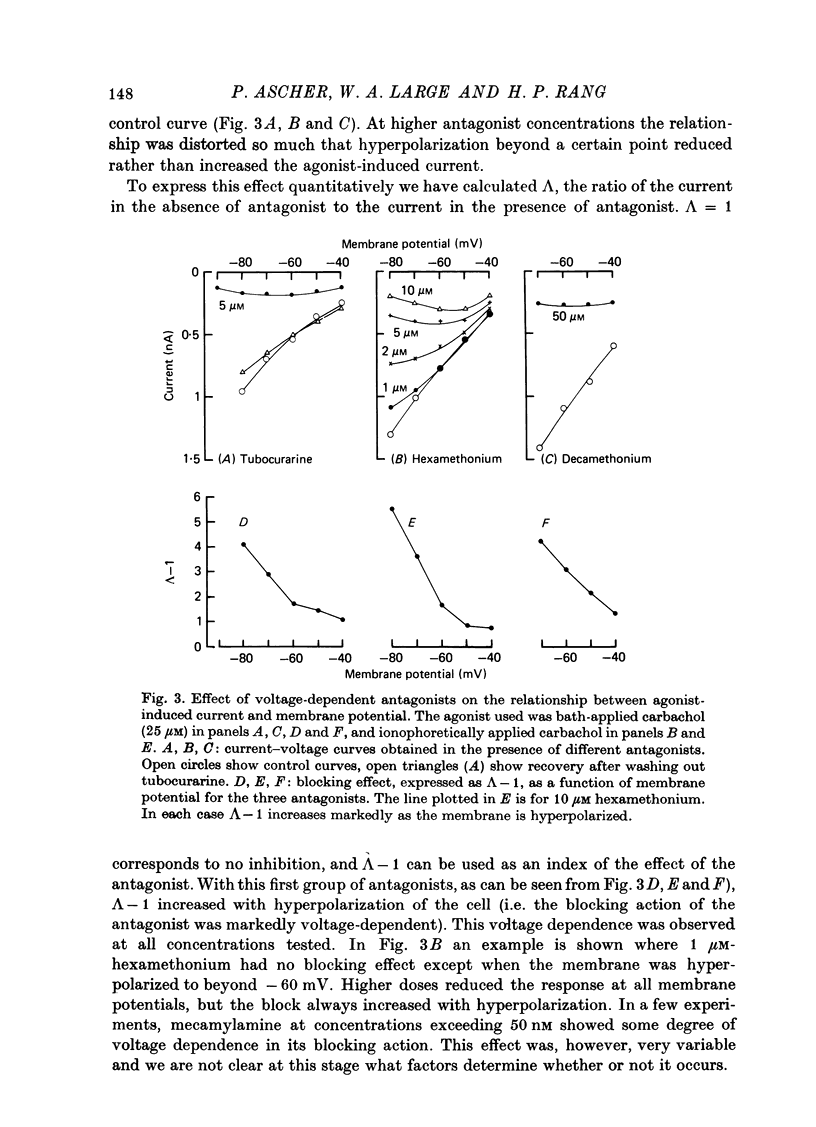
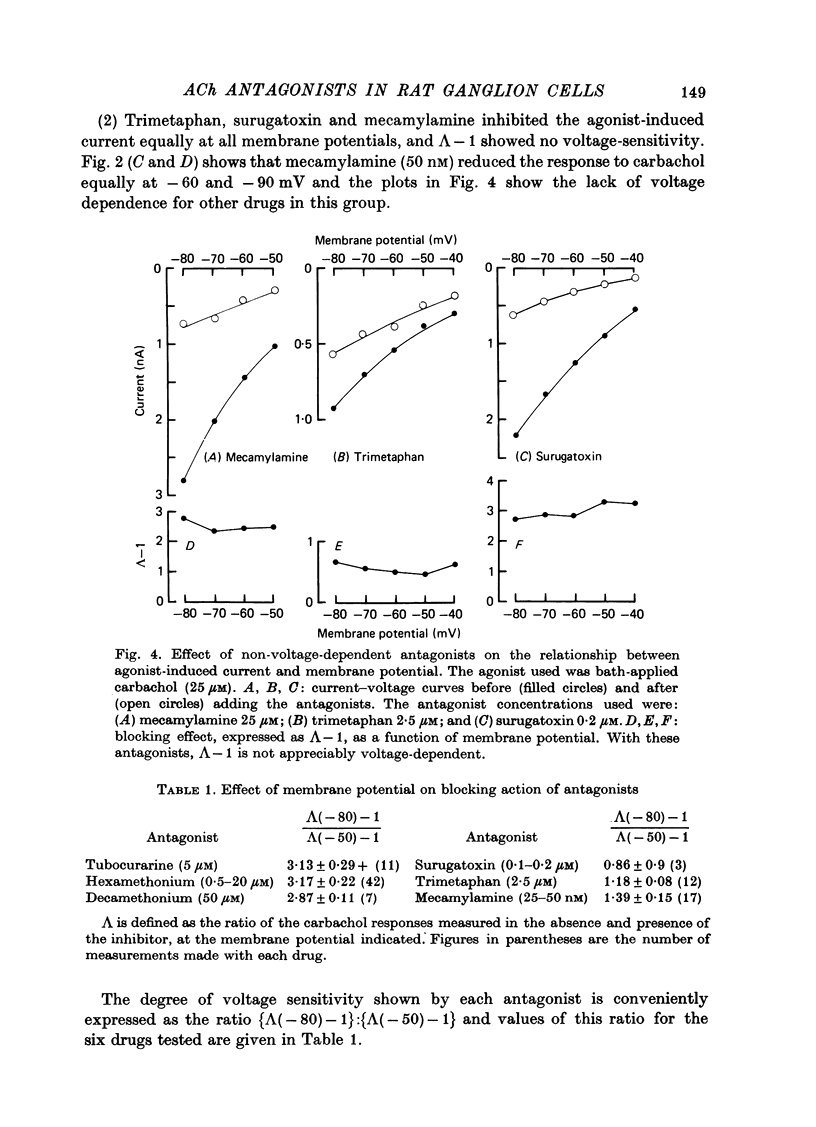
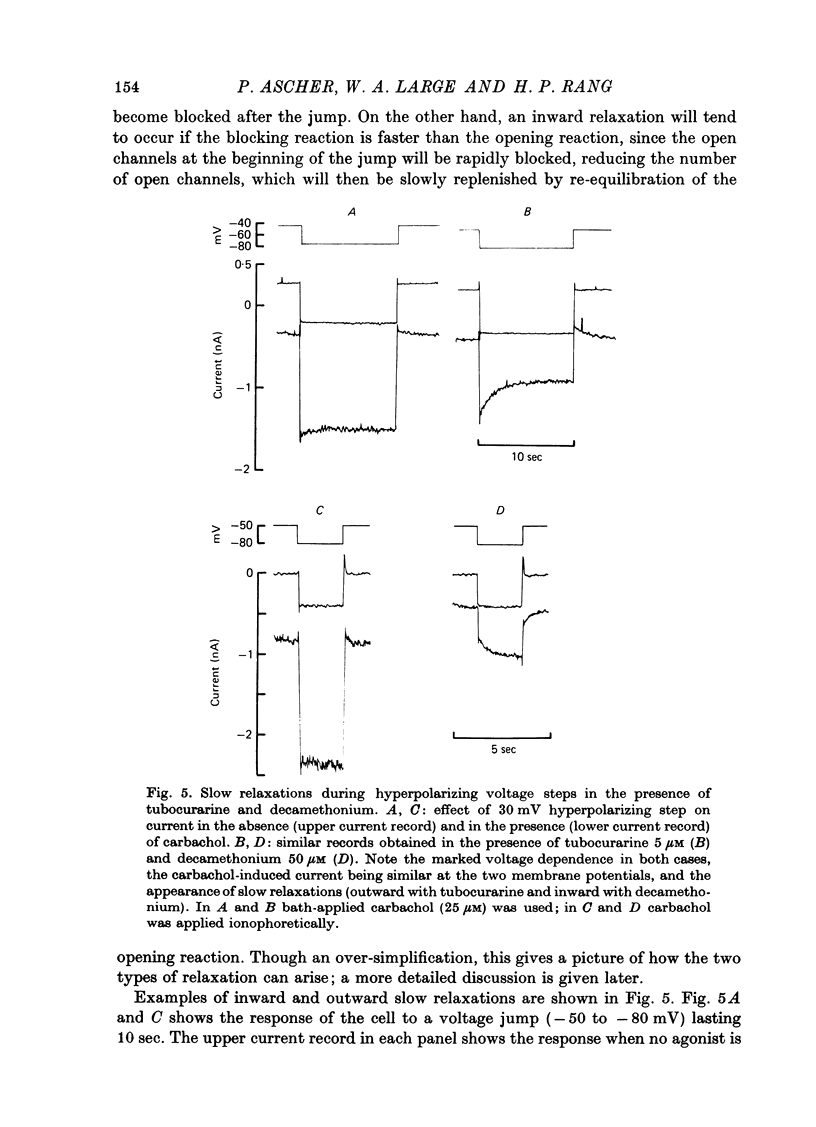
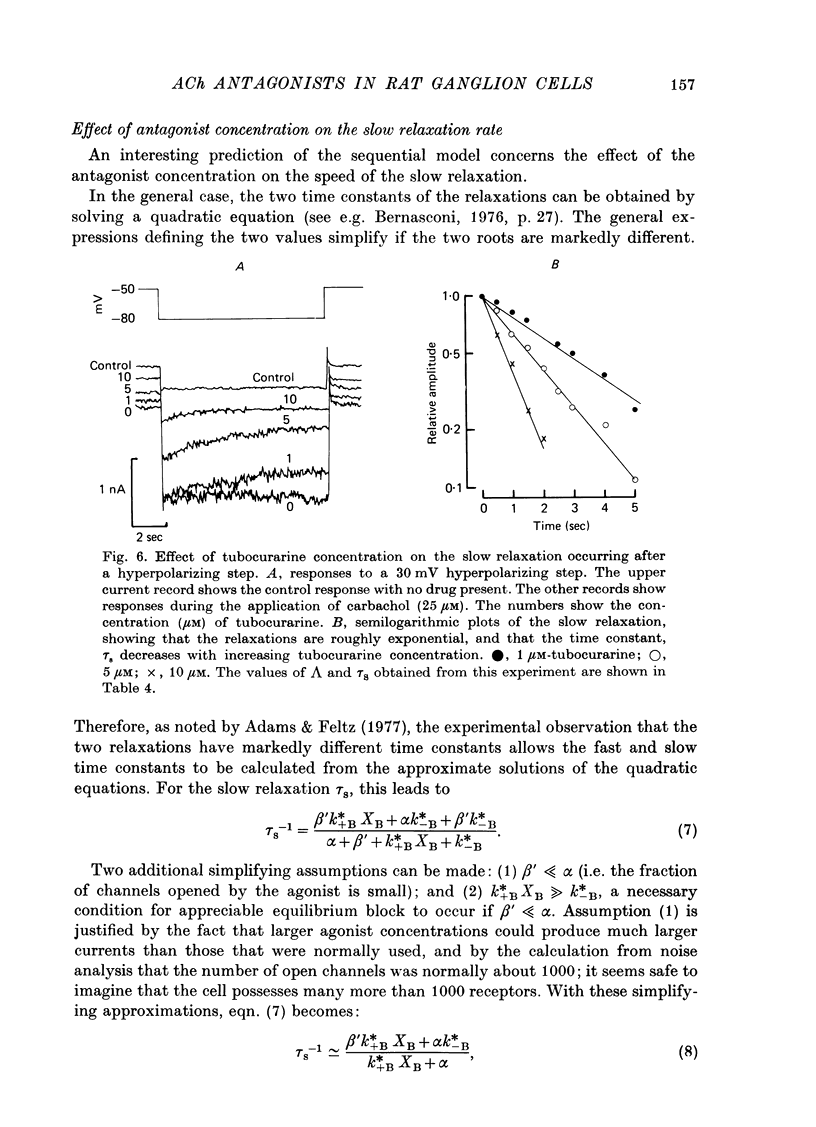
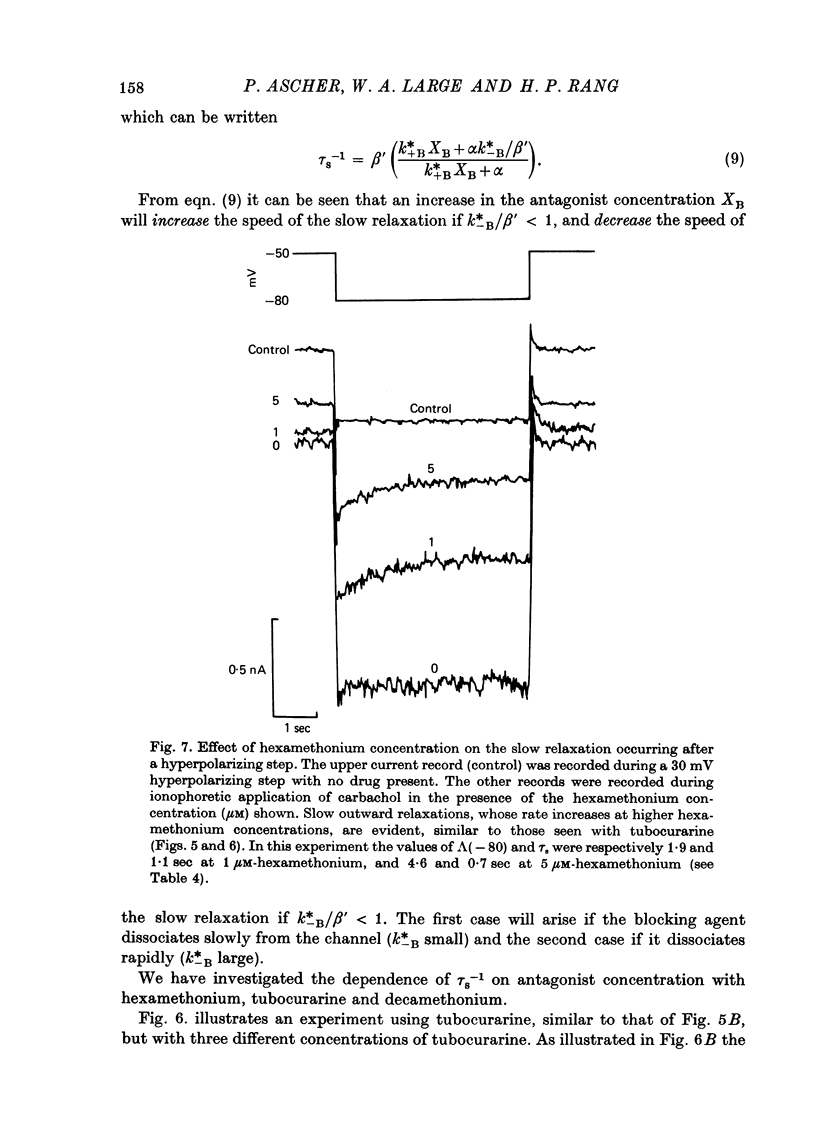
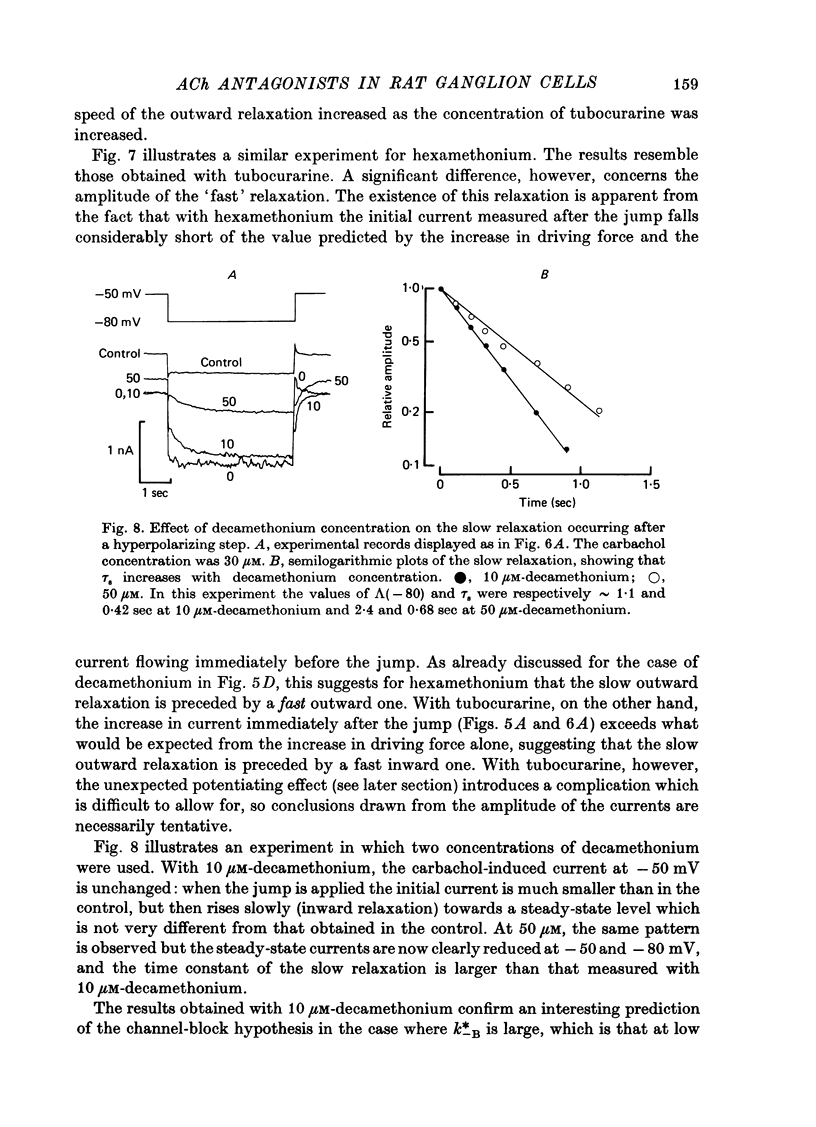
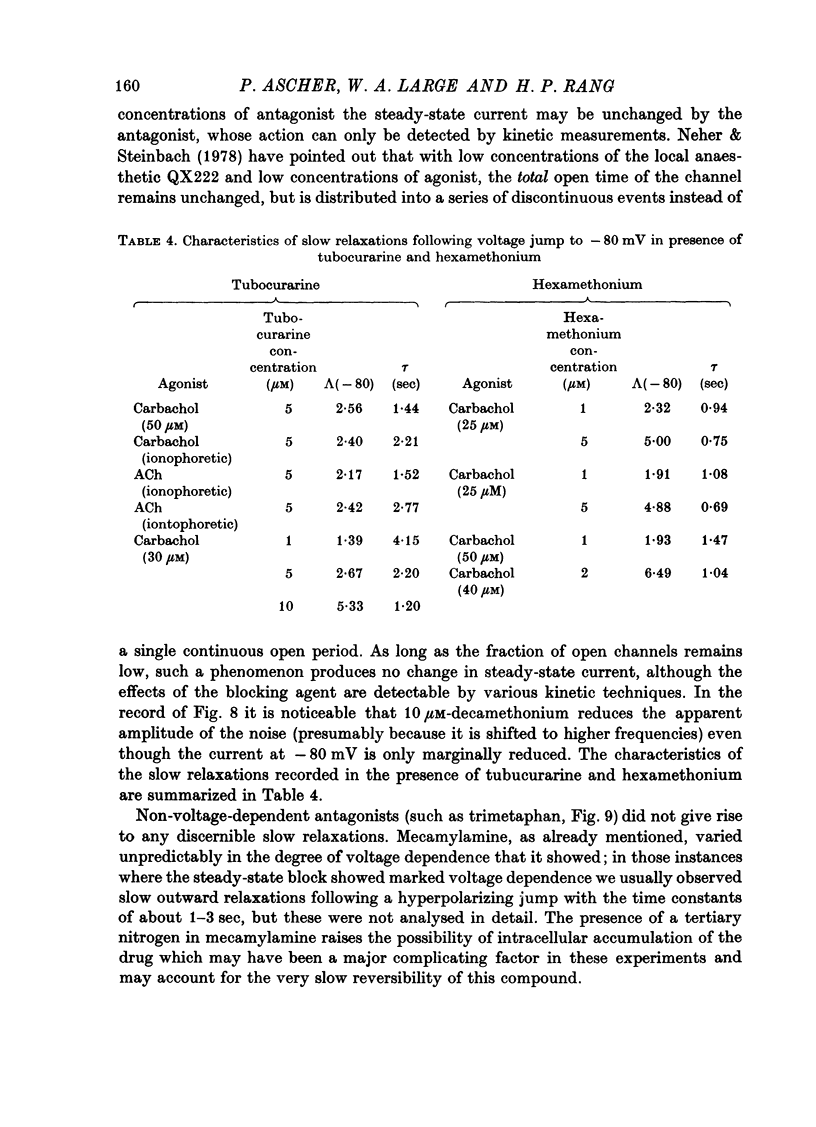
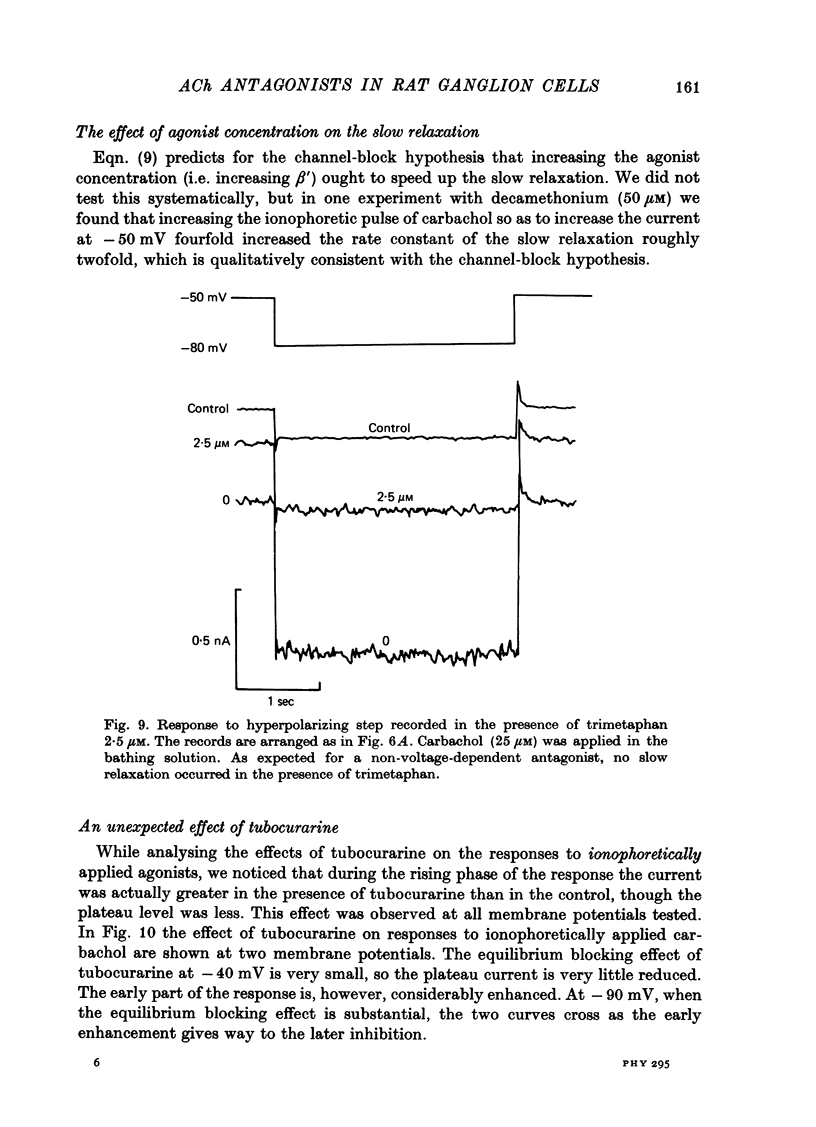
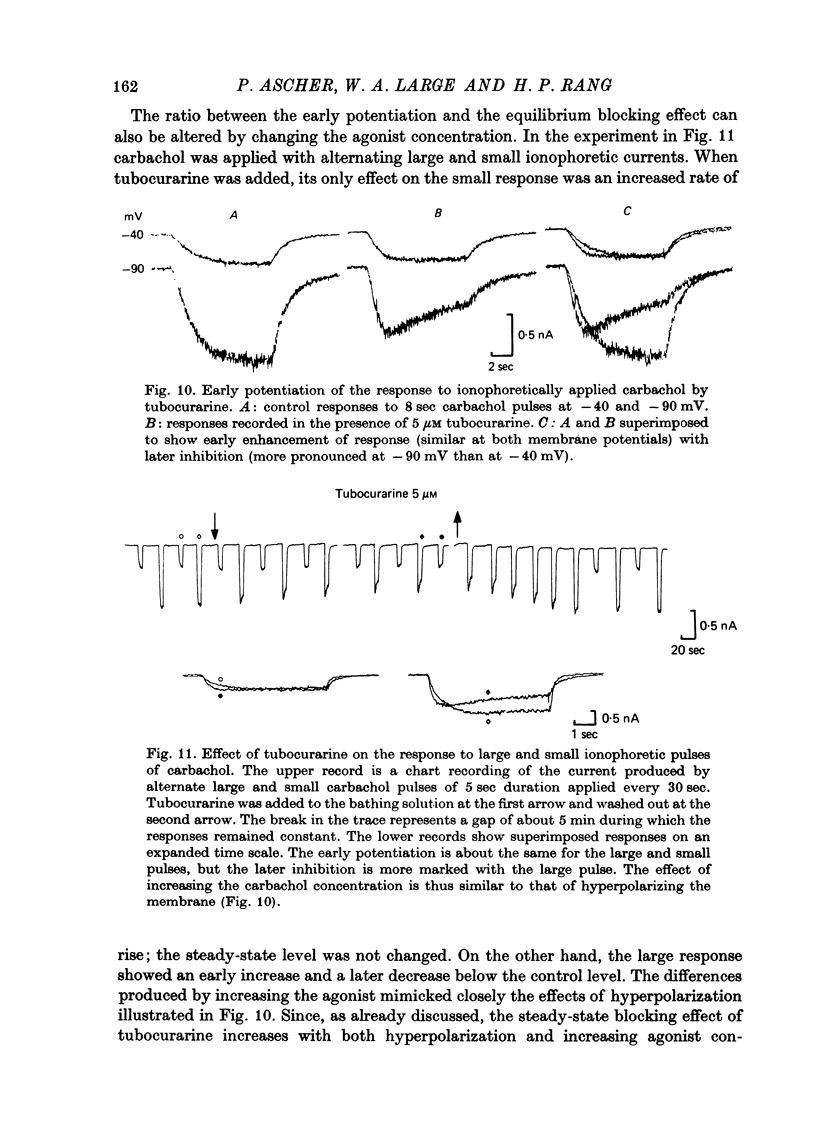
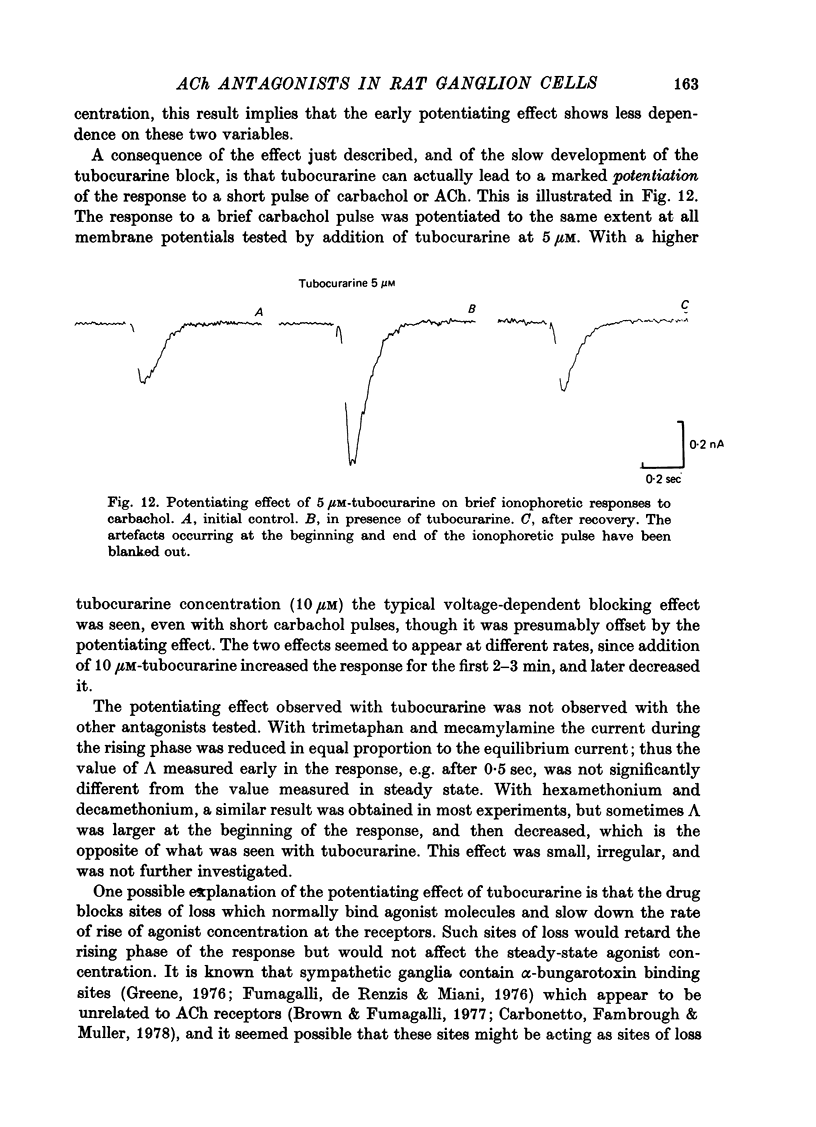
The mode of action of ACh antagonists on the parasympathetic neurones of the submandibular ganglion of the rat was studied by means of a two-micro-electrode voltage-clamp technique. The currents produced by various agonists (carbachol, ACh, suberylcholine) were studied in steady state and after voltage steps, before and after perfusion of various antagonists. 2. For three antagonists (tubocurarine, hexamethonium, decamethonium) the blocking action increases with hyperpolarization. For three other antagonists (surugatoxin, trimetaphan, mecamylamine) the effects observed at low concentrations appear to be independent of membrane potential, although in some cases voltage dependence of the block was observed for mecamylamine. 3. The blocks the 'open' channel-reception complex. The block produced by tubocurarine, hexamethonium and decamethonium increases with the agonist concentration, an observation which supports a 'sequential' scheme in which the antagonist blocks the 'open' channel-receptor complex. The block produced by trimetaphan and mecamylamine decreases slightly with increased agonist concentration, which in turn suggests that these two compounds are competitive antagonists, preventing binding of the agonists to the closed channel-receptor complex. 4. In the cases where the block is voltage dependent, voltage jumps trigger slow relaxations which are not present in control conditions. In the case of tubocurarine and hexamethonium, the relaxation following a hyperpolarizing voltage jump corresponds to a decrease in conductance. In the case of decamethonium, the slow relaxation is in the opposite direction. 5. The slow relaxations observed with tubocurarine and hexamethonium are speeded by an increase of the antagonist concentration; the slow relaxations observed with decamethonium are slowed by an increase of the decamethonium concentration. 6. The steady-state observations and the relaxations can be interpreted in terms of a scheme in which tubocurarine, hexamethonium and decamethonium act mainly by blocking the channels opened by the cholinergic agonists. 7. The two types of slow relaxation are those predicted if tubocurarine and hexamethonium dissociate slowly from the channel, and decamethonium rapidly. 8. An additional effect of tubocurarine is described, which consists of a potentiation of the rising phase of the response to an ionophoretic pulse. Possible mechanisms of this effect are discussed.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Feltz A. Interaction of a fluorescent probe with acetylcholine-activated synaptic membrane. Nature. 1977 Oct 13;269(5629):609–611. doi: 10.1038/269609a0. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. The action of ganglion-blocking drugs studied by voltage clamp [proceedings]. J Physiol. 1978 Jul;280:17P–17P. [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Two modes of action of ganglionic blocking drugs [proceedings]. Br J Pharmacol. 1979 May;66(1):79P–79P. [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A quantitative description of end-plate currents in the presence of two lidocaine derivatives. J Physiol. 1976 Jun;258(2):301–322. doi: 10.1113/jphysiol.1976.sp011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Fumagalli L. Department of Pharmacology, The School of Pharmacy, University of London, London, Great Britain. Brain Res. 1977 Jun 24;129(1):165–168. doi: 10.1016/0006-8993(77)90981-7. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Garthwaite J. Action of surugatoxin on nicotinic receptors in the superior cervical ganglion of the rat. Br J Pharmacol. 1976 Sep;58(1):157–159. doi: 10.1111/j.1476-5381.1976.tb07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAGAS C. Studies on the mechanis of curarization. Ann N Y Acad Sci. 1959 Aug 28;81:345–357. doi: 10.1111/j.1749-6632.1959.tb49318.x. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. T., Fambrough D. M., Muller K. J. Nonequivalence of alpha-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1016–1020. doi: 10.1073/pnas.75.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. Modes of action of gallamine at the neuromuscular junction [proceedings]. Br J Pharmacol. 1979 May;66(1):78P–79P. [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Parsons R. L. Synaptic channel gating differences at snake twitch and slow neuromuscular junctions. Nature. 1978 Aug 31;274(5674):902–904. doi: 10.1038/274902a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Feltz A., Large W. A., Trautmann A. Analysis of atropine action at the frog neutromuscular junction. J Physiol. 1977 Jul;269(1):109–130. doi: 10.1113/jphysiol.1977.sp011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli L., De Renzis G., Miani N. Acetylcholine receptors: number and distribution in intact and deafferented superior cervical ganglion of the rat. J Neurochem. 1976 Jul;27(1):47–52. doi: 10.1111/j.1471-4159.1976.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A. Binding of alpha-bungarotoxin to chick sympathetic ganglia: properties of the receptor and its rate of appearance during developement. Brain Res. 1976 Jul 23;111(1):135–145. doi: 10.1016/0006-8993(76)91054-4. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi E., Yamada S. Pharmacological studies on surugatoxin, the toxic principle from Japanese ivory mollusc (Babylonia japonica). Br J Pharmacol. 1975 Feb;53(2):207–215. doi: 10.1111/j.1476-5381.1975.tb07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neild T., Ascher P. Voltage sensitivity of acetylcholine currents in Aplysia neurones in the presence of curare. Nature. 1976 Jun 10;261(5560):501–503. doi: 10.1038/261501a0. [DOI] [PubMed] [Google Scholar]
- Marty A. Noise and relaxation studies of acetylcholine induced currents in the presence of procaine. J Physiol. 1978 May;278:237–250. doi: 10.1113/jphysiol.1978.sp012301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kusano K. Hyperpolarizing potentials induced by Ca-mediated K-conductance increase in hamster submandibular ganglion cells. J Neurobiol. 1978 Sep;9(5):367–392. doi: 10.1002/neu.480090504. [DOI] [PubMed] [Google Scholar]


