Abstract
Staphylococcal enterotoxins (SE) are exoproteins produced by Staphylococcus aureus that act as superantigens and have been implicated as a leading cause of food-borne disease and toxic shock. Little is known about how these molecules penetrate the gut lining and gain access to both local and systemic immune tissues. To model movement in vitro of staphylococcal enterotoxins, we have employed a monolayer system composed of crypt-like human colonic T-84 cells. SEB and SEA showed comparable dose-dependent transcytosis in vitro, while toxic shock syndrome toxin (TSST-1) exhibited increased movement at lower doses. Synthetic peptides corresponding to specific regions of the SEB molecule were tested in vitro to identify the domain of the protein involved in the transcytosis of SE. A toxin peptide of particular interest contains the amino acid sequence KKKVTAQELD, which is highly conserved across all SE. At a toxin-to-peptide ratio of 1:10, movement of SEB across the monolayers was reduced by 85%. Antisera made against the SEB peptide recognized native SEB and also inhibited SEB transcytosis. Finally, the conserved 10-amino-acid peptide inhibited transcytosis of multiple staphylococcal enterotoxins, SEA, SEE, and TSST-1. These data demonstrate that this region of the staphylococcal enterotoxins plays a distinct role in toxin movement across epithelial cells. It has implications for the prevention of staphylococcal enterotoxin-mediated disease by design of a peptide vaccine that could reduce systemic exposure to oral or inhaled superantigens. Since the sequence identified is highly conserved, it allows for a single epitope blocking the transcytosis of multiple SE.
The organism Staphylococcus aureus is a gram-positive bacterium that is known for its production of potent toxins. Once introduced into a host's system, these toxins can act to profoundly stimulate the immune system. The proteins are known to act on host systems in three distinct ways: as enterotoxins, they induce emesis and diarrhea in humans and nonhuman primates (16); as exotoxins, they have been implicated in induction of toxic shock (22); and as superantigens, they induce extensive Vβ-specific T-cell stimulation (12) followed by anergy and apoptosis, which results in immunosuppression (27).
S. aureus is carried by close to 30% of the world's population (28). This high carrier rate may be a factor in the prevalence of S. aureus contamination of food. Poor hygiene and improper holding temperatures of meats and creamy dishes, such as salad dressings, have been implicated as the primary etiologies of food-borne disease caused by S. aureus (3). At temperatures below 60°C, most strains of the bacteria produce enterotoxins. Once these enterotoxins enter the intestines of a host, they have the ability to transcytose the intestinal wall and gain access to the immune system (10, 23). Within 4 h of ingestion, these toxins can be responsible for an array of symptoms, such as diarrhea, nausea, vomiting, and abdominal pain. The host should become symptom-free about 24 h after the ingestion time. However, anorexia has been shown to persist for approximately 5 days in monkeys challenged with an incapacitating dose of staphylococcal enterotoxin B (SEB) (M. Sipos and M. Jett [WRAIR], personal communication). The mechanism responsible for the emetic response to staphylococcal enterotoxins (SE) could be immune mediated, in that stimulation of T-cell proliferation is associated with massive cytokine production (6). In fact, the symptoms of food poisoning can be mimicked by administration of exogenous interleukin 2 (IL-2) (18). However, studies of SEA mutated at histidine 61 have shown that superantigenic and emetic activity can be separated (13). Thus, additional mechanisms may be involved in elicitation of emesis. Further, drugs that block receptors for serotonin completely ablated the vomiting, diarrhea, and prostration induced by SEA or SEB in piglets (Mani et al., submitted for publication).
Superantigens are able to stimulate unusually large numbers of T cells (up to 20% of the entire T-cell complement of the host), and SE are prototypical bacterial superantigens. In contrast to conventional antigens, superantigens are not internalized by antigen-presenting cells, do not undergo processing, and are not presented within the antigen binding groove. Instead, the intact superantigens bind to both major histocompatibility complex class II (MHC-II) molecules and T cells at sites distinct from the conventional antigen-binding sites (14). Indeed, it has been shown that interaction with superantigens primarily involves the variable region of the T-cell receptor (TCR) β chain (Vβ) (18). Subsequent to proliferation, most T cells whose cognate antigen is not present in the host will undergo clonal deletion, resulting in immunosuppression. By contrast, in susceptible individuals activated T cells may continue to be stimulated and precipitate autoimmune disease (8, 18).
S. aureus produces multiple serotypes of toxin: SEA, SEB, SEC1, SEC2, SEC3, SED, SEE through SEJ, and toxic shock syndrome toxin one (TSST-1) (15). SEB and SEA are the most clinically important and best-characterized bacterial superantigenic toxins. SEB is a single polypeptide chain with a molecular mass of approximately 28.5 kDa. The protein structure consists of 5 α-helices and 12 β-sheets organized into two domains (33). Subsequent to resolution of the SEB crystal structure, attempts have been made to identify the regions of the toxins important for their immunomodulatory effects. SE have been cocrystallized with either MHC-II or the TCR region to which they bind (14, 21). The important regions determined by the resolution of these crystal structures largely confirm the data obtained functionally from peptides and mutant toxins. The MHC-binding region of SEB consists of residues in helix 5 and β-sheet 2, while the TCR-binding regions include four discrete sequences at the top of the molecule, helix 2, the β-sheet 2-3 loop, the end of β-sheet 4, and the β10 to helix 5 loop. Thus, the sites of interaction of SE with immune cells have been well documented. In contrast, studies to determine the region of the SE involved in their entry into the body through gastrointestinal or pulmonary surfaces in order to gain access to local and systemic immune systems have been much more limited. The present work identifies a region of SEB distinct from MHC or TCR binding sites that is involved in transcytosis across epithelial cell monolayers. Further, a peptide corresponding to this region, SEB 152-161, could significantly inhibit movement of not just SEB but transcytosis of multiple other SE was inhibited as well.
MATERIALS AND METHODS
Toxins, antitoxins, and toxin peptides.
Toxins, antitoxins, and antitoxin conjugates were purchased from Toxin Technology, Inc. (Sarasota, Fla.). SEA, SEB, SEE, and TSST-1 were guaranteed 95% pure based on gel electrophoresis and subsequent Coomassie blue or silver staining.
Based on the published sequence of SEB (19), four 30-amino-acid-long synthetic peptides representing all but the amino and carboxy termini of the molecule were prepared by Peninsula Laboratories (Belmont, Calif.). SEB 152-161, SEB 130-160, and the two control peptides (SEB 225-234 and SEB 191-220) from regions of the molecule not implicated in SEB function were synthesized with an ABI 431A peptide synthesizer using Nα-fluoroenylmethyloxycarbonyl chemistry. Peptides were cleaved from the resins using trifluoroacetic acid/ethanedithiol/thioanisole/anisole at a ratio of 90:3:5:2, extracted in ether and ethyl acetate and subsequently dissolved in water and lyophilized. Reversed-phase high-performance liquid chromatography analysis of crude peptides indicated one major peak in each profile, and amino acid analysis indicated that actual composition corresponded closely to theoretical.
Anti-peptide antisera generation.
A portion of each of the synthesized peptides was conjugated to keyhole limpet hemocyanin (KLH). Then, 13 mg of peptide was conjugated to 9 mg of KLH using 200 mM glutaraldehyde, and the glutaraldehyde was removed by dialysis. After dialysis, the peptide-carrier solutions were diluted to a concentration of 5 mg/ml, sterile filtered, and stored at −80°C. Ten-week-old C57BL/6 mice were purchased from NCI Charles River Laboratory (Frederick, Md.) and injected intraperitoneally with 250 μl of a 1:1 mixture of antigen (200 μg/ml) and Freund's complete adjuvant. On days 14 and 28, the mice received a boost of 250 μl of a 1:1 mixture of antigen (200 μg/ml) and Freund's incomplete adjuvant. Mice were euthanatized on day 38, and blood was collected. Serum was removed and samples were assayed for immunoglobulin G (IgG) responses to SEB and other SE by enzyme-linked immunosorbent assay (ELISA) using toxin-coated plates. Titers were determined as the inverse of the dilution of serum that yielded a half-maximal response. Control sera made against KLH alone did not react with toxins.
Cells.
The human crypt-like colonic epithelial cell line T-84, a model Cl− secretory epithelium, and the human adenocarcinoma cell line Caco-2 were chosen as model epithelia. T-84 cells especially are used commonly in drug absorption modeling, and monolayers can achieve a predictable, high electrical resistance in culture. The stock cultures were obtained from the American Type Culture Collection (Manassas, Va.). T-84 cells were maintained in equal parts Dulbecco minimal essential medium and Ham's F-12 media supplemented with 10% fetal bovine serum (FBS) and 1.5% HEPES and used between passages 9 and 12. Cell suspensions (500 μl) at 5 × 105 cells/ml were added to the chambers of 12-mm-pore-size nitrocellulose filters (Millicell-HA; Millipore Corp., Bedford, Mass.) and placed into 24-well tissue culture plates containing 500 μl of culture medium. Medium was replaced every other day until confluent monolayers with tight junctions developed as assessed by a hydrostatic pressure test and transepithelial electrical resistance (TEER) of monolayers. The hydrostatic pressure test was performed on filters 24 h before an experiment. Each monolayer was filled with medium to the top and incubated overnight at 37°C. Any filter that showed gross examinable leakage was excluded from the trial. TEER across other membranes that passed the hydrostatic pressure test was measured with a Millicell-ERS instrument (Millipore Corp.). Only monolayers with TEER of ≥150 Ω cm2 after correction for intrinsic value of empty filter were used. At this point the medium in both the filter inserts (apical medium) and culture wells (basal medium) were replaced, and the cultures were used in the transcytosis modeling protocol.
Caco-2 cells were maintained in Earle's minimal essential medium (with l-glutamine) which was supplemented with 20% FBS, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. Caco-2 cell monolayers were prepared using the Biocoat HTS Caco-2 Assay System (Becton Dickinson, Franklin Lakes, N.J.). Each system contains a fibrillar collagen-coated 24-well plate. Cells were seeded at 4 × 105 and supplemented with basal seeding medium. After 24 h, media were replaced with “MITO+ Serum Extender.” On day 3, the monolayers were tested for confluence (as described above). The HTS system was employed because of its 3-day turnaround time.
Toxin transcytosis assay.
The ability of T-84 or Caco-2 cells to transcytose SE and TSST-1 was tested using confluent monolayers grown on filter inserts as described above. Media was removed from the selected filter area (apical or basal), and 500 μl of fresh culture medium (without FBS) containing various concentrations of toxin was then added. Another 500 μl of culture medium (without FBS) was added to the other filter area. After incubation at 37°C for the indicated period of time, apical or basal media were collected depending on where the toxins were added. Inhibition assays were conducted similarly, and potential inhibitors (peptides or anti-peptide antisera) were added to the wells that contained toxins at appropriate concentrations.
ELISAs.
Nunc-Immuno MaxiSorp plates were coated with 100 μl of a 0.01 mM carbonate buffer solution containing 10 μg of anti-toxin IgG/ml. Coated plates were placed in a humid chamber over night on a plate rocker at 37°C. After incubation, plates were washed three times with phosphate-buffered saline containing 2.5% Tween and then refilled a fourth time with phosphate-buffered saline/Tween and allowed to incubate for 15 min, as recommended by Toxin Technologies for detection of low toxin concentrations. Samples (50 μl) were then added to the wells of the plate in triplicate. A standard curve was created using known concentrations of the toxin. Plates were incubated in humidified chamber for 2 h at 37°C and subsequently washed three times. Then, 100 μl of horseradish peroxidase-conjugated antitoxin was then added to all wells and incubated as described above for 1 h. Plates were washed five times followed by addition of o-phenylenediamine dihydrochloride substrate. After color development, the reaction was stopped with 2 M sulfuric acid. Plates were then read on a microplate reader at a wavelength of 490 nm. Titers of antitoxin antisera were determined using standard ELISA procedure and anti-immunoglobulin antibodies (Sigma).
RESULTS
Toxin transcytosis across epithelial monolayers.
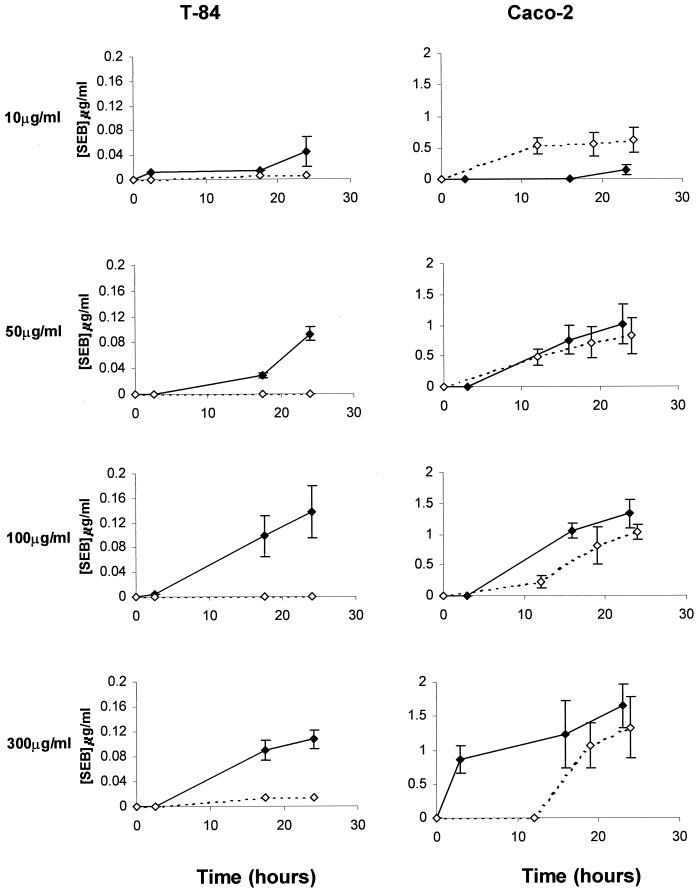
The movement of SEB across monolayers of T-84 and Caco-2 cells was determined over a 24-h period (Fig. 1). In both cell lines, SEB transcytosis increased with both increasing dose and time, but a higher percentage of administered toxin was transcytosed at lower concentrations. SEB transcytosis has been previously suggested to be a receptor-facilitated process (10), and in T-84 but not Caco-2 cells, saturation was observed at the 100-μg/ml toxin dose. In T-84 cells, transcytosis was biased in the apical to basal direction, which is anticipated in a polarized cell line like T-84 with unequal receptor distribution. Although there was little polarization observed in Caco-2 monolayers, this is consistent with previous observations and the demonstration of bidirectional receptors in this cell line (9, 10). Given the polarization of the T-84 monolayers, receptor saturation, and the consistent production of monolayers with high electrical resistance, T-84 cells were used for further experiments.
FIG. 1.
SEB movement across epithelial cell monolayers. SEB (10, 50, 100, or 300 μg/ml) was added to the apical or basal side of confluent T-84 or Caco-2 cell monolayers. The opposing chamber was sampled over a 24-h period, and the amount of toxin present was determined by sandwich ELISA. Apical to basal movement is depicted as a solid line, and basal to apical movement is depicted as a dashed line. Each point represents the mean of three replicate experiments ± standard error.
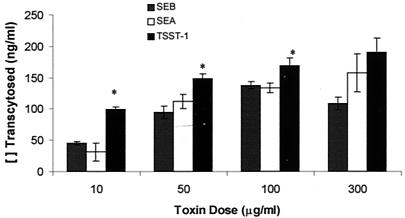
Movement of SEB was contrasted with that of SEA and TSST-1 (Fig. 2). SEA apical to basal transcytosis across T-84 monolayers was roughly parallel to the movement of SEB. It was only significantly higher at the 300-μg/ml concentration. Transcytosis of TSST-1 was slightly greater than that of SEB at all doses examined.
FIG. 2.
Toxins (10 to 300 μg/ml) were added to the apical chamber of confluent T-84 monolayer inserts and incubated for 24 h. The amount of toxin transcytosed to the basal chamber was determined by ELISA using only horseradish peroxidase-conjugated antitoxin antisera (Toxin Technologies), and results are expressed as the mean of two replicate experiments ± standard error. Significant differences between toxins were assessed by analysis of variance followed by Scheffe's F test (P < 0.05) and are indicated (∗).
Conserved SEB peptide blocks SEB transcytosis.
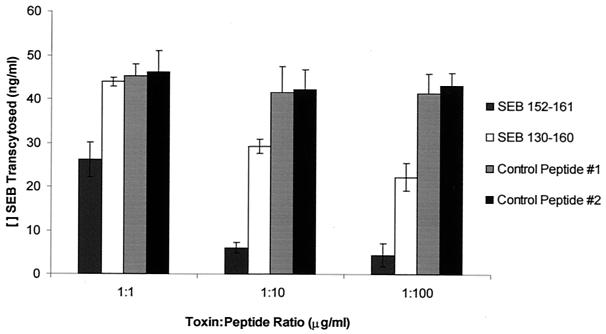
Synthetic peptides corresponding to various regions of SEB were used to attempt to block SEB transcytosis (Fig. 3). The peptides employed encompassed all but the amino- and carboxy-terminal regions. Only one peptide of the four examined, SEB 151-180, inhibited SEB movement across T-84 monolayers in a dose-dependent manner. Sequence analysis of the region corresponding to this peptide indicated that a 10-amino-acid sequence from residue 152 to 161 of SEB was highly conserved among other SE, and some conservation was even seen with TSST-1 (Fig. 4). Further, structural similarity of this 10-amino-acid region was observed among SE. In SEB, this region includes the end of the twisted beta sheet 7, beta sheet 8 (also twisted), and the beginning of helix 4 (33), and this conformation is retained in other SE and TSST-1. Therefore, we synthesized the conserved 10-amino-acid peptide, SEB 152-161. A longer peptide, SEB 130-160, with an extension into beta sheet 6, was also produced in an attempt to maintain the protruding position of valine 155, since longer peptides have been shown to better retain native structures. In addition, D161 was omitted to increase the overall charge of the peptide to +2, overcoming solubility problems. SEB 152-161 and SEB 130-160 along with control peptides of similar sizes from regions of SEB not previously identified as functionally significant were used to inhibit SEB transcytosis (Fig. 5). Both peptides containing the conserved sequence exhibited dose-dependent inhibition of SEB movement across T-84 cell monolayers. The control peptides were without effect. Unexpectedly, the conserved 10-mer was a more potent competitor than the longer peptide since even at a 1:1 ratio with toxin, significant inhibition of transcytosis was observed. Thus, the conserved sequence consisting of residues 152-161 of SEB appears to represent a region of the molecule involved in transcytosis of toxin across epithelial cell monolayers.
FIG. 3.
Peptide inhibition of SEB transcytosis. Peptides were incubated with 1 μg of SEB/ml at the indicated ratios for 24 h, and toxin transcytosis across T-84 cell monolayers was assessed by ELISA. Results from three replicates are presented as the mean concentration of SEB transcytosed ± standard deviation. As indicated on the axis, in the absence of peptide, 46.1 ± 13.4 ng of SEB/ml was transcytosed. SEB 61-92 at 1:10 (toxin to peptide) and SEB 151-180 at 1:10 and 1:100 were significantly different from toxin alone, as determined by analysis of variance followed by Scheffe's F test (P < 0.05).
FIG. 4.
Molecular models of SEB, SEA, SEE, and TSST-1. The amino acids highlighted in green signify the conserved sequence (KKKVTAQELD in SEB). The yellow amino acids on SEB identify the areas implicated in MHC-II binding, and the red amino acids correspond to the binding site of the TCR. All structures were obtained from the Protein Data Bank of the Research Collaboratory for Structural Bioinformatics. Downloaded files were subsequently manipulated using the RasMol program. The coordinates are based upon original publications about SEB (25), SEA (29), SEE (34), and TSST-1 (26).
FIG. 5.
Inhibition of SEB transcytosis by SEB 152-161 and SEB 130-160. Peptides were incubated with 1 μg of SEB/ml at the indicated ratios for 24 h, and toxin transcytosis was assessed. Results are presented as the mean ± standard error of SEB that was able to cross a monolayer of T-84 cells in the presence of the synthetic SEB peptides. In the absence of peptide, 46.1 ng of SEB/ml was transcytosed with a standard error of 9.6 ng/ml. Data were normalized based on transcytosis of toxin alone from three replicate experiments. SEB 130-160 at 1:10 and 1:100 toxin-to-peptide ratios and SEB 152-161 at all ratios were significantly different from toxin alone, as determined by analysis of variance followed by Scheffe's F-test (P < 0.05).
SEB 152-161 inhibits transcytosis of multiple SE.
SEB 152-161 was examined for its ability to affect transcytosis of SEA, SEE, and TSST-1 (Table 1). Consistent with the peptide inhibition of SEB transcytosis, SEB 152-161 inhibited movement across epithelial cell monolayers of all three other SE examined in a dose-dependent manner. Transcytosis of the more highly homologous SEA and SEE was reduced 73 and 68%, respectively. TSST-1 was also sensitive to inhibition with the SEB peptide, since at a toxin-to-peptide ratio of 1:100 its transcytosis was reduced by 59%.
TABLE 1.
SEB peptide inhibition of transcytosis of other SEa
| Toxin-peptide ratio | Level of transcytosis (mean ± SD) of:
|
||
|---|---|---|---|
| SEA | SEE | TSST-1 | |
| Toxin Alone | 60 ± 4.0 | 43 ± 12 | 31 |
| 1:1 | 46.9 ± 2.2 | 44.7 ± 0.5 | 35.2 ± 3.7 |
| 1:10 | 20.5 ± 0.9* | 19.1 ± 0.5* | 18.5 ± 1.8* |
| 1:100 | 15.9 ± 0.8* | 13.7 ± 2.3* | 12.7 ± 4.1* |
Peptides were incubated with 1 μg of SE/ml at the indicated ratios for 24 h, and toxin transcytosis was assessed by ELISA. Results are expressed as the mean of three replicate determinations ± standard deviation except for TSST-1. Significant differences from toxin controls were assessed by ANOVA followed by Scheffe's F test (P < 0.05) and are indicated (∗).
Antisera against conserved peptide inhibits transcytosis of SEB.
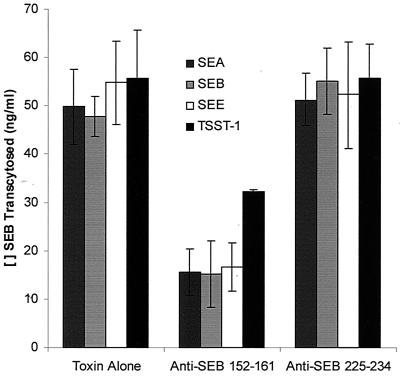
To confirm the region of the molecule encompassing SEB 152-161 as important for SEB transcytosis, antipeptide antisera were generated in mice and used to inhibit SEB movement. Antisera to the 10-mer peptide displayed greater reactivity with the parent toxin, SEB, than did antisera to the larger SEB 130-160 (Fig. 6). Cross-reactivity of the antipeptide antisera with other SE was also observed, especially with SEA, which could be anticipated based on the extent of homology. In contrast, little cross-reactivity to SEE was seen, which could be a function of the change in charge from a K in SEB to an E in SEE. Anti-SEB 152-161 was used in the SEB transcytosis assay because of its greater reactivity with SEB. Anti-SEB 152-161 significantly reduced transcytosis of not only SEB but also SEA, SEE, and TSST-1 while control antisera were without effect (Fig. 7). Therefore, the toxin peptide was antigenic and the antipeptide antisera inhibited movement of all SE examined.
FIG. 6.
Cross-reactivity of anti-SEB peptide antisera with other SE. The reactivity of anti-SEB 152-161 and anti-SEB 130-160 antibodies to SEE, SEB, SEA, and TSST-1 was assessed by ELISA. Data are represented as means of four replicate determinations ± standard deviation.
FIG. 7.
Anti-peptide antisera inhibition of multiple-toxin transcytosis. Antisera from mice immunized with SEB 152-161or SEB 225-234 conjugated to KLH were incubated at a 1:100 dilution with 1 μg of SE or TSST-1/ml, and toxin transcytosis was assessed. Results are presented as the mean of two replicate experiments ± standard error of SE or TSST-1 that was able to cross a monolayer of T-84 cells in the presence of anti-peptide antisera. The anti-SEB 152-161 antibody significantly inhibited transcytosis of all four toxins, as determined by analysis of variance followed by Scheffe's F test (P < 0.05).
DISCUSSION
The biological significance of superantigenic SE as causative agents of food poisoning and toxic shock contribute to the importance of this research (16, 22). Toxic shock caused by SE can follow wound infections and staphylococcal coinfections with influenza and could result from toxins used as agents of biological warfare. In contrast to their superantigenic effects, remarkably little is known about the fate of SE in the gut and at other mucosal surfaces and their reactions with local immune tissues. The hazards resulting from these interactions include not just toxic shock but sequelae, such as precipitation and exacerbation of autoimmune disease, in susceptible members of the population (8, 18). The paradoxical immune suppression, which normally follows T-cell stimulation, may be particularly devastating for individuals with impaired immune function, such as AIDS patients. Increased risk for both of these sequelae may occur at exposure levels below those required for emetic and toxic shock responses.
We have demonstrated a dose/response relationship between the SE and transcytosis through the gut epithelial monolayers. When the SEB concentration increased, the transcytosed concentration showed remarkable increases. However, at lower doses, a higher proportion of all the toxins examined was transcytosed. This has implications for the relationship between oral exposure levels to SEB in food and the risk of developing food poisoning. A dose of 100 μg of SEB is readily emetic in a monkey challenge assay, and in our study, when that dose was reduced 10-fold, the amount of toxin trancytosed showed only a 3-fold reduction. The SE have been found to retain immunologic activity after transcytosis across Caco-2 cell monolayers (10). Therefore, the limiting factor in systemic T-cell activation following mucosal exposure is the extent of toxin transcytosis.
Differences among the SE in movement across epithelial barriers have been noted both in vitro and in vivo. Facilitated transcytosis of SEB and TSST-1 across Caco-2 monolayers was observed previously and found to be dose dependent, but SEA moved across the monolayers at a much lower rate (10). We demonstrate that the Caco-2 and T-84 systems are not equivalent in that the T-84 monolayers develop a higher electrical resistance and do not display bidirectionality of movement. In the T-84 model, SEA and SEB transcytosis were roughly equivalent, while somewhat more TSST moved through the epithelial cell barrier. Oral delivery of 10 and 100 μg of TSST-1 to rabbits has been shown to induce lethality, while comparable doses of SEC1 and streptococcal pyrogenic exotoxin A (SPEA) did not (30). Thus, the rate of toxin transcytosis can determine the threshold dose for elicitation of biological activity and systemic effects.
Low doses (50 μg) of oral SEB have been shown to produce rapid expansion and activation of Vβ8+ cells in the mesenteric lymph nodes and Peyer's patches, but not systemically (31). This mucosal T-cell activation was associated with induction of IL-2 and gamma interferon mRNA, which may result in an acute enterotoxic poisoning in the absence of uptake of toxin into the blood. At low concentrations, only the local immune system is affected by toxin. In contrast, at high concentrations (high transcytosis levels), the superantigen can gain access to the systemic immune system, causing more serious effects.
The mechanism responsible for SE crossing epithelial barriers is unknown. Toxin-induced gross alterations in jejunal histopathology have been observed in vivo. Intraperitoneal administration of as little as 5 μg of SEB to BALB/c mice has been shown to cause vacuolation in the epithelial cells, reduce villus height, and increase crypt depth by 4 h after toxin exposure (4). Altered jejunal morphology persisted for 48 h with a 100-μg dose of toxin. These changes in the normal intestinal structure may contribute to increased systemic toxin access. In fact, toxin-induced increases in epithelial cell permeability have been demonstrated in vitro using T-84 cell monolayers (23). The SEB-induced increase in monolayer permeability was mediated by both gamma interferon and tumor necrosis factor alpha. CD4+ T cells have been demonstrated to be required for the elicitation of SEB effects on jejunal ion transport (24). In addition, SEB has been found to be directly cytotoxic to endothelial cells in the absence of effector cells or their mediators and altered their barrier function via a mechanism that was dependent on protein tyrosine kinases (5).
Alternatively, specific receptors may be involved in movement of SE across the gut epithelium. Since MHC-II is known to present SE to T cells, in vivo SEB may enter Peyer's patches via MHC-expressing M cells (1). On class II-negative Caco-2 cells, a low-affinity receptor that could bind SEB and TSST-1, not SEA, and orient the toxin appropriately for presentation to the TCR was postulated (10). On human kidney proximal tubular cells, SEB has been shown to bind to neutral glycosphingolipid (7). It has also been proposed that a non-MHC receptor for the SE may bind to large hydrophobic patches or a recognizable surface shape (30). The area of the toxins we have identified with our peptide inhibitor as involved in transcytosis is remarkably structurally conserved within the SE and may present the appropriate surface conformation to the transcytosis receptor.
The structural interactions of SE with both MHC and TCR have been well defined (14, 21). Mutations at SEB residue N23 that interacts with the TCR and F44 that binds MHC-II have been associated with reduced toxin transcytosis by Caco-2 cells (10). However, the ability of SE to stimulate T cells via MHC-TCR interaction cannot be directly correlated with their emetic activity (11), suggesting that alternative regions of the molecule may be involved in toxin activity in the gut. The sequence KKKVTAQEL in SEB has been recognized previously as being highly conserved among the SE, and it has been suggested that antisera directed against this region may neutralize toxin activity (17). Further, the longer SEB 130-160 peptide conjugated to KLH decreased SEB binding to lymphocytes (17). Recently, a variant of an SEB peptide representing amino acids 150 to 161, with the alanine and valine inverted and tyrosine replacing threonine 150, was found to protect mice against intraperitoneally SEB-induced lethal shock (2). The protective effect was observed when the peptide was injected intravenously 30 min before toxin or even 3 h after toxin administration. This time frame of activity is consistent with our observation that SEB 152-161 inhibits transcytosis, and the transcytosis time course experiments showed no significant toxin movement till well beyond the 3-h point. A/J and BALB/c mice were both protected by the peptide (2). This suggests that the transcytosis receptor is distinct from MHC-II molecules. Finally, they observed cross-protection by the peptide against shock induced by multiple SE, which is consistent with our demonstration of SEB peptide inhibition of transcytosis of SEA, SEE, and TSST-1. Thus, this newly identified functional domain encompassed by SEB 152-161 may be exerting its antagonistic effect on lethal shock and T-cell activation by inhibition of toxin transcytosis.
Because of the role of the SE in toxic shock, food poisoning, and immune system perturbations and its potential use in biological warfare, vaccine strategies have been developed based on information about functionally relevant toxin structural regions. SEB N23K and F44S mutants have been examined in a mouse SEB lethality model where the toxin was given intraperitoneally (38). Immunization with both mutant toxins protected more than 80% of challenged animals, but the mutants retained some level of emetic activity in monkeys. Use of a toxin synthetic peptide as a vaccine, however, may eliminate the emetic response. An SEB toxoid has been produced by formaldehyde treatment, incorporated into microspheres (35) or proteosomes (20), and used to elicit a protective antibody response against aerosolized SEB in monkeys. SEB neutralization was not a function of deletion of toxin-reactive T cells (35). Since mucosal immunization maximized protection, it may be that antibodies to the toxoid reacted with the transcytosis epitope currently identified.
A vaccine approach that would come into play if the toxin gained access to the systemic immune system has been to create recombinant SEB with triple mutations that interfere with binding to particular HLA molecules (36). Antibodies produced against these binding surfaces have been shown to cross-react with other related toxins (36) and are protective in a murine aerosol challenge model (32). It may be useful to incorporate this approach with the development of antibodies to the transcytosis epitope to reduce or eliminate contact of the toxin with systemic immune cells. Ongoing studies are directed toward in vivo transcytosis inhibition. It has been shown that systemic antibodies, which would be produced by immunization with SEB 152-161, do gain access to Peyer's patches and other mucosa-associated lymphoid tissues and inhibit superantigen-induced T-cell activation (37). Thus, a SEB 152-161 vaccine is being tested for its ability to generate antibodies that block transport-active regions of the SEB toxin in vivo, thereby preventing systemic immune cell activation.
Acknowledgments
This work was supported by a grant from the Joint Institute of Food Safety and Applied Nutrition, University of Maryland. Support was also provided by a grant from the Howard Hughes Medical Institute through the Undergraduate Biological Sciences Education Program.
We gratefully acknowledge the assistance of Stefanie Wetzel in performing the toxin ELISAs.
Editor: J. T. Barbieri
REFERENCES
- 1.Allan, C. H., D. L. Mendrick, and J. D. Trier. 1990. Rat intestinal M cells contain acidic endosomal-lysosomal compartments and express class II major histocompatibility complex determinants. Gastroenterology 104:698.. [DOI] [PubMed] [Google Scholar]
- 2.Arad, G., R. Levy, D. Hillman, and R. Kaempper. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414-421. [DOI] [PubMed] [Google Scholar]
- 3.Bean, N. H., J. S. Goulding, C. Lao, and F. J. Angulo. 1996. Surveillance for foodborne-disease outbreaks—United States, 1988-1992. Morb. Mortal. Wkly. Rep. 45:1-66. [PubMed] [Google Scholar]
- 4.Benjamin, M. A., J. Lu, G. Donnelly, P. Dureja, and D. M. McKay. 1998. Changes in murine jejunal morphology evoked by the bacterial superantigen Staphylococcus aureus enterotoxin B are mediated by CD4+ T cells. Infect. Immun. 66:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, W. N., M. Fitzpatrick, X. Ding, M. Jett, P. Gemski, and S. E. Goldblum. 1997. SEB is cytotoxic and alters EC barrier function through protein tyrosine phosphorylation in vitro. Am. J. Physiol. 273:L31-L39. [DOI] [PubMed]
- 6.Carlsson, R., and H. O. Sjogren. 1985. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell. Immunol. 96:175-183. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee, S., and M. Jett. 1992. Glycoshingolipids: the putative receptor for staphylococcus aureus enterotixin-B in human kidney proximal tubular cells. Mol. Cell. Biochem. 113:25-31. [DOI] [PubMed] [Google Scholar]
- 8.Cole, B. C. 1996. Mycoplasma interactions with the immune system: implications for disease pathology. ASM News 62:471-475. [Google Scholar]
- 9.Ellis, J. A., and J. P. Luzio. 1995. Identification and characterization of a novel protein (p137) which transcytoses bidirectionally in Caco-2 cells. J. Biol. Chem. 270:20717-20723. [DOI] [PubMed] [Google Scholar]
- 10.Hamad, A. R. A., P. Marrack, and J. W. Kappler. 1997. Transcytosis of staphylococcal superantigen toxins. J. Exp. Med. 185:1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris, T. O., D. Grossman, J. W. Kappler, P. Marrack, R. R. Rich, and M. J. Betley. 1993. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect. Immun. 61:3175-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann, T., S. Baschieri, R. K. Lees, and H. R. MacDonald. 1992. In vivo responses of CD4+ and CD8+ cells to bacterial superantigens. Eur J. Immunol. 22:1935-1938. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman, M., M. Tremaine, J. Mansfield, and M. Betley. 1996. Biochemical and mutational analysis of the histidine residues of staphylococcal enterotoxin A. Infect. Immun. 64:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jardetzky, T. S., J. H. Brown, J. C. Gorga, et al. 1994. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 368:188-192. [DOI] [PubMed] [Google Scholar]
- 15.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 16.Jett, M., W. Brinkley, R. Neill, P. Gemski, and R. Hunt. 1990. Staphylococcus aureus enterotoxin B challenge of monkeys: correlation of plasma levels of arachidonic acid cascade products with occurrence of illness. Infect. Immun. 58:3494-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jett, M., R. Neill, C. Welch, T. Boyle, E. Bernton, D. Hoover, G. Lowell, R. E. Hunt, S. Chatterjee, and P. Genski. 1994. Identification of staphylococcal enterotoxin B sequences important for the induction of lymphocyte proliferation by using synthetic peptide fragments of the toxin. Infect. Immun. 62:3408-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, H. M., J. K. Russell, and C. H. Pontzer. 1992. Superantigens in human disease. Sci. Am. 266:92-101. [DOI] [PubMed] [Google Scholar]
- 19.Jones, C. L., and S. A. Khan. 1986. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J. Bacteriol. 166:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowell, G. H., C. Colleton, D. Frost, R. W. Kaminski, M. Hughes, J. Hatch, C. Hooper, J. Estep, L. Pitt, M. Topper, R. E. Hunt, W. Baker, and W. B. Baze. 1996. Immunogenicity and efficacy against lethal aerosol staphylococcal enterotoxin B challenge in monkeys by intramuscular and respiratory delivery of proteosome-toxoid vaccines. Infect. Immun. 64:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malchiodi, E. L., E. Eisenstein, B. A. Fields, et al. 1995. Superantigen binding to a T cell receptor beta chain of known three-dimensional structure. J. Exp. Med. 182:1833-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrack, P., and J. W. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 249:705-711. [DOI] [PubMed] [Google Scholar]
- 23.McKay, D. M., and P. K. Singh. 1997. Superantigen-activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via interferon-γ and tumour necrosis factor-α. Inhibition of increased permeability, but not diminished secretory responses by transforming growth factor β2. J. Immunol. 159:2382-2390. [PubMed] [Google Scholar]
- 24.McKay, D. M., M. A. Benjamin, and J. Lu. 1998. CD4+ T cells mediate superantigen-induced abnormalities in murine jejunal ion transport. Am. J. Physiol. 275:G29-G38. [DOI] [PubMed]
- 25.Papageorgiou, A. C., H. S. Tranter, and K. R. Acharya. 1998. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 A resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J. Mol. Biol. 277:61-79. [DOI] [PubMed] [Google Scholar]
- 26.Prasad, G. S., R. Radhakrishnan, D. T. Mitchell, C. A. Earhart, M. M. Dinges, W. J. Cook, P. M. Schlievert, and D. H. Ohlendorf. 1997. Refined structures of three crystal forms of toxic shock syndrome toxin-1 and of a tetramutant with reduced activity. Protein Sci. 6:1220-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rellahan, B. L., L. A. Jones, A. M. Kruisbeek, A. M. Fry, and L. A. Matis. 1990. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J. Exp Med. 172:1091-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salyers, A. A., and D. D. Whitt. 1994. Bacterial pathogenesis, p. 136-139. ASM Press, Washington, D.C.
- 29.Schad, E. M., I. Zaitseva, V. N. Vaitsev, M. Dohlsten, T. Kalland, P. M. Schlievert, D. H. Ohlendorf, and L.A. Svensson. 1995. Crystal structure of the superantigen staphylococcal enterotoxin type A. EMBO J. 14:3292-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlievert, P. M., L. M. Jablonski, M. Roggiani, I. Sadler, S. Callantine, D. T. Mitchell, D. H. Ohlendorf, and G. A. Bohach. 2000. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect. Immun. 68:3630-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiekermann, G. M., and C. Nagler-Anderson. 1998. Oral administration of the bacterial superantigen staphylococcal enterotoxin B induced activation and cytokine production by T cells in murine gut-associated lymphoid tissue. J. Immunol. 161:5825-5831. [PubMed] [Google Scholar]
- 32.Stiles, B. G., A. R. Garza, R. G. Ulrich, and J. W. Boles. 2001. Mucosal vaccination with recombinantly attenuated staphylococcal enterotoxin B and protection in a murine model. Infect. Immun. 69:2031-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan, S., W. Furey, J. Pletcher, and M. Sax. 1992. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature 359:801-806. [DOI] [PubMed] [Google Scholar]
- 34.Swaminathan, S., W. Furey, J. Pletcher, and M. Sax. 1995. Residues defining V beta specificity in staphylococcal enterotoxins. Nat. Struct. Biol. 2:680-686. [DOI] [PubMed] [Google Scholar]
- 35.Tseng, J., J. L. Komisar, R. N. Trout, R. E. Hunt, J. Y.-J. Chen, A. J. Johnson, L. Pitt, and D. L. Ruble. 1995. Humoral immunity to aerosolized staphylococcal enterotoxin B (SEB), a superantigen, in monkeys vaccinated with SEB toxoid-containing microspheres. Infect. Immun. 63:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulrich, R. G., M. A. Olson, and S. Bavari. 1998. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine 16:1857-1864. [DOI] [PubMed] [Google Scholar]
- 37.Velin, D., G. Fotopoulos, J.-P. Kraehenbuhl, and H. Acha-Orbea. 1999. Systemic antibodies can inhibit mouse mammary tumor virus-driven superantigen response in mucosa-associated lymphoid tissues. J. Virol. 73:1729-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woody, M. A., T. Krakauer, and B. G. Stiles. 1997. Staphylococcal enterotoxin B mutants (N23K and F44S): biological effects and vaccine potential in a mouse model. Vaccine 15:133-139. [DOI] [PubMed] [Google Scholar]