Abstract
We have recently demonstrated that interleukin-10 (IL-10), produced by THP-1 monocytes in response to Borrelia burgdorferi lipoproteins, dampens the production of concomitantly elicited inflammatory cytokines. Thus, IL-10 could potentially down-regulate inflammatory and microbicidal effector mechanisms of the innate immune response to a B. burgdorferi infection, facilitating the establishment of the spirochete. To understand the mechanism(s) implicated in the regulation of the synthesis and release of IL-10 during early infection, we investigated the autocrine effects of IL-6, IL-12, tumor necrosis factor alpha (TNF-α), and IL-10 itself, as well as the exocrine effect of IFN-γ on the production of macrophage-derived IL-10 with lipoprotein as a stimulant. In addition, in view of the differences in the receptor and signal transduction pathways of lipopolysaccharide (LPS) and bacterial lipoproteins, we also investigated the effects described above with LPS as a stimulant. The THP-1 human monocytic cell line and purified recombinant lipidated OspA (L-OspA) were used as the model target cell and stimulant, respectively. TNF-α increased the production of IL-10, as elicited by lipoproteins. The production of IL-10 by THP-1 cells stimulated with L-OspA was autoregulated by a negative feedback mechanism involving the IL-10 receptor (IL-10R). Exogenous IFN-γ significantly inhibited the production of IL-10. Both autocrine (IL-10) and exocrine (IFN-γ) inhibition of IL-10 production resulted in an increase in the production of the proinflammatory cytokines IL-6 and IL-12. The same results were obtained when the stimulant was LPS. The results further illustrate that IL-10 may play a pivotal role in Lyme disease pathogenesis. Moreover, the regulation of its production with lipoprotein as a stimulant is indistinguishable from that observed when LPS acts as a stimulant.
Borrelia burgdorferi, the spirochete that causes Lyme disease, is spread to humans and other mammals through the bite of infected Ixodes ticks (7). Infection may involve multiple organs (3, 42) and may persist in certain tissues for prolonged periods of time (35, 48). Spirochetal persistence in the tissues has been associated with severe pathology (9, 16, 48) and both acute and chronic inflammatory conditions (37, 42). B. burgdorferi lacks lipopolysaccharide (LPS) (43), but its genome contains no fewer than 150 genes coding for putative lipoproteins (18); this amounts roughly to 11% of its genome coding capacity. Lipoproteins can directly elicit inflammatory responses both in vitro and in vivo (20, 36, 38, 39, 47). Recent findings that lipoproteins can elicit not only inflammatory but also anti-inflammatory mediators (e.g., interleukin-10 [IL-10]) from mononuclear cells present in peripheral blood (21, 22) and synovial fluid (49) have added support to the contention that lipoproteins play a crucial role in Lyme disease pathogenesis.
One of the hallmarks of Lyme disease is arthritis. This form of the disease has been studied extensively in the mouse model, with an emphasis on the role of cytokines in its etiology. Infection of different inbred mouse strains with B. burgdorferi results in distinct disease outcomes (2, 32, 48). B. burgdorferi infection elicits severe arthritis in C3H/HeN mice, but mild arthritis in C57BL/6N mice, although both strains harbor similar numbers of spirochetes in their ankles (32). These findings indicate that C57BL/6N mice either produce less proinflammatory cytokines or are better able to regulate inflammation in response to B. burgdorferi lipoproteins than C3H/HeN mice, resulting in a less intense inflammatory response and decreased arthritis severity. Recently, Brown and colleagues (6) corroborated this hypothesis by showing that macrophages from C57BL/6N mice, when stimulated with the lipoprotein outer surface protein A (OspA) or LPS, secreted significantly less proinflammatory cytokines (IL-6 and tumor necrosis factor alpha [TNF-α]) than C3H/HeN cells. In contrast, macrophages from C57BL/6N mice produced up to 10-fold-larger amounts of IL-10 than macrophages from C3H/HeN mice when the same stimulants were used, suggesting that IL-10 could contribute to the control of inflammation in Lyme disease. The role of IL-10 as a key mediator in anti-inflammatory immune responses induced by B. burgdorferi was also underscored in this study by showing that C57BL/6J mice deficient in IL-10 developed more severe arthritis than wild-type C57BL/6J mice (6). Recently, Ganapamo et al. (19) showed that lymph node cells from C3H/HeJ and C57BL/6J mice produced comparable IL-10 levels when the cells were restimulated in vitro with B. burgdorferi spirochetes. However, IL-10 downregulated inflammatory cytokines elicited by B. burgdorferi more efficiently in C57BL/6J mice than in C3H/HeJ mice (19).
Evidence for the role of IL-10 in human Lyme disease comes from Yin et al. (49), who showed that IL-10 can be produced concomitantly with Th1 cytokines when synovial fluid mononuclear cells from infected patients are incubated with sonicated proteins of B. burgdorferi. The same study showed that this endogenously produced IL-10 can inhibit both Borrelia-specific lymphocyte proliferation and production of TNF-α and IFN-γ. Studies by Diterich et al. (12) and Giambartolomei et al. (21) showed enhanced secretion of IL-10 by Borrelia-stimulated mononuclear cells in patients with borreliosis and in normal subjects, respectively.
Recently, we demonstrated that endogenously synthesized IL-10 significantly dampened the production of inflammatory cytokines, as elicited from human monocytes by lipoproteins (34). This finding suggested that IL-10 could facilitate the establishment of B. burgdorferi by downregulating inflammatory and microbicidal effector mechanisms of the innate immune response. The same study indicated that IL-10 production by lipoprotein-stimulated monocytes was regulated by mediators elicited from or present in the cells. Regulation of IL-10 production by LPS-stimulated monocytes has been studied extensively (8, 13, 29), but this phenomenon has received scant attention in the context of lipoprotein stimulation. LPS and lipoproteins share CD14 as a coreceptor on cells of the myeloid lineage (20, 39, 47) as well as the MyD88 adapter protein (1), while their respective receptors, namely TLR-4 and TLR-2, differ (5, 24, 30). In addition, the toll-IL-1 receptor (IL-1R) domain-containing adapter protein (TIRAP) controls activation of MyD88-independent signaling pathways downstream of TLR-4, but not downstream of TLR-2 (25). The reported differences between the LPS and lipoprotein signaling receptors and pathways prompted us to determine if LPS- and lipoprotein-induced IL-10 might be differentially regulated by cytokines in macrophages. We investigated the effect of exogenous IL-6, IL-12, and TNF-α on the production of macrophage-derived IL-10. We also determined if endogenously produced IL-10 could modulate its own production through a pathway involving IL-10R. Since it is known that gamma IFN (IFN-γ) modulates the production of IL-10 in other infectious diseases (8, 13, 29), we assessed whether IFN-γ can regulate IL-10 production by L-OspA-stimulated cells. We used the THP-1 human monocytic cell line and purified recombinant lipidated OspA (L-OspA) as the model target cell and stimulant, respectively (34). Although L-OspA appears not to be expressed by B. burgdorferi in the early phases of infection (4, 10, 37), the use of this (or any other) lipoprotein as a model is justified in so far as its immunological effects are elicited by the lipid, not the protein, moiety. The lipid moiety is likely shared by all B. burgdorferi lipoproteins. The effect of L-OspA was compared with that of LPS.
MATERIALS AND METHODS
Stimulants.
Recombinant L-OspA was the same as that already reported (20, 21, 34) and was obtained from John Dunn, Brookhaven National Laboratories, Brookhaven, N.Y. The L-OspA preparation contained less than 0.25 endotoxin units per mg of protein, as assessed by Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, Mass). LPS from Escherichia coli strain 026:B6 was obtained from Sigma Chemical Company (L-XXXX; St. Louis, Mo.) and contained less than 5% E. coli protein contamination.
Cytokines, reagents, and antibodies.
Human recombinant IL-6 (rIL-6), rIL-12, and rTNF-α (catalog no. 1966IV, 1993IV, and 1976IT, respectively) were all purchased from PharMingen (San Diego, Calif.). Human rIFN-γ (catalog no. PHC403-C) was purchased from Biosource International, Camarillo, Calif.). Mouse anti-human IL-10R antibody (catalog no. MAB274) was purchased from R & D Systems (Minneapolis, Minn.). Mouse anti-human TNF-α (catalog no. 18630D) and normal mouse immunoglobulin G1 (IgG1) (catalog no. 20610D) were purchased from PharMingen. IL-10, IL-6, and IL-12 cytokine reagents were purchased from PharMingen (34).
Cell culture and stimulation of cytokine production.
The THP-1 monocyte cell line was the same as described previously (20, 34) and was obtained from the American Type Culture Collection (Manassas, Va.). THP-1 cells were pretreated with 0.05 μM 1α, 25-dihydroxyvitamin D3 (Calbiochem-Nova Biochem Int., La Jolla, Calif.) for 48 h (26) prior to being used in this study. For cytokine production, THP-1 cells at 106/ml were stimulated with 1 μg of L-OspA or LPS per ml for 48 h, except where indicated in the text. Cultures were subsequently centrifuged at 400 × g for 10 min at 4°C, and cell supernatants were collected, aliquoted, and stored at −70°C until they were used.
Effect of exogenous IL-6, IL-12, TNF-α, and IFN-γ on IL-10 production.
To study the effect of exogenous IL-6, IL-12, TNF-α, and IFN-γ on IL-10 production, THP-1 cells were stimulated with 1 μg of L-OspA or LPS per ml in the presence or absence of various concentrations (0.1, 1, 10, and 100 ng/ml) of rIL-6, rIL-12, and rTNF-α. rIFN-γ was used at concentrations of 0.01, 0.1, 1, and 10 ng/ml. To determine the effect of endogenous TNF-α on IL-10 production, cells were stimulated with L-OspA and LPS in the presence or absence of neutralizing mouse anti-human TNF-α antibody at 10, 20, and 40 μg/ml. Normal mouse IgG1 was used as control. All cultures were incubated for 48 h, except where indicated, after which time cell supernatants were collected following centrifugation at 400 × g for 10 min at 4°C. Supernatants were aliquoted and stored at −70°C until they were used.
Effect of blocking the IL-10R on IL-10 production.
To determine the effect of blocking the IL-10R on IL-10 production, cells were stimulated with 1 μg of L-OspA or LPS per ml in the presence or absence of a mouse anti-human IL-10R monoclonal antibody (MAb) at 10 μg/ml. Normal mouse IgG1 at the same concentration of the IL-10R MAb was used as a control antibody. Antibodies were added at either the same time as the stimulants or 8 h afterwards. Cultures were incubated for 2, 8, 24, 48, and 120 h, after which cell supernatants were collected following centrifugation at 400 × g for 10 min at 4°C. Supernatants were aliquoted and stored at −70°C until they were used.
Measurement of cytokine concentrations.
IL-6, IL-10, and IL-12 cytokines were measured in culture supernatants by sandwich enzyme-linked immunosorbent assay (ELISA) with paired cytokine-specific MAbs as previously described (34).
Kinetics of cytokine production.
For the study of the kinetics of cytokine production, cell supernatants were collected after THP-1 cells had been stimulated for 2, 4, 8, 24, 48, and 120 h. The concentration of stimulants used was 1 μg of L-OspA or LPS per ml.
Detection of IL-10 mRNA by semiquantitative RT-PCR.
Reverse transcription (RT)-PCR was performed as previously described (21). Results were expressed in terms of fold increase over the mRNA levels of cells cultured in the absence of L-OspA and LPS.
Statistics.
Student's t test was used to compare the data. Significance was established at a P level of 0.05.
RESULTS
Effect of exogenous and endogenous TNF-α and exogenous IL-6 and IL-12 on production of IL-10 induced by L-OspA and LPS.
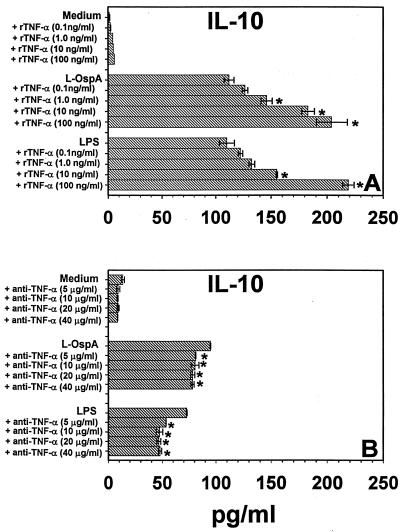
We examined whether exogenous IL-6, IL-12, and TNF-α added at the initiation of culture were able to affect the concentration of IL-10 measured at 48 h after L-OspA or LPS stimulation. This time point was chosen because kinetic studies of IL-10 production in this system showed that at 48 h poststimulation (p.s.), IL-10 concentrations either reached a plateau or were changing at a relatively low rate (34). THP-1 cells were incubated with stimulants in the absence or presence of rIL-6, rIL-12, and rTNF-α, each at a concentration of 0.1, 1, 10, or 100 ng/ml. IL-10 levels were below the detection limits in the absence of stimulants and in the presence of recombinant cytokines alone. The IL-10 concentration was significantly increased (P < 0.02 to P < 0.006) in the presence of 1 to 100 ng of rTNF-α per ml in L-OspA-stimulated cultures and in 10 and 100 ng of rTNF-α per ml in LPS-stimulated culture supernatants (Fig. 1A). Moreover, neutralizing anti-TNF-α antibody added to the culture together with the stimulants caused a small but significant reduction (P < 0.05 to P < 0.0004) in the concentration of IL-10 in both L-OspA- and LPS-stimulated cultures (Fig. 1B). Isotype control antibody had no effect (not shown). Our results indicate that TNF-α increases the production (transcription, processing, and/or export) of IL-10 by THP-1 cells when these cells are stimulated with L-OspA or LPS. Addition of rIL-6 or rIL-12 did not significantly affect (P > 0.05) the concentration of endogenous IL-10 in supernatants of L-OspA- and LPS-treated cells (data not shown).
FIG. 1.
Effect of exogenous TNF-α (A) and anti-TNF-α antibody (B) on IL-10 production. Vitamin D3-treated THP-1 cells (106/ml) were incubated with L-OspA (1 μg/ml) or LPS (1 μg/ml) in the presence or absence of various concentrations of human rTNF-α and anti-TNF-α antibody. After 48 h, IL-10 in cell supernatants was quantified by antibody-capture ELISA. The lower limit of detection of the ELISA was 15 pg/ml. Asterisks indicate significant differences from cells incubated with L-OspA and LPS alone (P < 0.05 to P < 0.0004). P values were calculated by use of Student's t test. Each bar represents the mean ± standard error of duplicate cultures. Data are representative of two separate experiments.
Effect of blocking the IL-10R on the concentrations of IL-10, IL-6, and IL-12 induced by L-OspA and LPS stimulation.
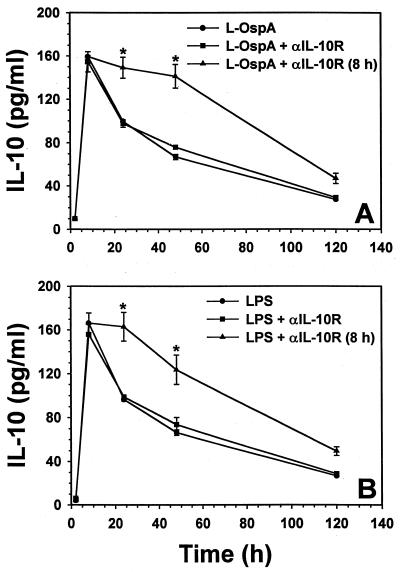
We investigated if endogenously produced IL-10 could modulate its own production via negative or positive feedback through the IL-10R when stimulated with either L-OspA or LPS. THP-1 cells were cultured with stimulants in the presence or the absence of anti-IL-10R MAb added either at the time of stimulation or 8 h after the initiation of cultures. The IL-10 concentration was determined at 2, 8, 24, 48, and 120 h after addition of the stimulants to the culture. As previously reported (34), the IL-10 concentration peaked between 8 and 16 h p.s. and declined thereafter. Addition of anti-IL-10R MAb together with the stimulants had no effect on the production of IL-10 at any of the time points examined. However, anti-IL-10R MAb added 8 h p.s. significantly increased (P < 0.02) the L-OspA- and the LPS-induced concentration of IL-10 from 8 h p.s. onwards when compared with cell supernatants taken at the same time points, but in the absence of the anti-IL-10R MAb (Fig. 2A and B).
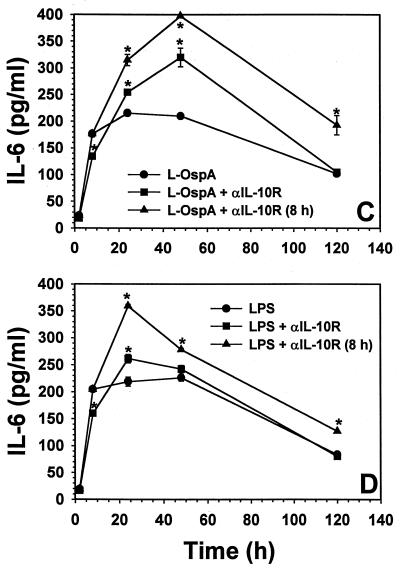
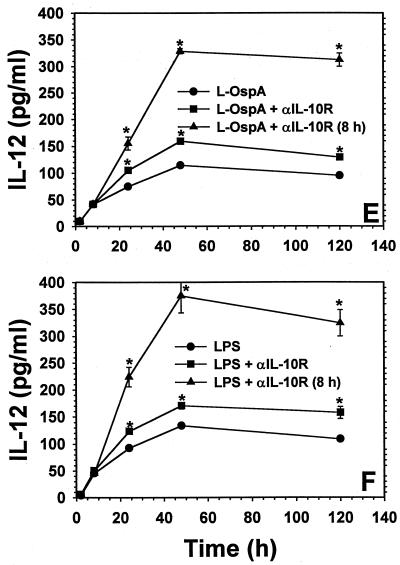
FIG. 2.
Effect of blocking the IL-10R on IL-10 (A and B), IL-6 (C and D), and IL-12 (E and F) production. Vitamin D3-treated THP-1 cells (106/ml) were incubated with L-OspA (1 μg/ml) or LPS (1 μg/ml) in the presence of 10 μg of anti-IL-10R per ml. Anti-IL-10R antibody was added either at the same time as the stimulants or 8 h afterwards. Cultures were incubated for 2, 8, 24, 48, and 120 h, after which specific cytokines in cell supernatants were collected and quantified by antibody-capture ELISA. The lower limit of detection of the ELISA was 15 pg/ml. Asterisks indicate significant differences from cells incubated with L-OspA and LPS alone (P < 0.01 to P < 0.0002). P values were calculated by use of Student's t test. Each bar represents the mean ± standard error of duplicate cultures. The data are representative of two separate experiments.
Because we had demonstrated that IL-10 was able to downmodulate the production of IL-6 and IL-12, as elicited by B. burgdorferi lipoproteins (34), we conducted further experiments to determine the effect of blocking the IL-10R on the production of IL-6 and IL-12 as induced by L-OspA and LPS stimulation. Cytokine concentrations were determined at 2, 8, 24, 48, and 120 h after addition of the stimulants to the culture. The concentration of IL-6 increased rapidly and peaked at about 24 h p.s. (Fig. 2C and D). That of IL-12 increased slowly after stimulation and reached a maximum by 48 h p.s. (Fig. 2E and F). A significant increase (P < 0.02 to P < 0.008) in the production of IL-6 was seen only at 24 and 48 h p.s. in L-OspA and at 24 h p.s. in LPS-stimulated cultures when anti-IL-10R MAb was added at the beginning of the culture period (Fig. 2C and D). Addition of anti-IL-10R MAb at the beginning of the culture caused a significant increase (P < 0.01 to P < 0.0008) in the production of IL-12 in L-OspA- and LPS-stimulated cultures (Fig. 2E and F). The increase was greater when the anti-IL-10R MAb was added 8 h p.s. in both L-OspA- and LPS-stimulated cultures. The concentrations of both IL-6 and IL-12 increased significantly (P < 0.01 to P < 0.00002) from 8 h p.s. onwards when compared with that in cell supernatants taken at the same time points either in the presence of initially added anti-IL-10R MAb or in its absence (Fig. 2C to F). The isotype-matched control antibody had no effect on the production of all cytokines studied (data not shown). Our results indicate that THP-1 cells with their IL-10R blocked lose their capacity to downmodulate IL-10 production when stimulated with L-OspA or LPS. This suggests that lipoprotein- and LPS-induced IL-10 can modulate its own production (transcription, processing, and/or export) via negative feedback through the IL-10R. The results also indicate that IL-10 modulates the production of L-OspA- and LPS-induced IL-6 and IL-12 through the same mechanism.
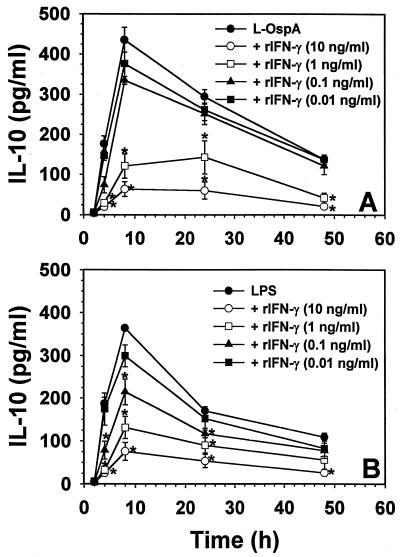
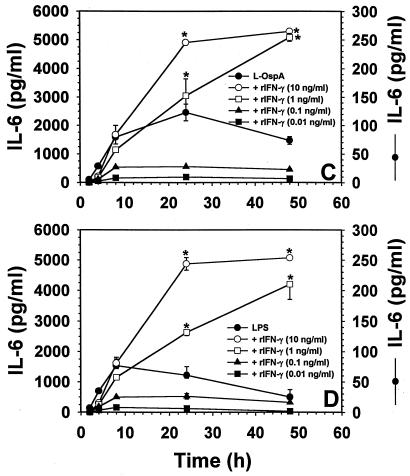
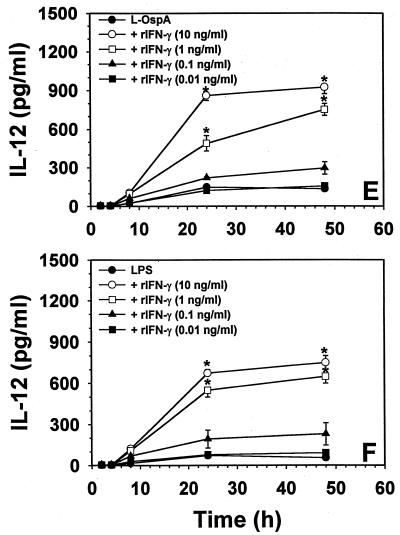
Effect of exogenous IFN-γ on the rate and level of production of IL-10, IL-6, and IL-12 induced by L-OspA and LPS stimulation.
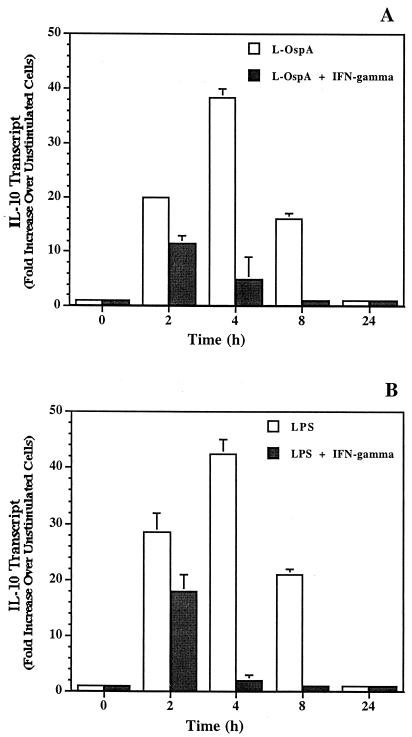
IFN-γ is known to downregulate LPS-induced IL-10 production by monocytes in other infectious disease systems (8, 13, 29). Moreover, IFN-γ has the ability to enhance IL-12 and IL-6 production in monocytes stimulated by LPS (15, 23, 31). Thus, the effect of exogenous IFN-γ on the rate and level of L-OspA- and LPS-induced production of IL-10, IL-6, and IL-12 was studied. THP-1 cells were incubated with 1 μg of either L-OspA or LPS per ml in the absence of rIFN-γ or in the presence of various concentrations of this cytokine (0.01, 0.1, 1, and 10 ng/ml). The concentrations of IL-10, IL-6, and IL-12 were determined at 2, 4, 8, 24, and 48 h after addition of the stimulants and rIFN-γ to the cultures. Both the rate of production and the concentration of IL-10 produced diminished significantly (P < 0.05 to P < 0.0009) in the presence of rIFN-γ at 1 and 10 ng/ml (Fig. 3A). As expected (13), similar results were obtained when LPS was used as a stimulant (Fig. 3B). These results suggest that IFN-γ is affecting the rate of transcription, processing, and/or export of IL-10. When the kinetics of IL-6 and IL-12 production was studied, rIFN-γ at 1 and 10 ng/ml caused an enhanced production (P < 0.03 to P < 0.003) of both IL-6 and IL-12 by L-OspA (Fig. 3C and E) and LPS (Fig. 3D and F) only at 24 h after the initiation of culture. Given that rIFN-γ modulated the production of IL-10, and IL-10 is known to downregulate the production of IL-12 and IL-6 in both L-OspA and LPS cultures (34), these findings suggest that the early IFN-γ inhibition of IL-10 production could have resulted in the enhanced production of IL-6 and IL-12. To determine the mechanism of rIFN-γ downmodulation of IL-10 in L-OspA- or LPS-stimulated cells, a kinetic study of the IL-10 gene transcription was performed by RT-PCR. IL-10 mRNA levels were markedly increased at 2 h p.s. with L-OspA, peaked at 4 h p.s., and began to decrease at 8 h p.s. In the presence of 10 ng of rIFN-γ per ml, IL-10 mRNA levels were low at 2 h and markedly reduced at 4 h when compared to levels induced by L-OspA or LPS alone, suggesting that rIFN-γ inhibits the production of IL-10 induced by L-OspA and LPS in THP-1 cells at the transcriptional level (Fig. 4).
FIG. 3.
Effect of exogenous IFN-γ on the rate and level of production of IL-10 (A and B), IL-6 (C and D), and IL-12 (E and F) induced by L-OspA and LPS stimulation. Vitamin D3-treated THP-1 cells (106/ml) were incubated with L-OspA (1 μg/ml) or LPS (1 μg/ml) in the presence or absence of various concentrations of human rIFN-γ. Cultures were incubated for 2, 8, 24, 48, and 120 h, after which specific cytokines in cell supernatants were collected and quantified by antibody-capture ELISA. The lower limit of detection of the ELISA was 15 pg/ml. Asterisks indicate significant difference from cells incubated with L-OspA and LPS alone (P < 0.01 to P < 0.0002). P values were calculated by use of Student's t test. Each bar represents the mean ± standard error of duplicate cultures. Data are representative of two separate experiments.
FIG. 4.
Effect of exogenous IFN-γ on expression of IL-10 mRNA in THP-1 cells stimulated with L-OspA (A) and LPS (B). Vitamin D3-treated THP-1 cells (106/ml) were incubated with L-OspA (1 μg/ml) or LPS (1 μg/ml) in the presence (black bars) or absence (white bars) of 10 ng of human rIFN-γ per ml. At different times p.s., the mRNA levels of IL-10 were determined by RT-PCR. Responses are shown as fold increase over the level in unstimulated cells. All values were normalized with respect to GAPDH mRNA levels. Results represent the mean value of two determinations (each from a separate RNA batch) ± standard error.
DISCUSSION
Of the three monocyte/macrophage-derived proinflammatory cytokines we investigated, namely IL-6, IL-12, and TNF-α, only the latter had a regulatory effect on IL-10. Addition of exogenous TNF-α caused an increase in IL-10 production and, as would be expected, inhibition of the endogenously produced cytokine with anti-TNF-α antibody had a suppressive effect. In a previous study of the kinetics of monocyte-derived cytokine production in the context of lipoprotein stimulation, we had noticed that TNF-α was abundantly secreted several hours earlier than IL-10 (34). This lag time in the onset of IL-10 production could offset the potential of IL-10 to exert an anti-inflammatory effect with respect to TNF-α. We speculated that TNF-α might, conversely, enhance IL-10 production (34), as had been observed in the context of LPS stimulation (45). This speculation was borne out by the results obtained herein.
As with LPS before (8), as well as in the present study, no regulatory effect of IL-6 was observed on IL-10 production in lipoprotein-stimulated monocytes. Other cytokines that do not inhibit the production of IL-10 in LPS-stimulated monocytes include IL-1, IL-2, IL-3, IL-4, IL-7, transforming growth factor β (TGF-β), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (8). In view of the LPS-lipoprotein parallelisms observed by us thus far, we speculate that these cytokines also will have no effect on IL-10 production by monocytes stimulated with lipoproteins. Our data permit us to add IL-12 to the list of cytokines that do not affect production of IL-10 by monocytes, regardless of whether these cells are stimulated by LPS or lipoprotein.
The production of IL-10 by monocytes stimulated with L-OspA appeared to be regulated by a negative feedback mechanism. Blocking the IL-10R complex with a specific antibody led to a significant increase in the production of IL-10 in lipoprotein-stimulated monocytes. Our results, obtained with either L-OspA or LPS as a stimulant, agree with those obtained by deWaal Malefyt et al. (11) and Knolle et al. (27), who demonstrated that IL-10 expression is autoregulated at the transcriptional level in monocytes and Kupffer cells stimulated with LPS. The negative autoregulation of IL-10 production in monocytes stimulated by lipoproteins adds to the panoply of regulatory mechanism at play during spirochetal infection.
Curiously, the effect exerted by the anti-IL-10R MAb was observed only when the antibody was added to the culture 8 h p.s. Since the antibody added at the beginning of the culture should have been present 8 h thereafter, the fact that its addition was ineffective when added together with the stimulants suggests that the antibody was bound to the monocytes by a nonfunctional form of the IL-10R and was thus ineffectively consumed. Conversely, when the antibody was added several hours after stimulation had been initiated, it was effective in enhancing IL-10 production. We take this as an indication that at this time the antibody was able to bind to a functional form of the IL-10R. Functional IL-10R complexes are tetramers consisting of two IL-10R1 polypeptide chains and two IL-10R2 chains (14). Stimulation of murine fibroblasts with LPS induces cellular IL-10 binding activity and IL-10R mRNA synthesis in a dose- and time-dependent manner (46). If there were a lag time between IL-10R monomer synthesis and assembly of the receptor complex within the cell membrane, the antibody could bind to the monomers and thus be consumed without having an effect on IL-10 production. When added later in the stimulatory process, it would bind to assembled and fully functional receptor complexes.
As with LPS (13), exogenous IFN-γ significantly inhibited the lipoprotein-induced production of IL-10 in monocytes. IFN-γ exerted its effect at the transcriptional level by downmodulating IL-10 gene transcription in monocytes stimulated with L-OspA or LPS. Our results agree with the observations made by Chomarat and colleagues (8) and Donnelly et al. (13), who demonstrated that among various cytokines tested, only IFN-γ strongly reduced IL-10 synthesis by LPS-activated monocytes. As with lipoproteins, this inhibition also was effected at the transcriptional level (13, 40).
It is noteworthy that inhibition of the biological activity of IL-10, either by suppressing its synthesis via IFN-γ or by blocking its receptor, resulted in an increase in the production of the proinflammatory cytokines IL-6 and IL-12 in macrophages stimulated by both L-OspA and LPS. Similar observations have been made in models of other infectious diseases, where IFN-γ was shown to differentially regulate IL-10 and IL-12 production, resulting in upregulation of IL-12 release and downregulation of IL-10 release in response to Mycobacterium leprae stimulation (29) and in human herpesvirus 6 infections (28). Given that IFN-γ has the ability to enhance IL-12 and IL-6 production in monocytes stimulated by LPS (15, 23, 31), the increase in IL-12 and IL-6 concentrations that was observed in the IFN-γ-treated cells could be due to either the direct effect of IFN-γ on IL-12 and IL-6 production or the indirect upregulation of both cytokines following the decrease in IL-10 production induced by IFN-γ. The increase in IL-12 and IL-6 production observed in the experiments conducted with anti-IL-10R in the absence of IFN-γ underscores the direct role of IL-10 in modulating IL-12 and IL-6 as induced by lipoproteins.
There have been a number of reports describing how IL-10 and IFN-γ antagonize each other with regard to their production and function (13, 17, 28, 29, 41, 44), including studies of monocytes/macrophages stimulated with LPS (8, 33, 40). This cross-regulatory effect of IL-10 and IFN-γ, which our study has now uncovered as well in lipoprotein-stimulated monocytes, may help to explain differences in pathology and disease outcome in human Lyme disease. It was recently demonstrated that blood cells from borreliosis patients release smaller amounts of the proinflammatory cytokines IFN-γ and TNF-α than cells from healthy volunteers when stimulated with either LPS or borrelial antigens. In contrast, the release of the anti-inflammatory cytokine IL-10 was slightly (though not significantly) higher in ex vivo-stimulated blood from borreliosis patients than in the control group (12). One possible interpretation of these results is that persistent infection with B. burgdorferi is associated with an attenuation of the capacity of the cells to produce proinflammatory cytokines, such as IFN-γ and TNF-α. This attenuation could be due to the preferential induction of the anti-inflammatory cytokine IL-10 by borrelial antigens, which is known to downregulate the production of IFN-γ (8) and other proinflammatory cytokines, such as IL-6, IL-12, and TNF-α (34). This modulation of proinflammatory cytokines could create conditions that favor the persistence of the spirochete in the infected host. In fact, the same study demonstrated that cells from healthy individuals that were stimulated in vitro with antigens of B. burgdorferi sensu lato induced reduced release of IFN-γ and TNF-α and enhanced secretion of IL-10 and G-CSF when compared to the pattern induced by bacterial endotoxins (12).
In summary, autocrine regulation of IL-10 production by THP-1 monocytes was afforded by IL-10 itself and by TNF-α whereas IFN-γ exerted its regulatory effect in an exocrine fashion, because this cytokine is not commonly produced by macrophages/monocytes. We could find no differences in these regulatory effects regardless of whether the cells were stimulated with L-OspA or LPS, in spite of the differences already pointed out between the receptors and signaling pathways of these two bacterial stimulants.
Acknowledgments
This work was supported by grant U50/CCU606604 from the Centers for Disease Control and Prevention and by grant RR00164 from the National Center for Research Resources, National Institutes of Health. Special thanks go to the American Society of Microbiology for providing an International Fellowship to G. H. Giambartolomei. G.H.G. is a member of the Research Career of CONICET (Argentina). Special thanks go to the Department of Biotechnology, India, for providing a Senior Overseas Associateship to P.K.M.
We thank John Dunn (Brookhaven National Laboratory) for purified recombinant OspA. We also thank Avery Labat for her excellent secretarial help.
Editor: R. N. Moore
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W., E. Fikrig, L. K. Bockenstedt, and D. H. Persing. 1995. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect. Immun. 63:2255-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and M. Wooten. 1999. Dual role of interleukin 10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgdorfer, W. A., G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease: a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 8.Chomarat, P., M. C. Rissoan, J. Banchereau, and P. Miossec. 1993. Interferon gamma inhibits interleukin 10 production by monocytes. J. Exp. Med. 177:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyle, P. K. 1993. Lyme disease, p.179-183. In S. Manning (ed.), Pathogenesis of Lyme disease. Mosby Year Book Co., St. Louis, Mo.
- 10.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deWaal Malefyt, R., J. Haanen, H. Spits, M. G. Roncolo, A. TeVelde, C. Fidgor, K. Johnson, R. Kastelein, H. Yssel, and J. E. DeVries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diterich, I., L. Harter, D. Hassler, A. Wendel, and T. Hartung. 2001. Modulation of cytokine release in ex vivo-stimulated blood from borreliosis patients. Infect. Immun. 69:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly, R. P., S. L. Freeman, and M. P. Hayes. 1995. Inhibition of IL-10 expression by IFN-gamma up-regulates transcription of TNF-alpha in human monocytes. J. Immunol. 155:1420-1427. [PubMed] [Google Scholar]
- 14.Donnelly, R. P., H. Dickensheets, and D. S. Finbloom. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563-573. [DOI] [PubMed] [Google Scholar]
- 15.Durum, S. K., and J. J. Oppenheim. 1993. Proinflammatory cytokines and immunity, p. 801. In W. E. Paul (ed.), Fundamental immunology, 3rd ed. Raven Press, New York, N.Y.
- 16.England, J. D., R. P. Bohm, Jr., E. D. Roberts, and M. T. Philipp. 1997. Mononeuropathy multiplex in rhesus monkeys with chronic Lyme disease. Ann. Neurol. 41:375-384. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 18.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter et al. 1997. Genomic sequence of a Lyme disease spirochaete Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 19.Ganapamo, F., V. A. Dennis, and M. T. Philipp. 2000. Early induction of gamma interferon and interleukin-10 production in draining lymph nodes from mice infected with Borrelia burgdorferi. Infect. Immun. 68:7162-7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 1999. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 67:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giambartolomei, G. H., V. A. Dennis, and M. T. Philipp. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 66:2691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haupl, T., S. Landgraf, P. Netusil, N. Biller, C. Capiau, P. Desmons, P. Hauser, and G. R. Burmester. 1997. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol. Med. Microbiol. 19:15-23. [DOI] [PubMed] [Google Scholar]
- 23.Hayes, M. P., J. Wang, and M. A. Norcross. 1995. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood 86:646-650. [PubMed] [Google Scholar]
- 24.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J.Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 25.Horng, T., G. M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835-841. [DOI] [PubMed] [Google Scholar]
- 26.Kitchens, R. L., and R. S. Munford. 1995. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J. Biol. Chem. 270:9904-9910. [DOI] [PubMed] [Google Scholar]
- 27.Knolle, P. A., A. Uhrig, U. Protzer, M. Trippler, R. Duchmann, K. H. Meyer zum Buschenfelde, and G. Gerken. 1998. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology 27:93-99. [DOI] [PubMed] [Google Scholar]
- 28.Li, C., J. M. Goodrich, and X. Yang. 1997. Interferon-gamma (IFN-gamma) regulates production of IL-10 and IL-12 in human herpesvirus-6 (HHV-6)-infected monocyte/macrophage lineage. Clin. Exp. Immunol. 109:421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libraty, D. H., L. E. Airan, K. Uyemura, D. Jullien, B. Spellberg, T. H. Rea, and R. L. Modlin. 1997. Interferon-gamma differentially regulates interleukin-12 and interleukin-10 production in leprosy. J. Clin. Investig. 99:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 31.Ma, X., J. M. Chow, G. Gri, G. Carra, F. Gerosa, S. F. Wolf, R. Dzialo, and G. Trinchieri. 1996. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 183:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchant, A., C. Bruyns, P. Vandenabeele, M. Ducarme, C. Gerard, A. Delvaux, D. De Groote, D. Abramowicz, T. Velu, and M. Goldman. 1994. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur. J. Immunol. 24:1167-1171. [DOI] [PubMed] [Google Scholar]
- 34.Murthy, P. K., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 2000. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 68:6663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 36.Norgard, M. V., L. L. Arndt, D. R. Akins, L. L. Curetty, D. A. Harrrich, and J. D. Radolf. 1996. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NK-κB. Infect. Immun. 64:3845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philipp, M. T., and B. J. B. Johnson. 1994. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 2:431-437. [DOI] [PubMed] [Google Scholar]
- 38.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 39.Sellati, T. J., D. A. Bouis, R. L. Kitchens, R. P. Darveau, J. Pugin, R. J. Ulevitch, S. C. Gangloff, S. M. Goyert, M. V. Norgard, and J. D. Radolf. 1998. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160:5455-5464. [PubMed] [Google Scholar]
- 40.Shakhov, A. N., G. Woerly, B. D. Car, and B. Ryffel. 1996. Interferon gamma enhances TNF-alpha production by inhibiting early phase IL-10 transcription. Eur. Cytokine Netw. 7:741-750. [PubMed] [Google Scholar]
- 41.Silva, J. S., P. J. Morrissey, K. H. Grabstein, K. M. Mohler, D. Anderson, and S. G. Reed. 1992. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J. Exp. Med. 175:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 21:586-596. [DOI] [PubMed] [Google Scholar]
- 43.Takayama, K., R. J. Rothenberg, and A. G. Barbour. 1987. Absence of lipopolysaccharide in the Lyme disease spirochete Borrelia burgdorferi. Infect. Immun. 55:2311-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieira, P., R. de Waal-Malefyt, M. N. Dang, K. E. Johnson, R. Kastelein, D. F. Fiorentino, J. E. deVries, M. G. Roncarolo, T. R. Mosmann, and K. W. Moore. 1991. Isolation and expression of human cytokine synthesis inhibitory factor (CSIF/IL-10) cDNA clones: homology to EBV open reading frame BCRFI. Proc. Natl. Acad. Sci. USA 88:1172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanidworanum, C., and W. Strobet. 1993. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J. Immunol. 151:6853-6861. [PubMed] [Google Scholar]
- 46.Weber-Nordt, R. M., M. A. Meraz, and R. D. Schreiber. 1994. Lipopolysaccharide-dependent induction of IL-10 receptor expression on murine fibroblast. J. Immunol. 153:3734-3744. [PubMed] [Google Scholar]
- 47.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 48.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin, Z., J. Braun, L. Neure, P. Wu, U. Eggens, A. Krause, T. Kamradt, and J. Sieper. 1997. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 40:69-79. [DOI] [PubMed] [Google Scholar]