Abstract
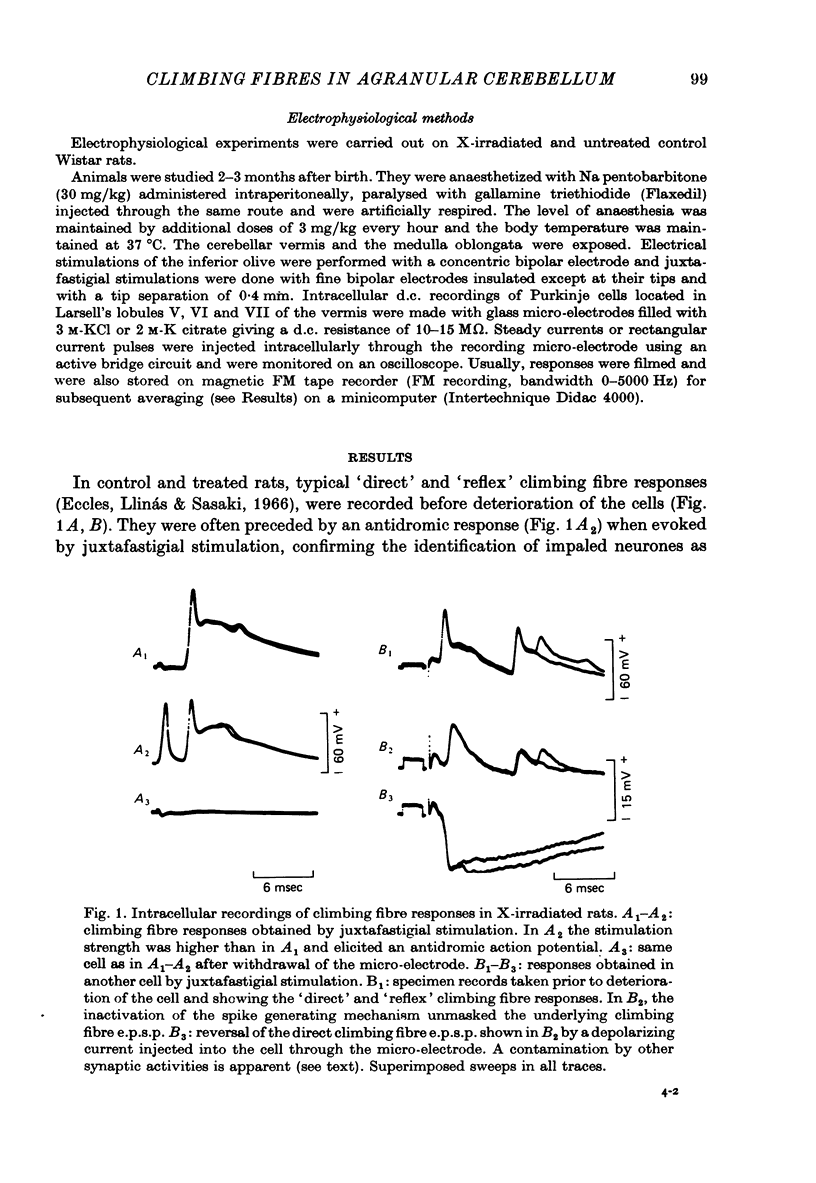
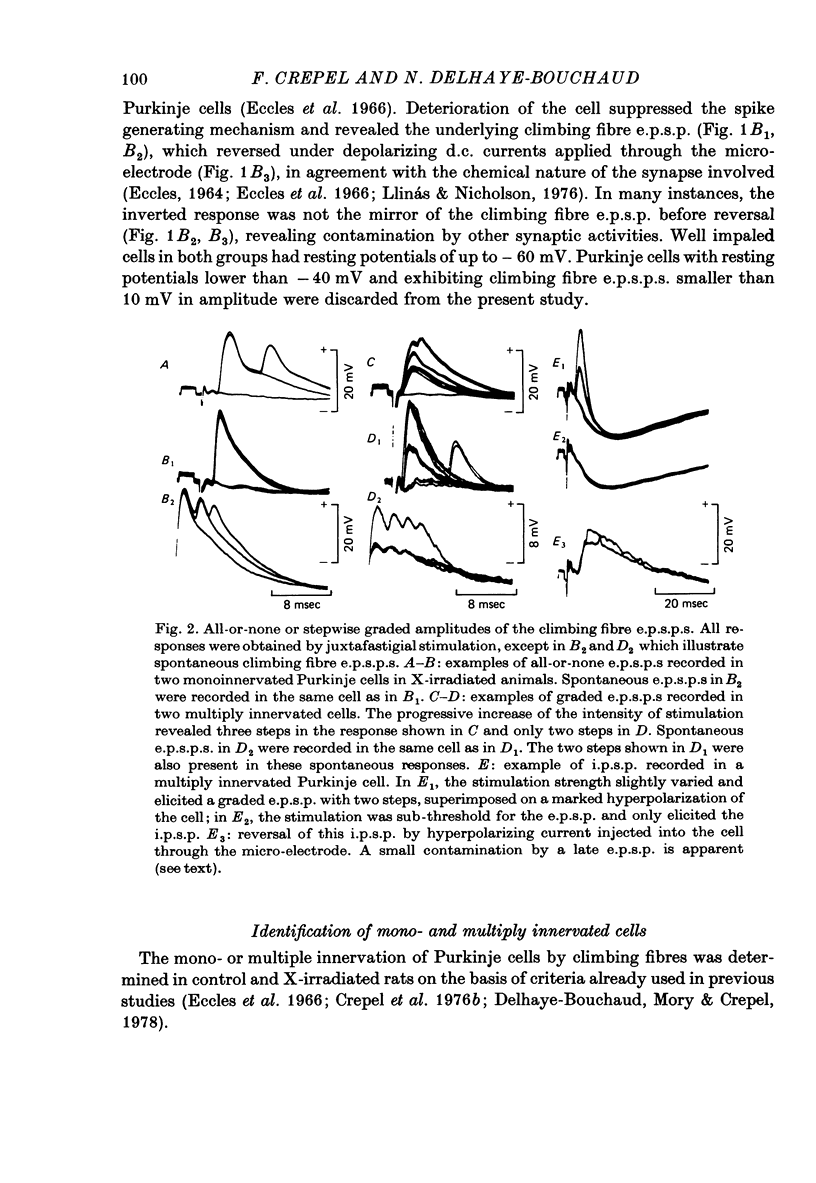
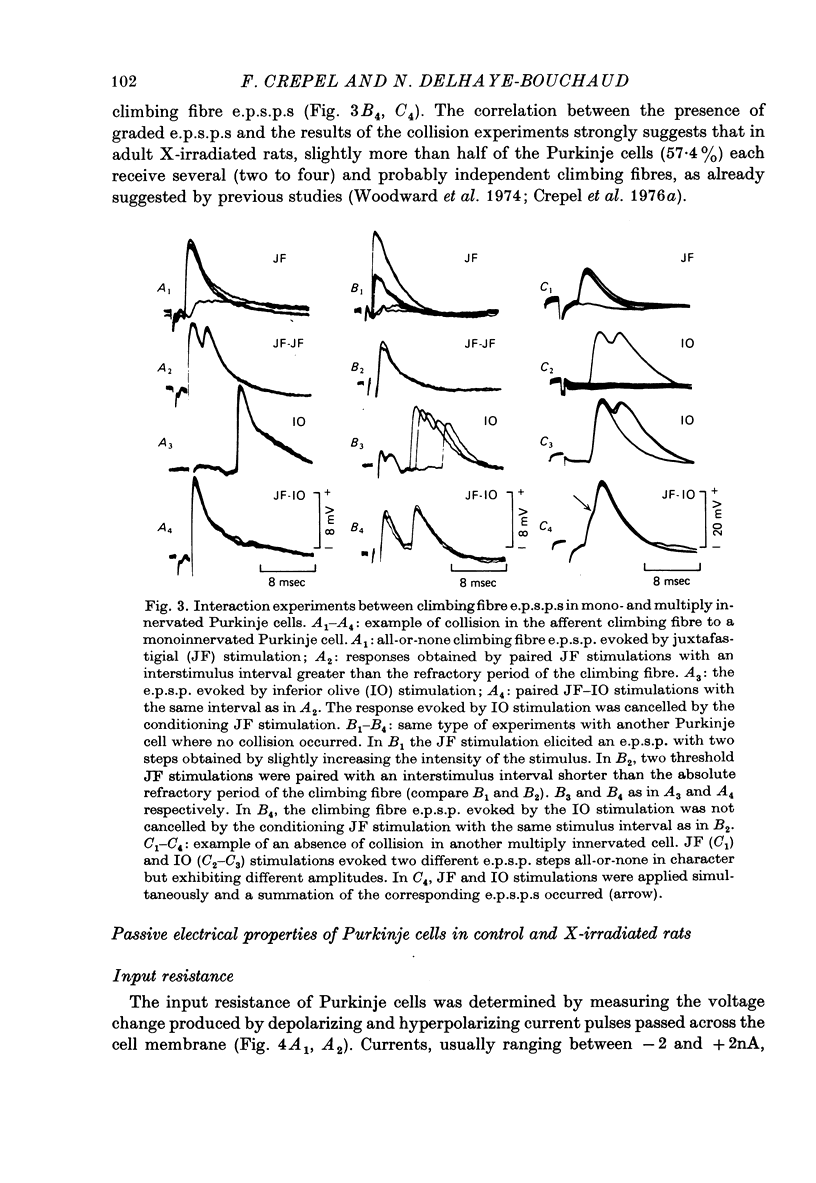
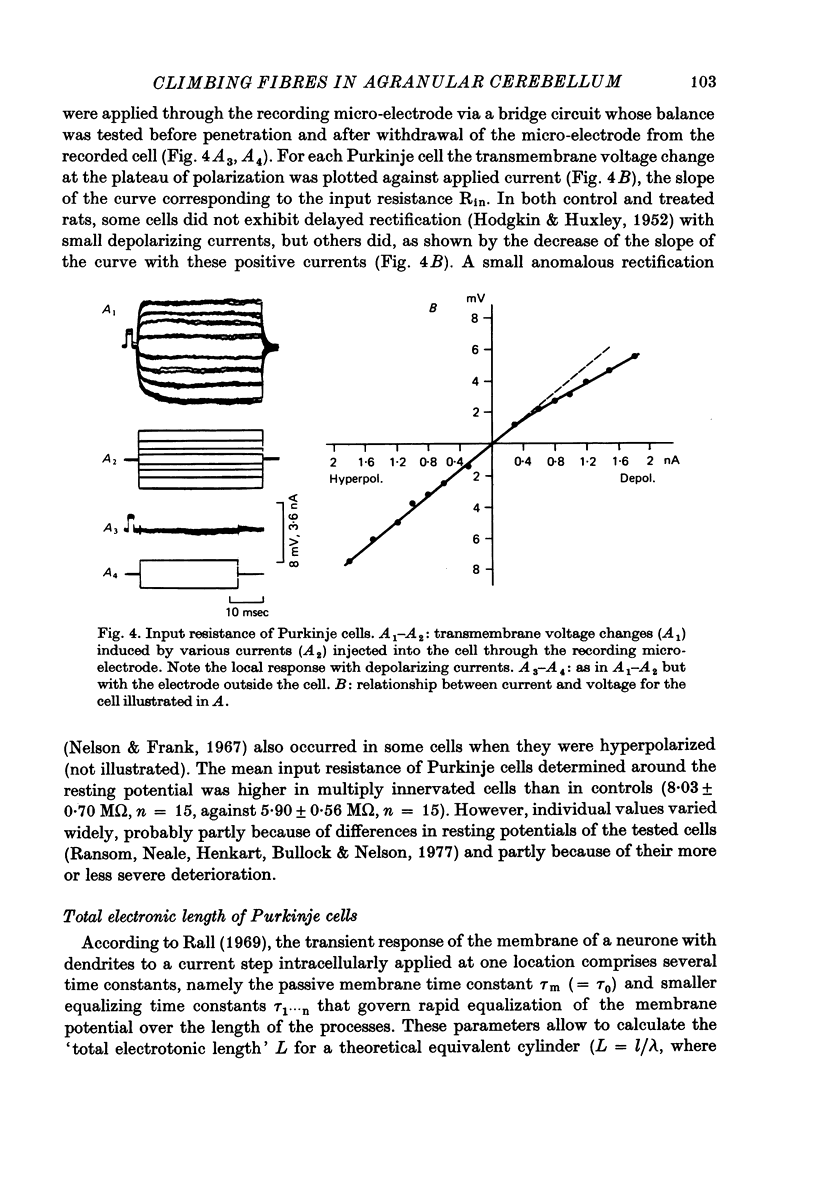
1. The distribution of climbing fibres on cerebellar Purkinje cells has been studied with intracellular recordings in X-irradiated and normal rats. 2. In the treated rats, multiple steps in the post-synaptic potential were elicited in 57% of the Purkinje cells by graded stimulation of the climbing fibres, the response was all-or-none in character in the other cells and in all Purkinje cells recorded in normal animals. In the neurones exhibiting the former type of response, no collision was seen along the afferent fibres during interaction experiments between just-threshold juxtafastigial and maximal olivary stimulations, whereas a collision always occurred when all-or-none responses were recorded. 3. These results show that in X-irradiated rats, the majority of Purkinje cells have a multiple innervation by two to four climbing fibres, instead of the one-to-one relationship seen normally. 4. Input resistances and total electrotonic lengths of Purkinje cells were measured in normal and treated rats. Mean values for these two parameters were higher than normal in multiply innervated cells. 5. Mean time course and mean current for reversal of the post-synaptic potential elicited in Purkinje cells by stimulation of the climbing fibres were nearly the same in mono- and in multiply innervated neurones. In multiply innervated cells, time courses and currents for reversal were independent of the size of the response or varied slightly with it, suggesting that the climbing fibres involved innervated territories whose electrotonic distance from the recording site were either the same or slightly different. 6. Interactions between two all-or-none steps of the graded post-synaptic potential evoked in multiply innervated cells by juxtafastigial and olivary stimulations revealed either a very weak or a very marked shunting effect between synapses of the two climbing fibres involved. 7. These results indicate that the over-all distribution of climbing fibre synapses on multiply innervated Purkinje cells is not grossly abnormal and that two fibres contacting a given cell can be either intermingled on the same dendrites, or segregated on distinct dendritic branches. 8. In general, the present study does not suggest the existence of a strong competition among climbing fibres innervating each Purkinje cell during development at least when granule cells are absent.
Full text
PDF


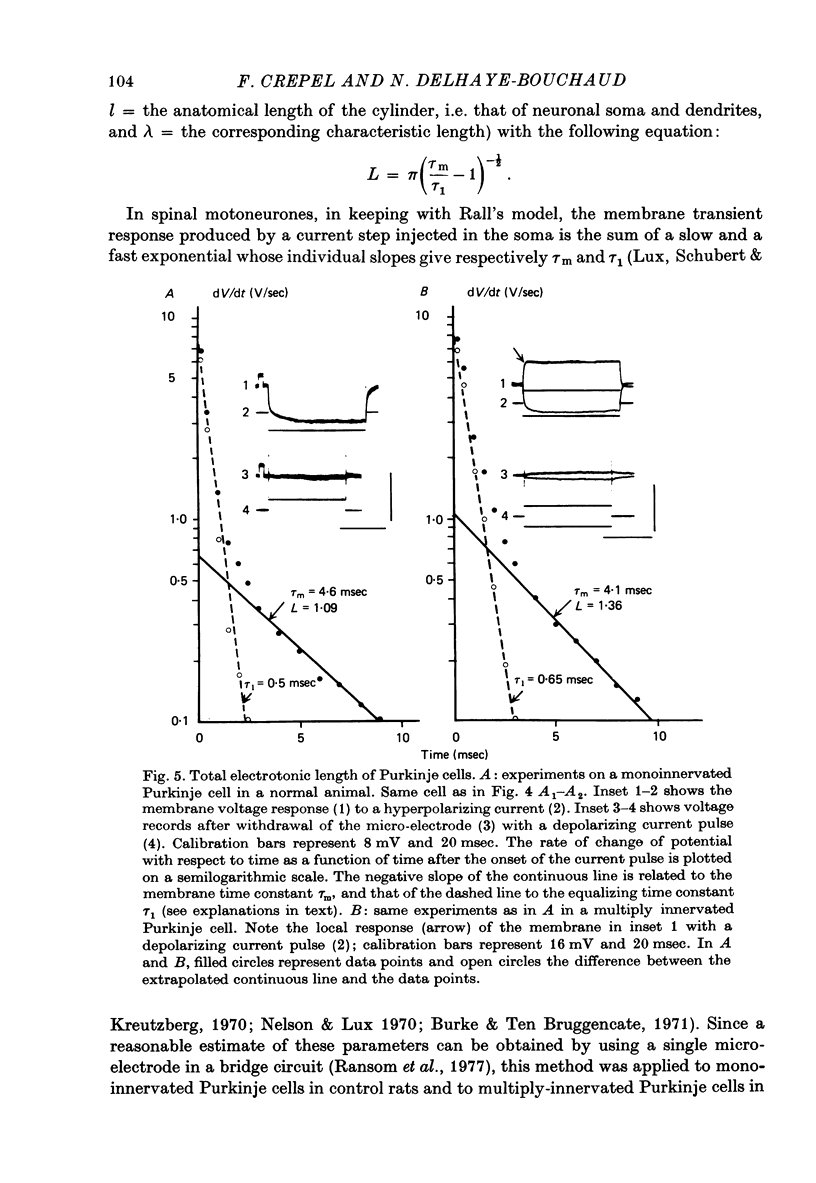

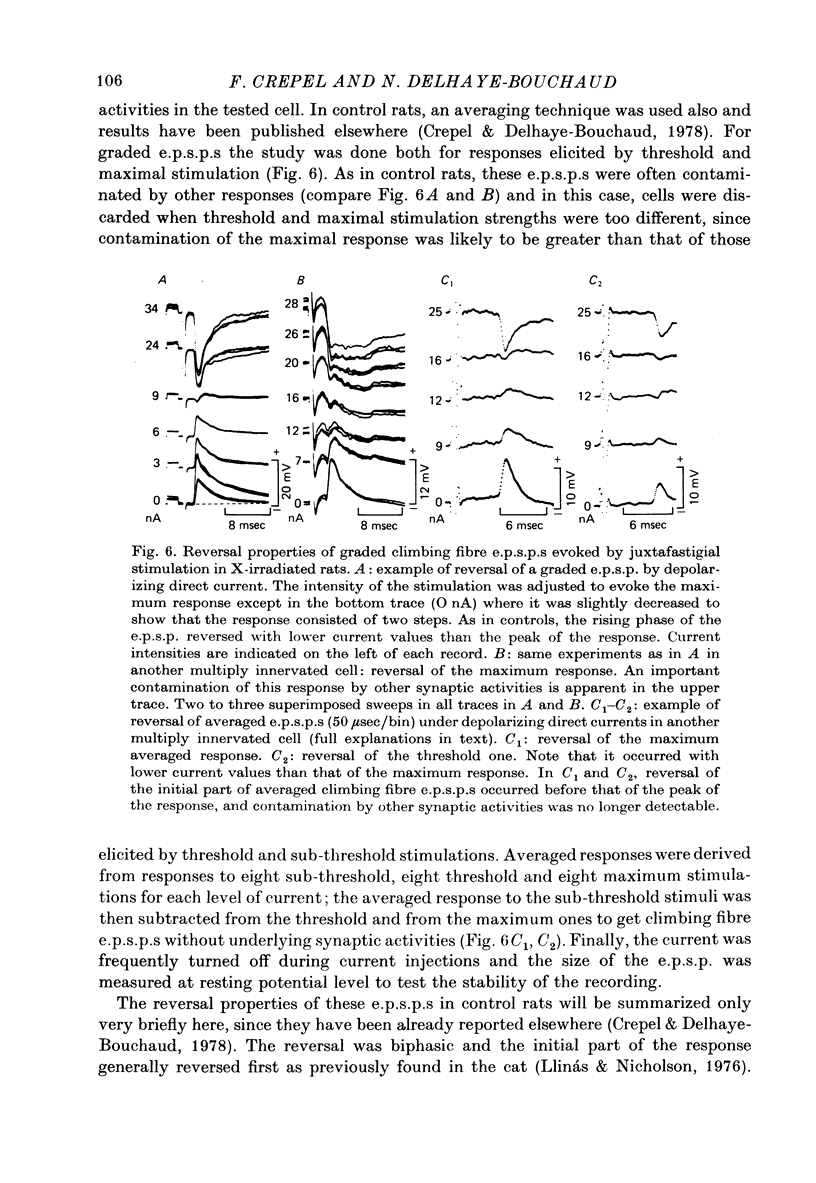

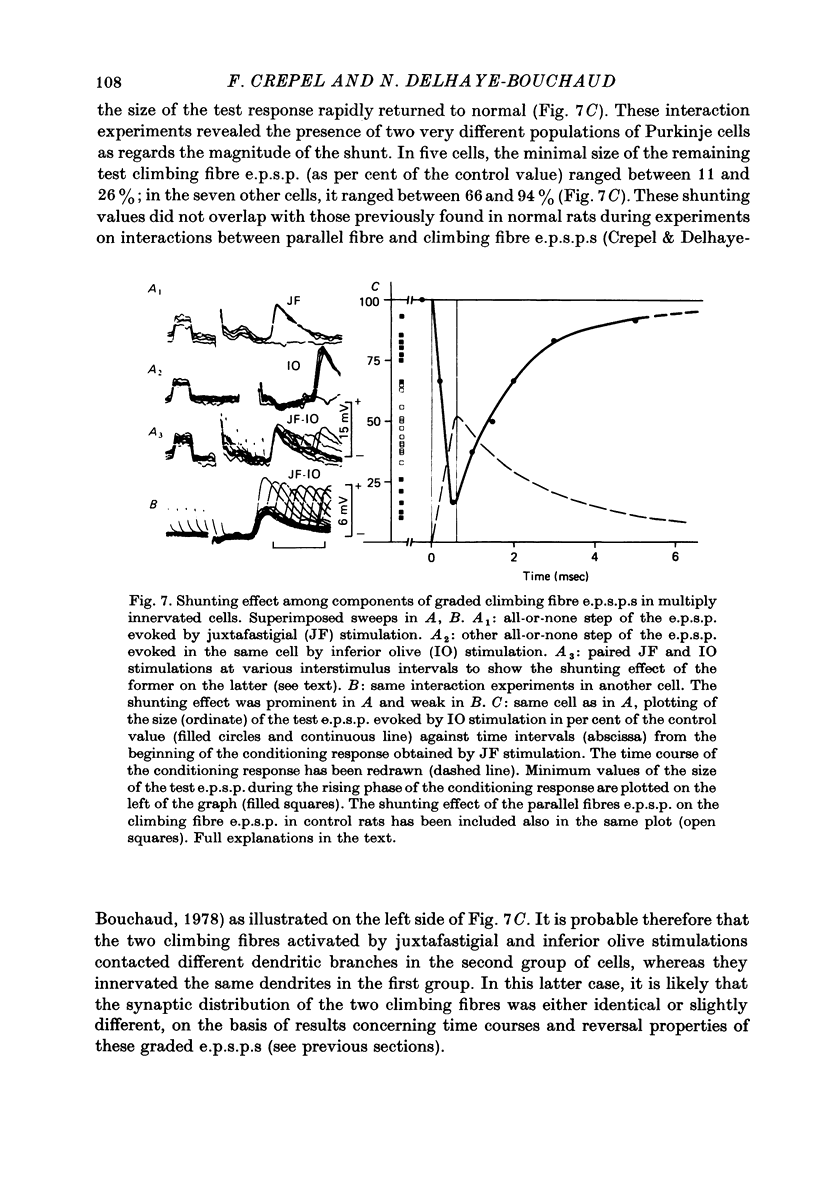




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagust J., Lewis D. M., Westerman R. A. Polyneuronal innervation of kitten skeletal muscle. J Physiol. 1973 Feb;229(1):241–255. doi: 10.1113/jphysiol.1973.sp010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Berry M., Bradley P. The growth of the dendritic trees of Purkinje cells in irradiated agranular cerebellar cortex. Brain Res. 1976 Nov 12;116(3):361–387. doi: 10.1016/0006-8993(76)90487-x. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H. Dendritic synapses and reversal potentials: theoretical implications of the view from the soma. Exp Neurol. 1969 Jun;24(2):248–264. doi: 10.1016/0014-4886(69)90018-1. [DOI] [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N. Intracellular analyses of synaptic potentials in cerebellar Purkinje cells of the rat. Brain Res. 1978 Oct 20;155(1):176–181. doi: 10.1016/0006-8993(78)90321-9. [DOI] [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N., Legrand J. Electrophysiological analysis of the circuitry and of the corticonuclear relationships in the agranular cerebellum of irradiated rats. Arch Ital Biol. 1976 Feb;114(1):49–74. [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Crepel F., Mariani J. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the Weaver Mutant Mouse. J Neurobiol. 1976 Nov;7(6):579–582. doi: 10.1002/neu.480070610. [DOI] [PubMed] [Google Scholar]
- Delhaye-Bouchaud N., Crepel F., Mariani J. Mise en évidence d'une multi-innervation temporaire des cellules de Purkinje du cervelet par les fibres grimpantes au cours du développement chez le rat. C R Acad Sci Hebd Seances Acad Sci D. 1975 Sep 29;281(13):909–912. [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K. Voltage attenuation within Aplysia neurons: the effect of branching pattern. Brain Res. 1975 May 2;88(2):325–332. doi: 10.1016/0006-8993(75)90394-7. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen H., Jansen J. K. Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibres in newborn rats. J Neurocytol. 1976 Oct;5(8):591–604. doi: 10.1007/BF01175572. [DOI] [PubMed] [Google Scholar]
- Kuffler D., Thompson W., Jansen J. K. The elimination of synapses in multiply-innervated skeletal muscle fibres of the rat: dependence on distance between end-plates. Brain Res. 1977 Dec 16;138(2):353–358. doi: 10.1016/0006-8993(77)90752-1. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Hillman D. E., Precht W. Neuronal circuit reorganization in mammalian agranular cerebellar cortex. J Neurobiol. 1973;4(1):69–94. doi: 10.1002/neu.480040106. [DOI] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976 Mar;39(2):311–323. doi: 10.1152/jn.1976.39.2.311. [DOI] [PubMed] [Google Scholar]
- Mariani J., Crepel F., Mikoshiba K., Changeux J. P., Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1977 Nov 2;281(978):1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Lux H. D. Some electrical measurements of motoneuron parameters. Biophys J. 1970 Jan;10(1):55–73. doi: 10.1016/S0006-3495(70)86285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Spira M. E., Werman R., Bergmann F. Non-homogeneous conduction in giant axons of the nerve cord of Periplaneta americana. J Exp Biol. 1969 Jun;50(3):635–649. doi: 10.1242/jeb.50.3.635. [DOI] [PubMed] [Google Scholar]
- Puro D. G., Woodward D. J. The climbing fiber system in the Weaver mutant. Brain Res. 1977 Jun 24;129(1):141–146. doi: 10.1016/0006-8993(77)90976-3. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol. 1973 May;230(3):509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G. Regional differentiation of the axon: a review with special reference to the concept of the multiplex neuron. Brain Res. 1972 Dec 12;47(2):269–288. doi: 10.1016/0006-8993(72)90639-7. [DOI] [PubMed] [Google Scholar]
- Westerfield M., Joyner R. W., Moore J. W. Temperature-sensitive conduction failure at axon branch points. J Neurophysiol. 1978 Jan;41(1):1–8. doi: 10.1152/jn.1978.41.1.1. [DOI] [PubMed] [Google Scholar]
- Woodward D. J., Hoffer B. J., Altman J. Physiological and pharmacological properties of Purkinje cells in rat cerebellum degranulated by postnatal x-irradiation. J Neurobiol. 1974;5(4):283–304. doi: 10.1002/neu.480050402. [DOI] [PubMed] [Google Scholar]


