Abstract
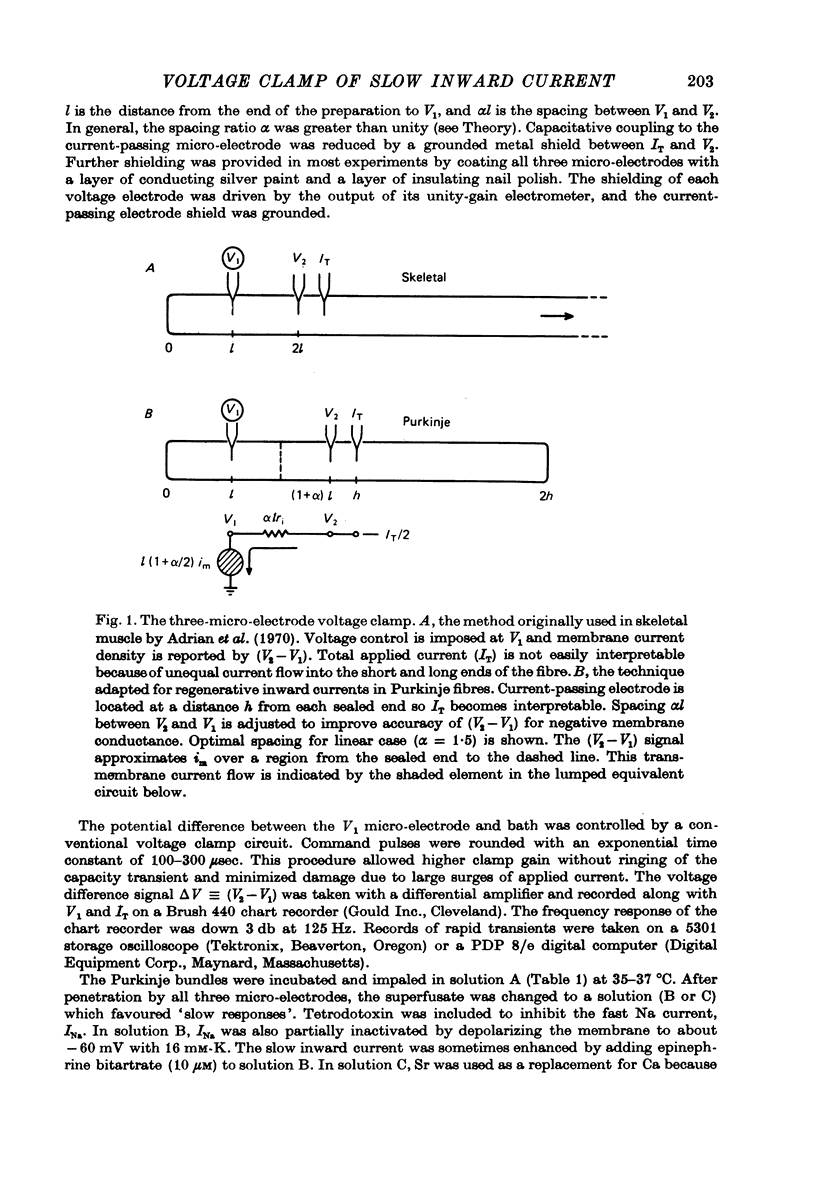
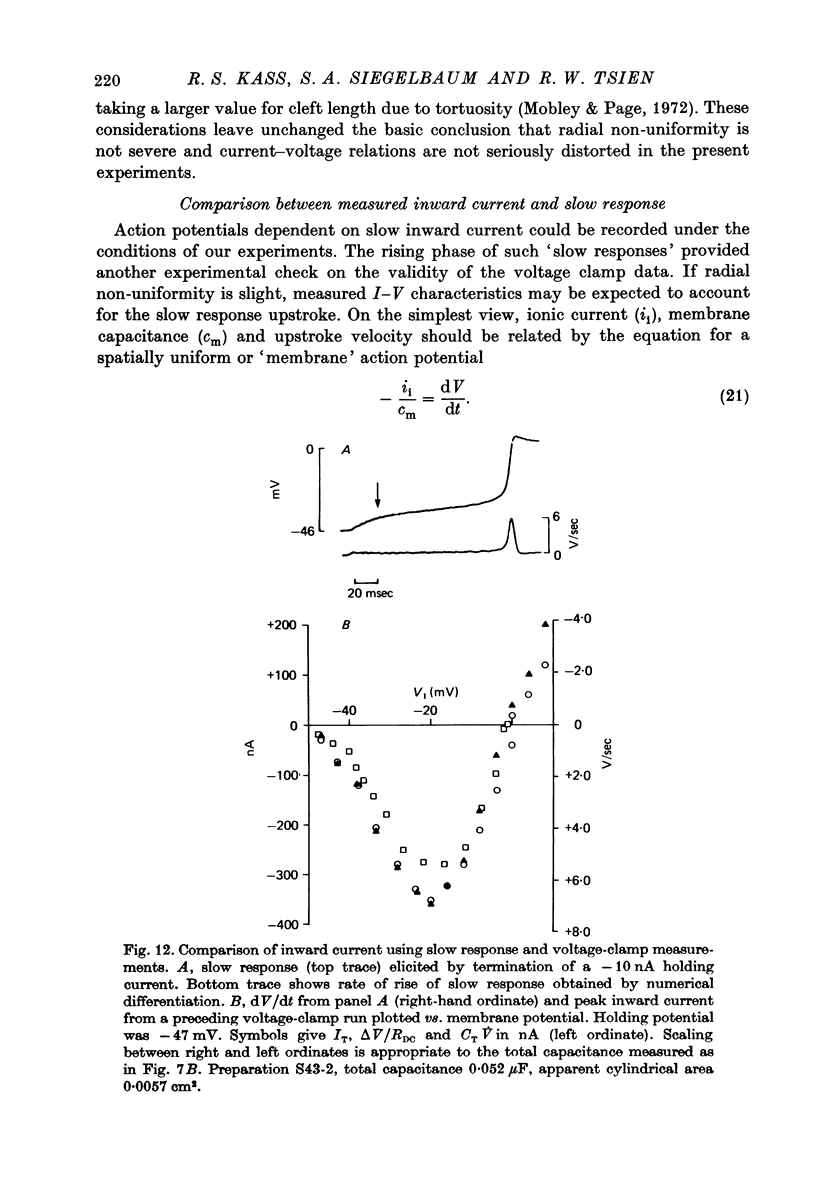
1. The three-micro-electrode voltage clamp method (Adrian, Chandler & Hodgkin, 1970) was adapted for the study of regenerative inward currents in cardiac muscle. The adequacy of measurements of slow inward current in cardiac Purkinje fibres was assessed. 2. Membrane current density is reported simultaneously by total applied current (IT), along with a longitudinal voltage difference signal (delta V), recorded between two intracellular micro-electrodes. 3. Non-linear cable calculations show that delta V is a more faithful measure of membrane current density than IT as peak inward current increases. Quantitative agreement between delta V and IT only occurs when both signals report the membrane characteristics that would be obtained with an ideal longitudinal space clamp. 4. Agreement between delta V and IT is thus a useful criterion for satisfactory experimental measurements which we applied to the slow inward current. This component was elicited by depolarizing steps from a holding potential near =45 mV in the presence of tetrodotoxin. The IT signal was compared directly with delta V/R, where R is an effective longitudinal resistance that was experimentally determined. 5. delta V/R and IT showed very good agreement in both peak amplitude and time course at all potentials studied. 6. Radial non-uniformity during the measured peak slow inward current was estimated by calculations assuming clefts 200 A wide with a uniform distribution of ionic channels. The calculated voltage span from surface to centre was always less than 5 mV, and the measured I-V characteristics showed little distortion. 7. In another check, I-V characteristics and slow response membrane action potentials were compared. The measured peak current showed good agreement with the product (total preparation capacitance) x (rate of rise). 8. The experimental and theoretical analysis suggest that the measurements of slow inward current are a good approximation to genuine membrane properties.
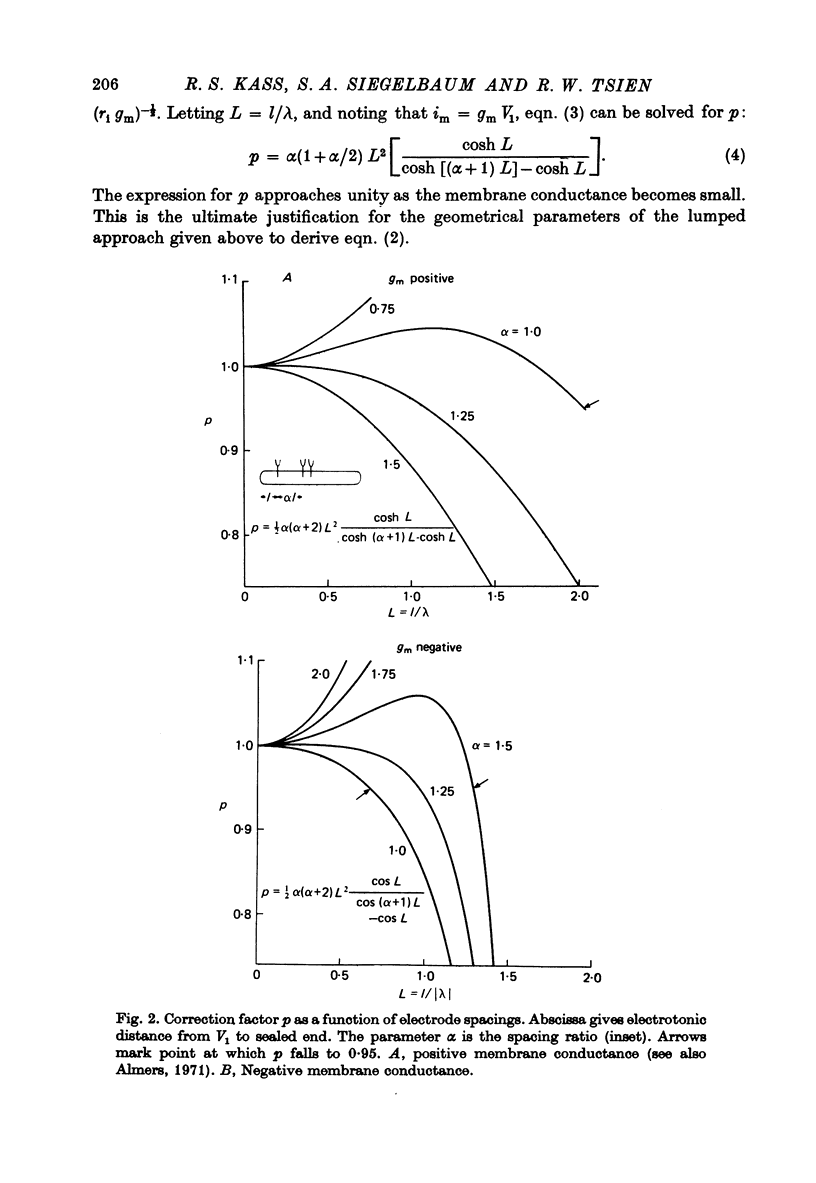
Full text
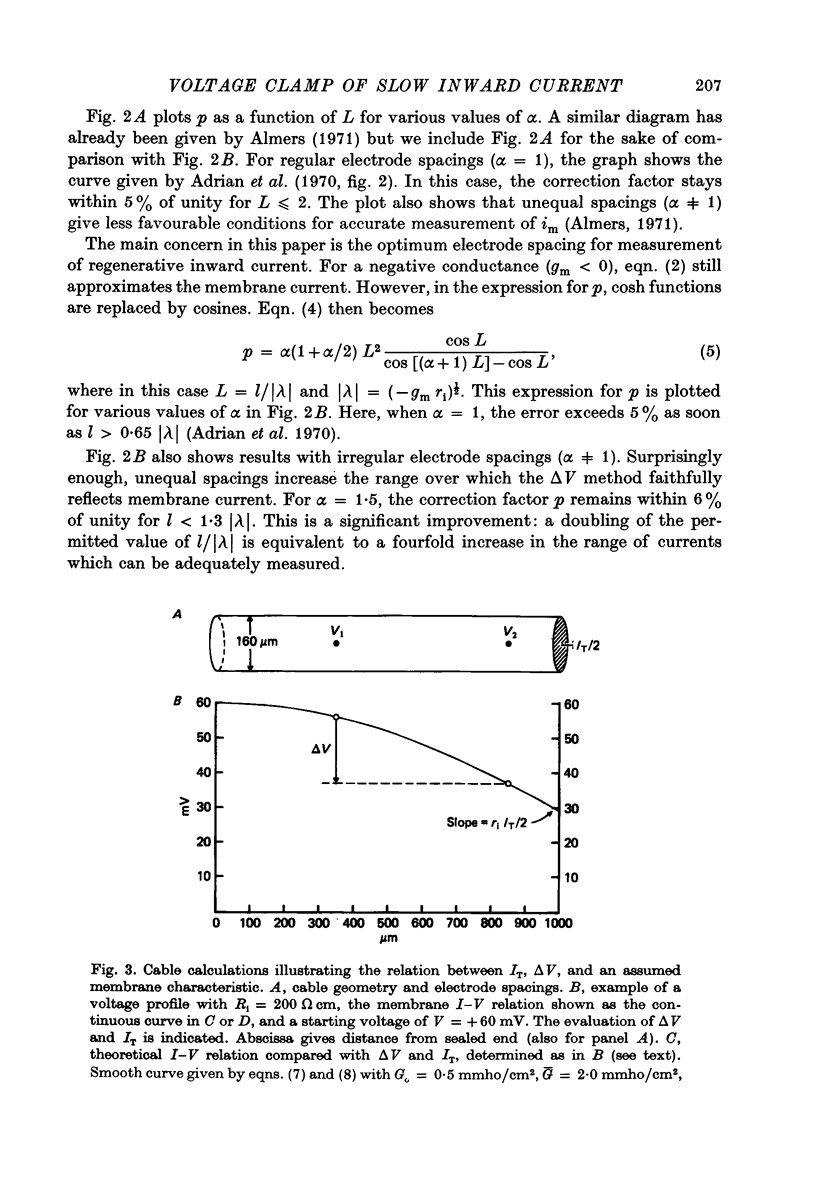
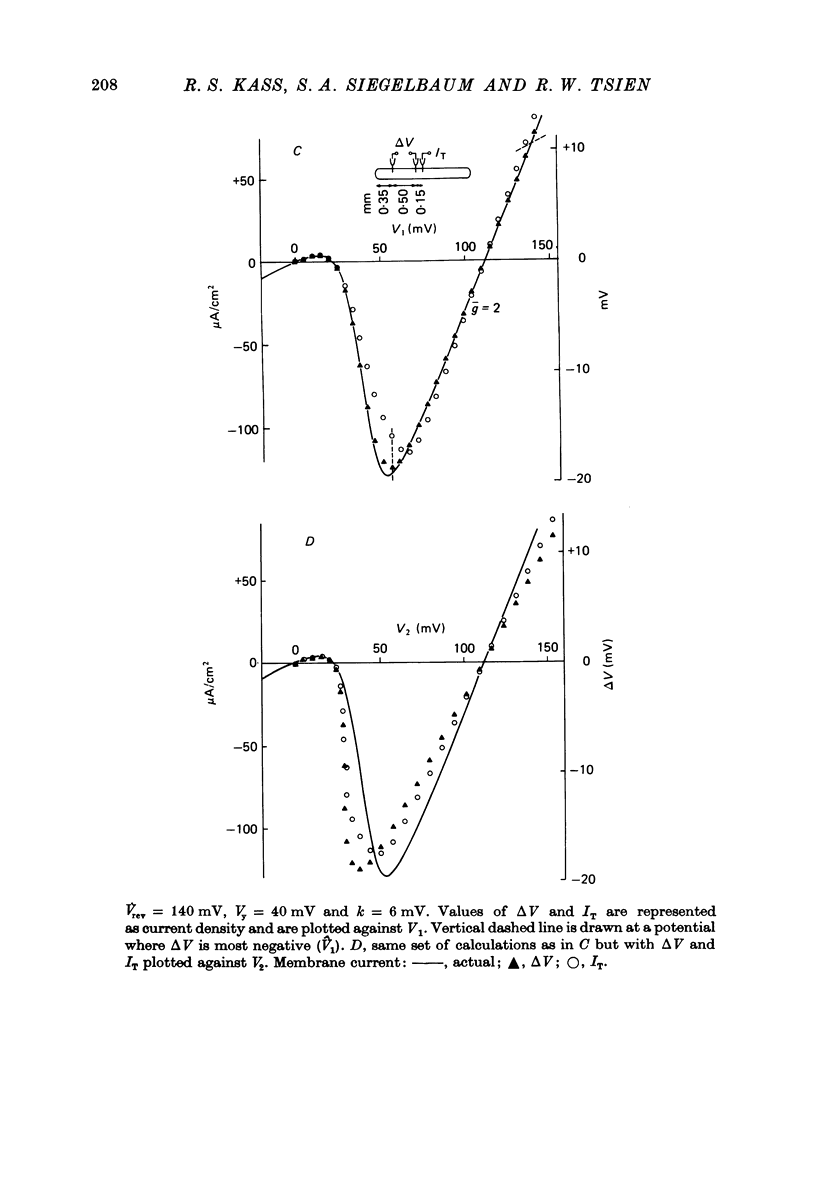
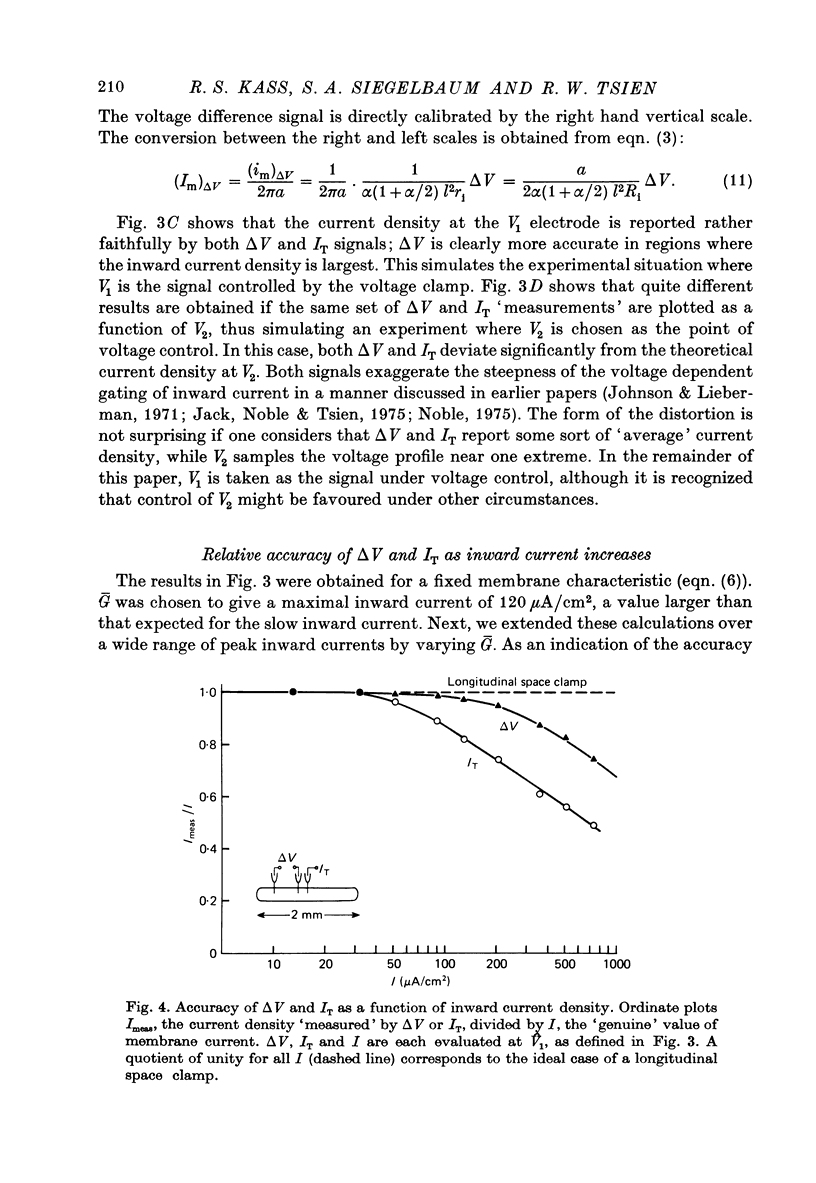
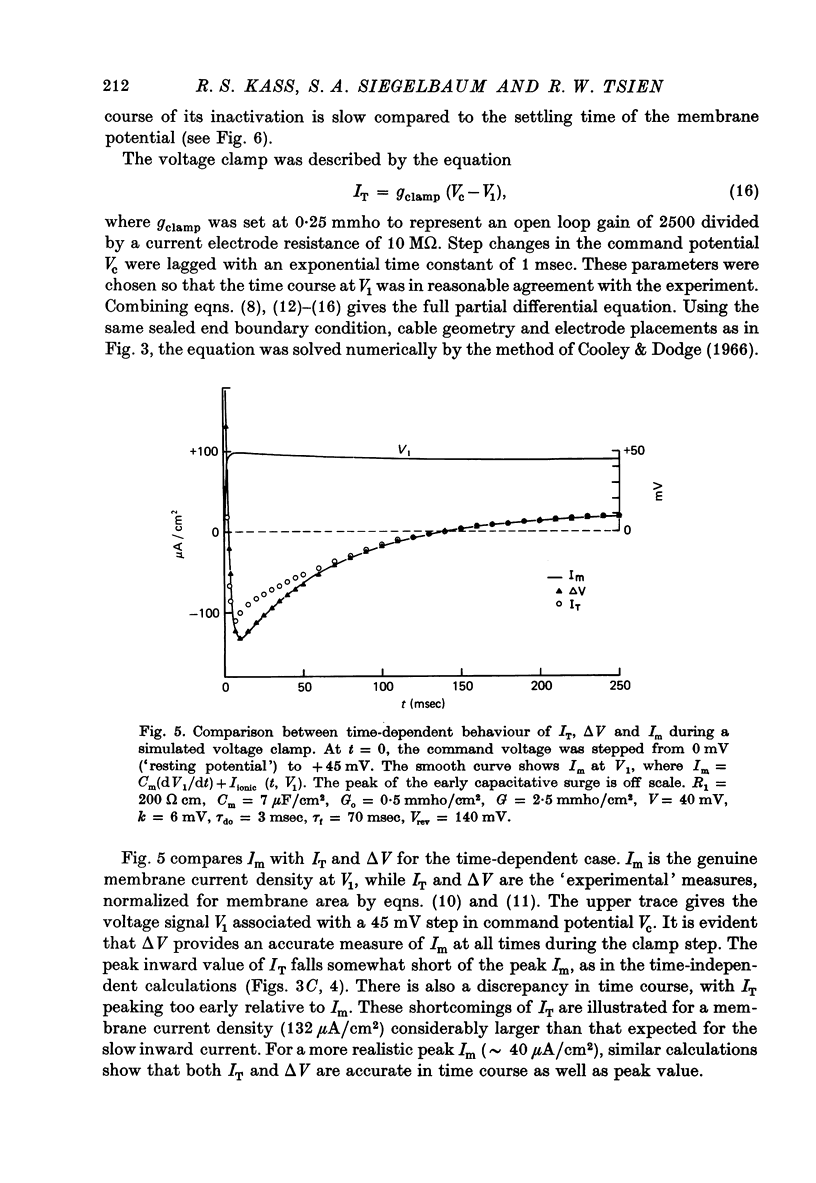
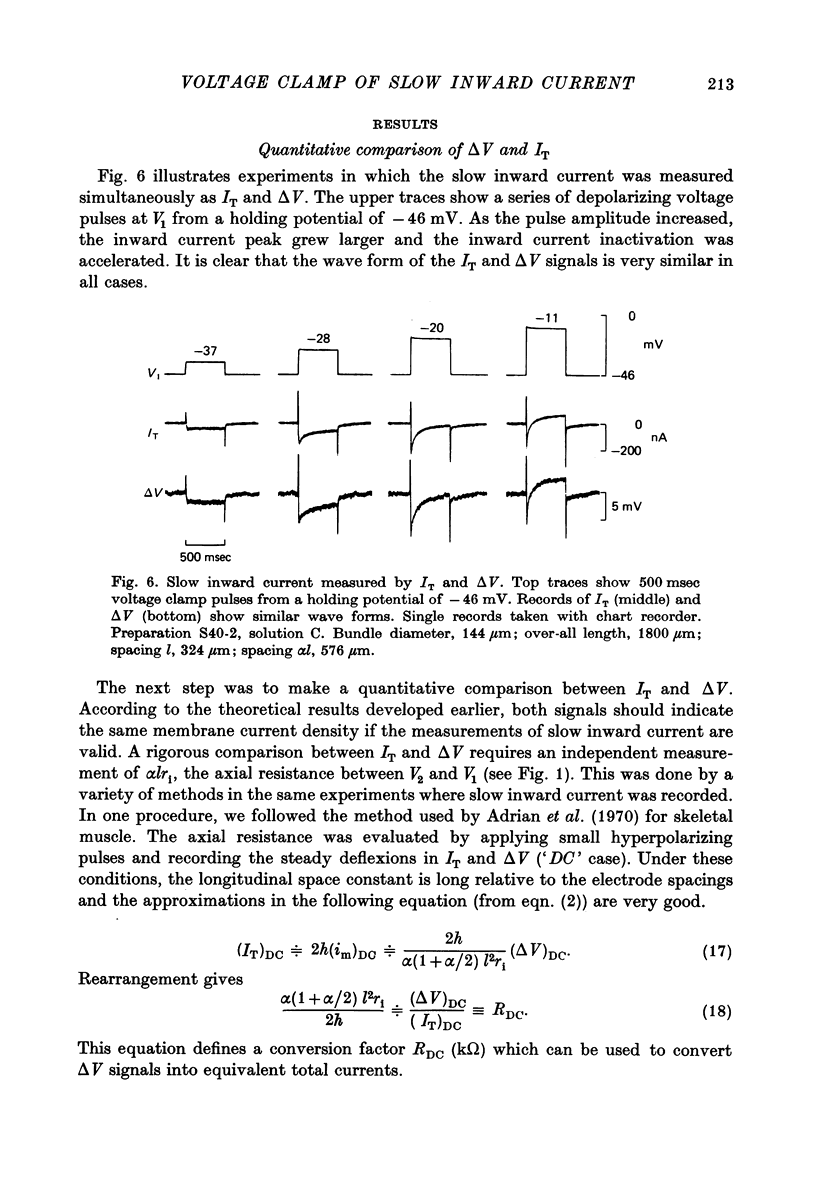
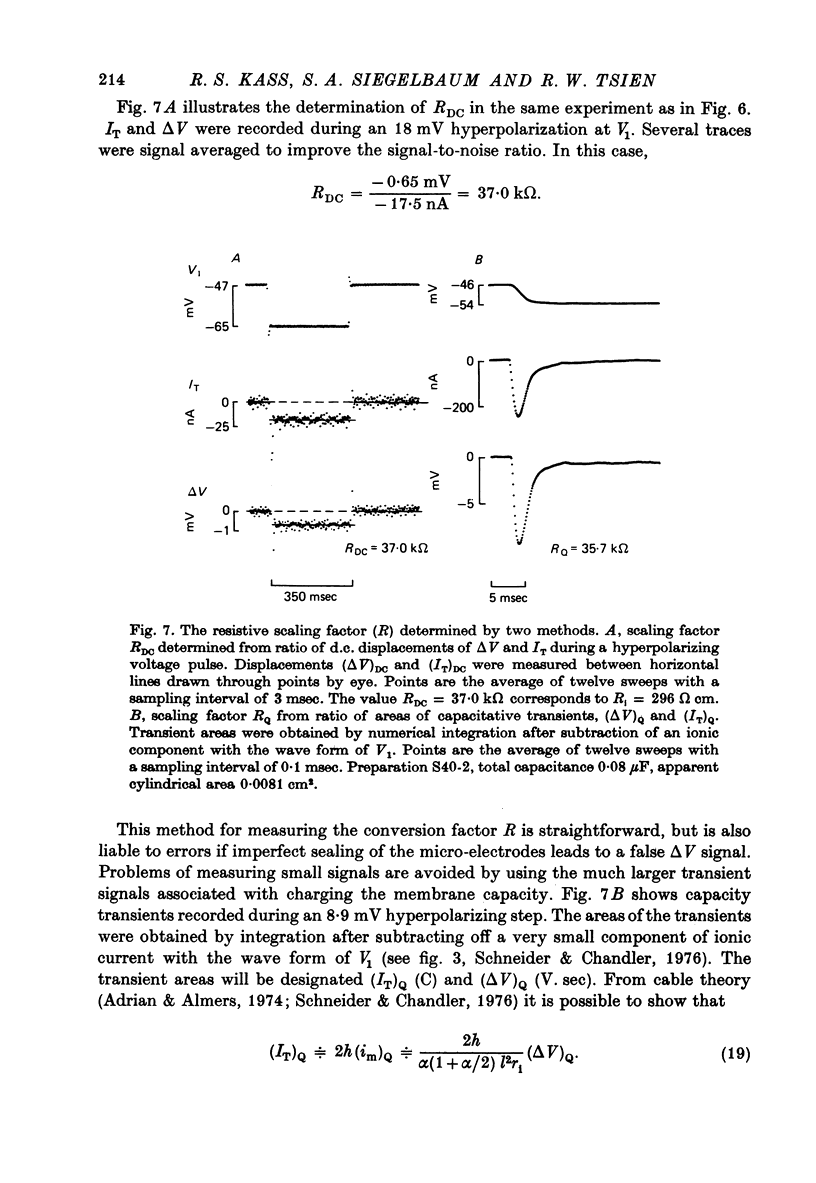
PDF






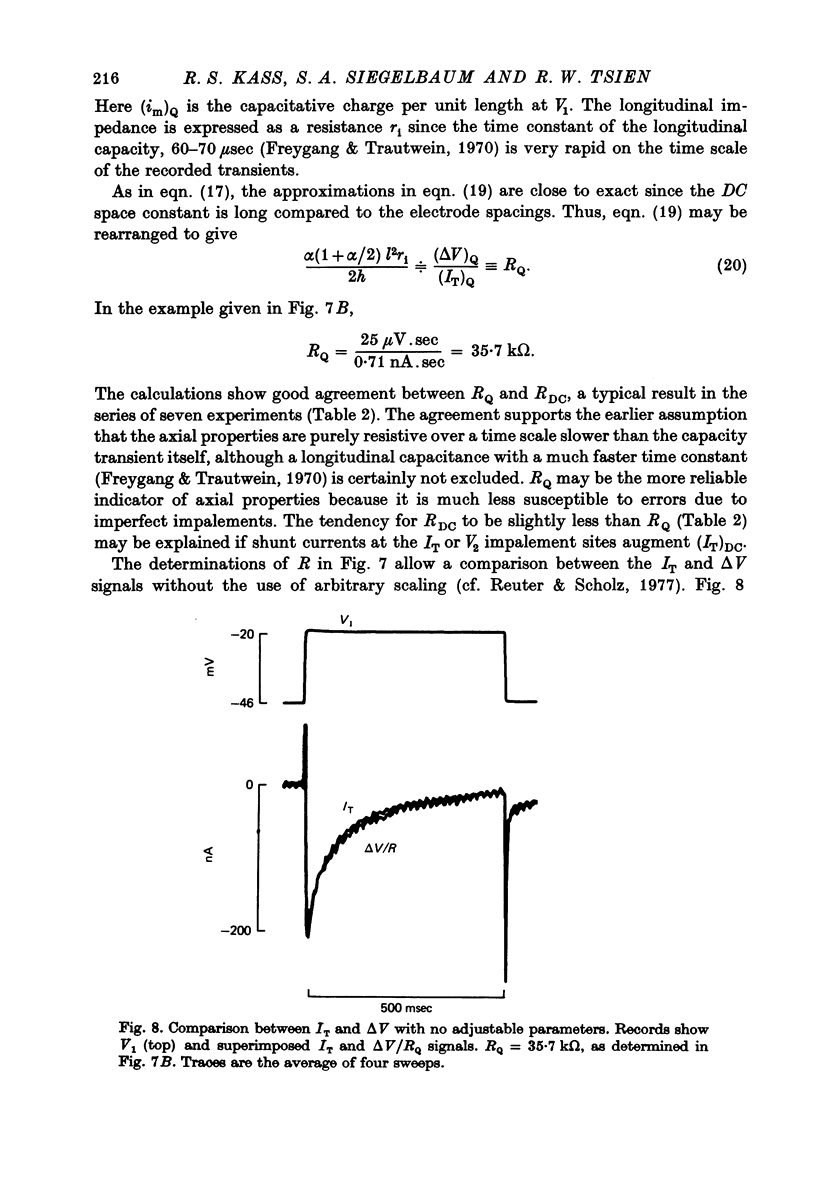
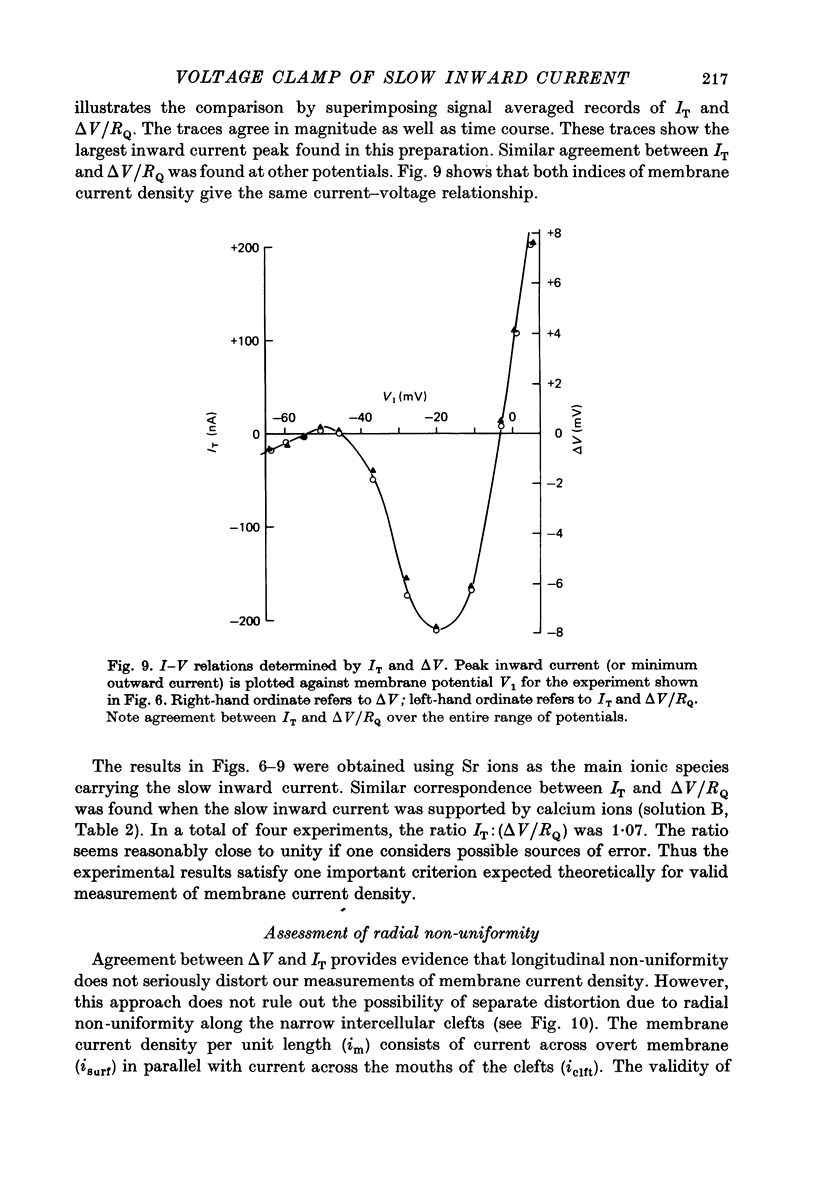
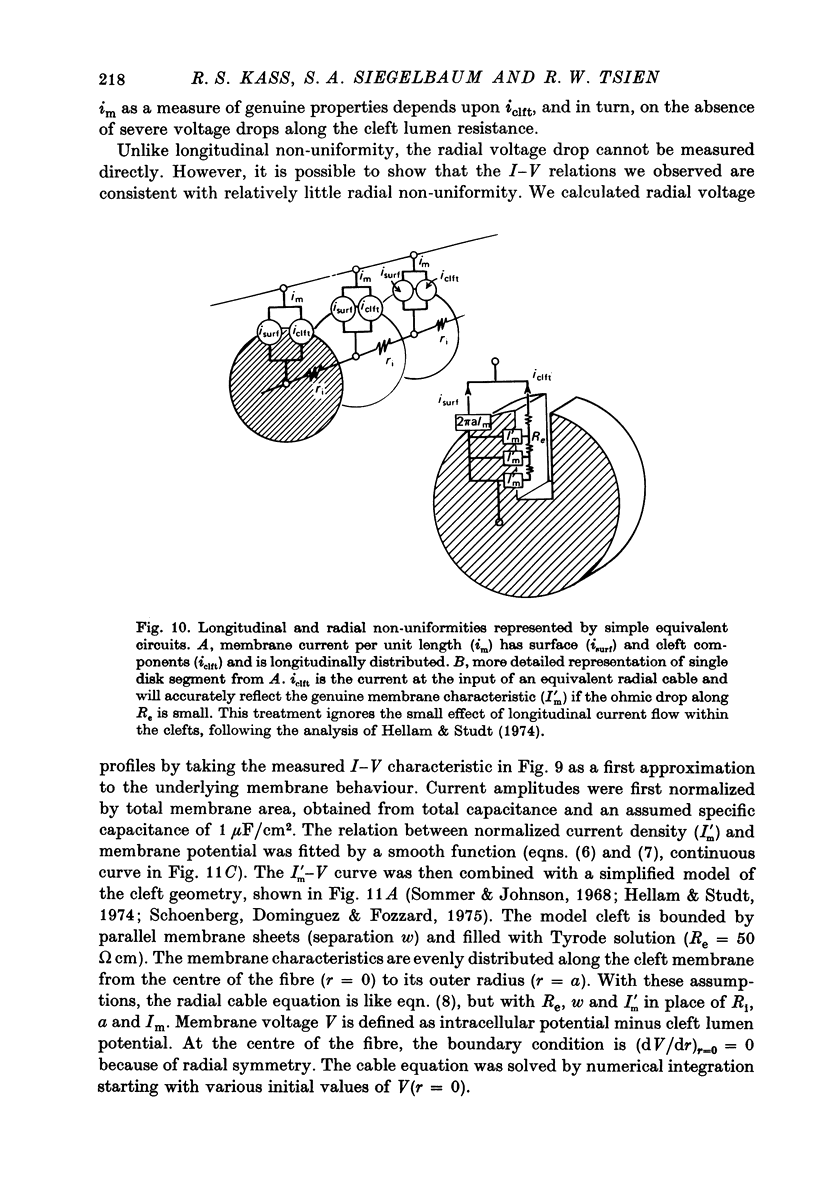
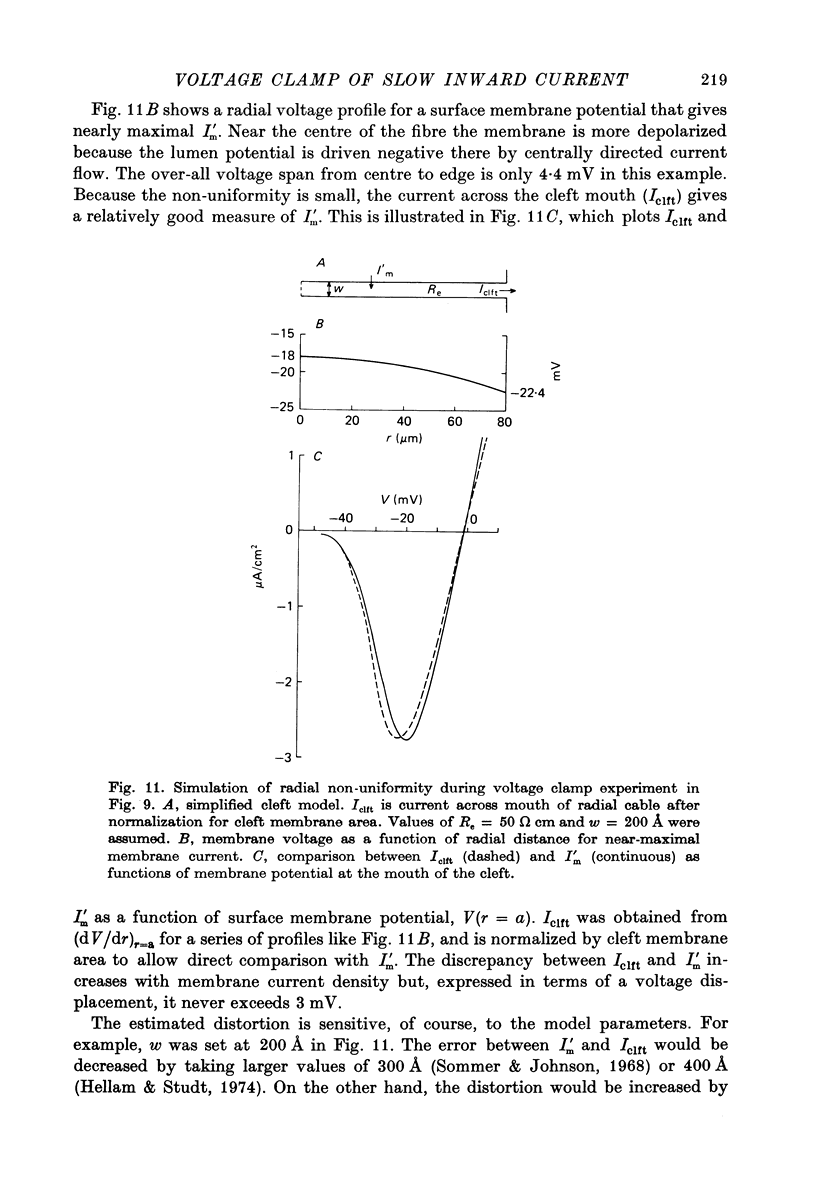





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Cohen I. The voltage clamp of multicellular preparations. Prog Biophys Mol Biol. 1977;31(3):201–245. doi: 10.1016/0079-6107(78)90009-3. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Willems J. The frequency dependent character of the membrane capacity in cardiac Purkyne fibres. J Physiol. 1971 Feb;213(1):85–93. doi: 10.1113/jphysiol.1971.sp009369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky T. J., Tsien R. W. Electrical properties associated with wide intercellular clefts in rabbit Purkinje fibres. J Physiol. 1979 May;290(2):227–252. doi: 10.1113/jphysiol.1979.sp012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Barr L., Jakobsson E. Electrical characteristics of frog atrial trabeculae in the double sucrose gap. Biophys J. 1975 Oct;15(10):1047–1067. doi: 10.1016/S0006-3495(75)85882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley J. W., Dodge F. A., Jr Digital computer solutions for excitation and propagation of the nerve impulse. Biophys J. 1966 Sep;6(5):583–599. doi: 10.1016/S0006-3495(66)86679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Beeler G. W., Jr The voltage clamp and cardiac electrophysiology. Circ Res. 1975 Oct;37(4):403–413. doi: 10.1161/01.res.37.4.403. [DOI] [PubMed] [Google Scholar]
- Freygang W. H., Trautwein W. The structural implications of the linear electrical properties of cardiac Purkinje strands. J Gen Physiol. 1970 Apr;55(4):524–547. doi: 10.1085/jgp.55.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Studt J. W. A core-conductor model of the cardiac Purkinje fibre based on structural analysis. J Physiol. 1974 Dec;243(3):637–660. doi: 10.1113/jphysiol.1974.sp010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976 Dec;263(2):73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Goldman Y. Letter to the Editors: Clarification of membrane conductance measurements in ventricular heart muscle. J Mol Cell Cardiol. 1976 Feb;8(2):169–172. doi: 10.1016/0022-2828(76)90028-6. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Effects of membrane potential on the capacitance of skeletal muscle fibers. J Gen Physiol. 1976 Feb;67(2):125–163. doi: 10.1085/jgp.67.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Dominguez G., Fozzard H. A. Effect of diameter on membrane capacity and conductance of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Apr;65(4):441–458. doi: 10.1085/jgp.65.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W., Kass R. S. Role of intracellular calcium in the transient outward current of calf Purkinje fibres. Nature. 1977 Oct 13;269(5629):611–613. doi: 10.1038/269611a0. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968 Mar;36(3):497–526. doi: 10.1083/jcb.36.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M., Trank J. W. An assessment of the double sucrose-gap voltage clamp technique as applied to frog atrial muscle. Biophys J. 1974 Sep;14(9):627–643. doi: 10.1016/S0006-3495(74)85940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F. Membrane conductance measurements in cat ventricular muscle. J Mol Cell Cardiol. 1978 Apr;10(4):387–394. doi: 10.1016/0022-2828(78)90385-1. [DOI] [PubMed] [Google Scholar]
- Vereecke J., Carmeliet E. Sr action potentials in cardiac Purkyne fibres. I. Evidence for a regenerative increase in Sr conductance. Pflugers Arch. 1971;322(1):60–72. doi: 10.1007/BF00586665. [DOI] [PubMed] [Google Scholar]


