Abstract
A multivalent vaccine containing amino-terminal M protein fragments from 26 different serotypes of group A streptococci was constructed by recombinant techniques. The vaccine consisted of four different recombinant proteins that were formulated with alum to contain 400 μg of protein per dose. Rabbits were immunized via the intramuscular route at 0, 4, and 16 weeks. Immune sera were assayed for the presence of type-specific antibodies against the individual recombinant M peptides by enzyme-linked immunosorbent assay and for opsonic antibodies by in vitro opsonization tests and indirect bactericidal tests. The 26-valent vaccine was highly immunogenic and elicited fourfold or greater increases in antibody levels against 25 of the 26 serotypes represented in the vaccine. The immune sera were broadly opsonic and were bactericidal against the majority of the 26 different serotypes. Importantly, none of the immune sera cross-reacted with human tissues. Our results indicate that type-specific, protective M protein epitopes can be incorporated into complex, multivalent vaccines designed to elicit broadly protective opsonic antibodies in the absence of tissue-cross-reactive antibodies.
Group A streptococcal pharyngitis is one of the most common bacterial infections in school age children. In addition, invasive streptococcal infections afflict thousands of children and adults each year, often resulting in death or significant morbidity (37). Although the incidence of acute rheumatic fever (ARF), a nonsuppurative sequela of streptococcal pharyngitis, has declined in developed countries, the disease is rampant in developing countries (40). Efforts to develop a vaccine that would prevent group A streptococcal infections have been ongoing for more than 8 decades (22, 28). New molecular techniques (8) and a better understanding of the biology of group A streptococci (11) have allowed the previous obstacles associated with vaccine development to be overcome.
Previous studies have shown that the surface M protein is the major virulence determinant and the major protective antigen of group A streptococci (29). The type specificity of each M protein, of which more than 100 are now known, is largely determined by the epitopes located in the amino-terminal 40 to 50 amino acid residues (3, 8, 14, 27). These regions of M proteins have been shown to evoke antibodies with the greatest bactericidal (protective) activity and are least likely to cross-react with human tissues (2, 14, 20). Thus, our approach has been to combine small amino-terminal M protein peptides to make multivalent vaccines that would elicit opsonic antibodies against epidemiologically important serotypes of group A streptococci (12, 21).
In the present study, we constructed a 26-valent M protein-based vaccine by recombinant technology. The vaccine is composed of four different fusion proteins that contain six or seven M protein fragments linked in tandem. Each component protein of the vaccine was designed to serve as its own carrier, thus obviating the need for unrelated proteins. When formulated with alum, the vaccine was highly immunogenic in rabbits and evoked broadly protective antibodies, as determined by opsonization and indirect bactericidal activity assays. Our results demonstrate the feasibility of using highly complex, multivalent M protein-based group A streptococcal vaccines to evoke broadly protective antibodies.
MATERIALS AND METHODS
Selection of group A streptococcal serotypes to include in the 26-valent vaccine.
The rationale for the selection of vaccine serotypes was based on three criteria: (i) serotypes that are frequent causes of uncomplicated pharyngitis, (ii) serotypes that are commonly recovered from normally sterile sites (invasive strains) in the ongoing Active Bacterial Core Surveillance of the Emerging Infections Program Network supported by the Centers for Disease Control and Prevention (CDC) (7, 36), and (iii) serotypes that are currently considered or have historically been considered “rheumatogenic” (9). Also included in the vaccine is the amino-terminal peptide fragment of Spa, a new protective antigen that is expressed by at least several serotypes of group A streptococci (19).
Criteria for selection of M peptides.
The amino acid sequences of the M proteins selected for inclusion in the vaccine were obtained from the CDC emm typing center website (www.cdc.gov/ncidod/biotech/strep/emmtypes.html). The amino-terminal regions of the mature M proteins (and Spa) were searched by BlastP for homology against human proteins in the GenBank database. Amino-terminal regions having five or more contiguous amino acid matches with human proteins were excluded. The selected regions of the M peptides and Spa were then analyzed by the method of Hopp and Woods (24) to ensure the integrity of hydrophilic peaks.
Construction and expression of hexavalent and septavalent vaccine proteins.
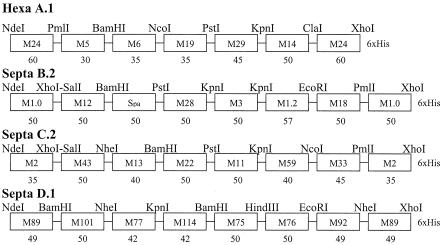
Once the specific 5′ sequences of each emm gene and the spa gene had been selected for inclusion in the vaccine, they were used to design four hybrid DNA molecules, each containing six or seven emm gene fragments linked in tandem by unique restriction enzyme sites (Fig. 1). Each protein sequence encoded by the hybrid DNA molecules was again analyzed by BlastP to ensure that there were no significant homologies with human proteins. Restriction enzyme sites were selected to avoid potential cross-reactive epitopes.
FIG. 1.
Schematic diagram of the four recombinant fusion proteins contained in the 26-valent M protein-based vaccine. The oligonucleotide primers used to amplify each 5′ emm gene fragment by PCR were synthesized to include the unique restriction enzyme sites indicated. The number of codons contained in each emm gene fragment is indicated below the M type. M101 was formerly stNS5, and M114 was formerly st2967. The M13 is strain M13W, which has been newly designated M94.
The four hybrid DNA molecules were constructed by using PCR-generated M protein or Spa fragments that were amplified from genomic DNA of the corresponding serotype by using oligonucleotide primers that were synthesized to contain flanking coding sequences for unique restriction enzyme sites. The PCR-generated fragments were purified, cut with the appropriate restriction enzymes, and then ligated together sequentially by methods previously described (12, 18). In each hybrid DNA molecule, the 5′ emm fragment was also ligated to the 3′ end based on previous studies that showed that an amino-terminal M protein fragment reiterated on the carboxy-terminal end of the protein appeared to enhance or protect the immunogenicity of the adjacent M protein fragments (12). Prior to ligation, the 3′ emm fragment was similarly generated by using an oligonucleotide primer that also contained codons for six histidine residues at the 3′ end. The completed hybrid DNA molecules were ligated into expression plasmid pT5, which was then used to transform Escherichia coli strain JM105. The integrity of the PCR-generated sequences was verified by sequencing both strands by the ABI dye termination method. Expression of each multivalent fusion protein was detected by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis using whole-cell lysates before and after isopropyl-β-d-thiogalactopyranoside (IPTG) induction.
Construction and expression of individual recombinant dimeric M peptides.
Individual recombinant dimeric peptides comprising the vaccine component peptides were expressed and purified for use as serologic reagents in these studies. Each emm gene fragment included in the hybrid DNA molecules described above was independently amplified by PCR, purified, and cloned sequentially into pT5 as an in-frame dimer with a restriction enzyme site in the middle. Each sequence was verified by sequencing both strands of the dimeric DNA molecules. Expression of each peptide in transformed E. coli JM105 was detected by SDS-PAGE analysis as described above.
Purification of recombinant multivalent vaccine component fusion proteins.
Each component fusion protein was purified separately. Cell paste of E. coli JM105 expressing recombinant His-tagged protein was lysed in phosphate-buffered saline (PBS) by Microfluidation (Microfluidics, Inc., Newton, Mass.). After centrifugation, the clarified lysate was batch adsorbed to nickel-loaded affinity chelate resin (Tosoh Biosep, Montgomeryville, Pa.), washed, and eluted with a step gradient of imidazole in PBS. Fractions containing the eluted recombinant protein were pooled, pumped through a 5-ml HiTrap Q anion-exchange cartridge (Amersham Pharmacia Biotech, Piscataway, N.J.), and concentrated in a stir cell (Millipore, Bedford, Mass.). The concentrated recombinant protein was further purified by size exclusion chromatography with a Superdex 200 column (600-cm length; Amersham Pharmacia Biotech) equilibrated with PBS. Fraction purity was monitored by SDS-PAGE, and fractions containing pure recombinant protein were pooled and stored at −20°C until use. The purity, identity, and concentration of each fusion protein were further assessed by reverse-phase high-performance liquid chromatography, amino acid analysis, and electrospray mass spectrometry.
Purification of individual recombinant dimeric M peptides.
E. coli JM105 expressing recombinant His-tagged protein was lysed in PBS containing 8 M urea for 3 h with stirring. After centrifugation, the clarified lysate was batch adsorbed to nickel-loaded affinity chelate resin (Tosoh Biosep) and washed and eluted with a step gradient of imidazole in PBS. The eluted protein was then loaded onto a preparative reverse-phase C4 column (Vydac, Hesperia, Calif.), washed, and eluted with increasing concentrations of acetonitrile in water containing 0.1% trifluoroacetic acid. Fractions of eluted protein were monitored by SDS-PAGE. Fractions containing purified protein were pooled and dialyzed against PBS before storage at −20°C.
Vaccine formulation and immunization of rabbits.
The four vaccine polypeptides A.1, B.2, C.2, and D.1 were mixed in equimolar amounts and adsorbed to alum (Rehydragel, low viscosity; Reheis, Inc., Berkeley Heights, N.J.) to achieve a final protein concentration of 800 μg/ml and a final alum concentration of 1.5 mg/ml in PBS. New Zealand White rabbits were each immunized with 400 μg of the vaccine via the intramuscular route at either 0, 4, and 8 weeks or 0, 4, and 16 weeks (12). Serum was obtained prior to the first injection and 2 weeks after the final injection.
Detection of type-specific antibodies.
Enzyme-linked immunosorbent assays (ELISAs) were performed with preimmune and immune rabbit sera by methods previously described (33). The purified recombinant dimeric M peptides comprising the vaccine subunits were used as solid-phase antigens.
Opsonization assays and indirect bactericidal tests.
Opsonic antibodies were detected by in vitro opsonization assays as previously described (6). The test mixture consisted of 0.05 ml of a standard suspension of streptococci grown to mid-log phase, 0.05 ml of test serum, and 0.2 ml of whole, heparinized (10 U/ml), nonimmune human blood. For these assays, the number of streptococcal CFU per leukocyte was approximately 10. The tubes were rotated end over end for 45 min at 37°C. Smears were then made on glass slides and stained with Wright's stain (Sigma Diagnostics, St. Louis, Mo.). Opsonization was quantitated by counting 50 consecutive neutrophils and calculating the percentage with associated streptococci (percent opsonization).
Bactericidal assays were performed similarly (30), except that 0.05 ml of Todd-Hewitt broth containing fewer bacteria was added to 0.1 ml of test serum and 0.35 ml of blood and the mixture was rotated for 3 h at 37°C. Then, 0.1-ml aliquots of this mixture were added to melted sheep blood agar, pour plates were prepared, and viable organisms (CFU) were counted after overnight incubation at 37°C. For each serotype tested, three different inocula were used to ensure that the growth in blood containing preimmune serum was optimal and quantifiable. The results were expressed as percent killing, which was calculated by using the following formula: {[(CFU after 3 h of growth with preimmune serum) − (CFU after 3 h of growth with immune serum)] ÷ CFU after 3 h of growth with preimmune serum} × 100. Only those assays that resulted in growth of the test strain for at least eight generations in the presence of preimmune serum were used to express percent killing in the presence of immune serum.
Assays for tissue-cross-reactive antibodies.
Immune sera were tested for the presence of tissue-cross-reactive antibodies by indirect immunofluorescence assays using frozen sections of human heart, brain, kidney, and cartilage by methods previously described (15).
RESULTS
Surveillance data supporting the selection of group A streptococcal serotypes for the 26-valent vaccine.
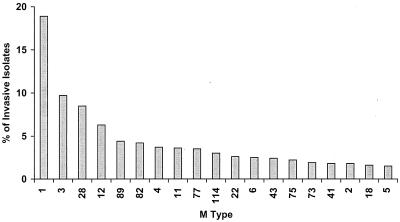
The multivalent group A streptococcal vaccine contains amino-terminal regions of 26 different M types. The selection of M types for inclusion in the 26-valent vaccine was based on epidemiologic data from several sources. The overall goal was to include the majority of serotypes that are common causes of streptococcal pharyngitis, those serotypes that are frequently isolated from cases of invasive disease, and the serotypes that are either currently associated or have historically been associated with ARF. The most comprehensive data on M types responsible for invasive streptococcal disease have been provided by the Active Bacterial Core Surveillance of the Emerging Infections Program Network supported by the CDC. The most recent sequence analysis of the emm genes from 1,922 invasive strains of group A streptococci submitted in the years 1998 to 2000 showed that 19 deduced serotypes accounted for 84% of the total isolates (Fig. 2). In addition, very little sequence variation was observed within the type-specific regions of the majority of these types. A comparison of the serotypes selected for inclusion in the 26-valent vaccine (Fig. 1) with all of the M types identified in the surveillance program showed that the vaccine serotypes accounted for 78% of invasive infections.
FIG. 2.
Summary of the 19 most common invasive emm types of group A streptococci isolated in the United States between 1998 and 2000. These data are part of ongoing studies conducted by the Active Bacterial Core Surveillance Program of the CDC. The 19 emm-types accounted for 84% of the 1,922 invasive isolates submitted during this period of time.
There is also an ongoing study to determine the serotype distribution of group A streptococci recovered from pediatric cases of pharyngitis in the United States (directed by S. T. Shulman and R. R. Tanz, Northwestern University Medical School, Chicago, Ill., and B. Beall, CDC, Atlanta, Ga.). More than 800 clinical isolates were submitted for emm typing from nine primary-care pediatric sites between the fall of 2000 and the spring of 2001. Sixteen different serotypes accounted for 97% of the pharyngitis-related isolates submitted. It is noteworthy that the serotypes contained in the 26-valent vaccine accounted for 80% of the cases of acute pharyngitis (B. Beall, personal communication).
The vaccine was also designed to contain protective M peptides from serotypes of group A streptococci that have been epidemiologically linked to ARF (9). Some of these serotypes have recently been associated with ARF, such as types 18 (38, 39) and 5 (25), while others, such as types 19 and 24, are rarely encountered today but have historically been characterized as rheumatogenic strains (9).
Immunogenicity of the 26-valent vaccine in rabbits.
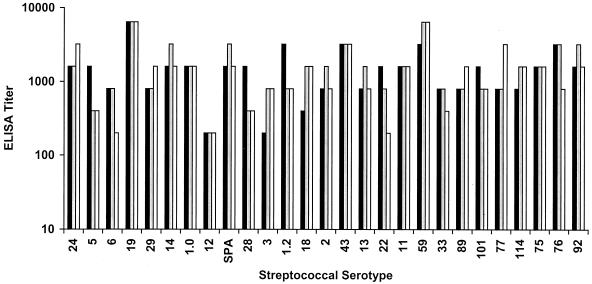
The component fusion proteins of the vaccine (Fig. 1) were purified separately, mixed together in equimolar concentrations, and formulated with alum to contain 400 μg of protein and 750 μg of alum in each 0.5-ml dose. Initial experiments were performed to determine the immunogenicity of the vaccine in groups of three rabbits that each received three intramuscular injections of the vaccine at either 0, 4, and 8 weeks or 0, 4, and 16 weeks. ELISAs were performed with preimmune sera and immune sera (obtained 2 weeks after the final injection) against the purified recombinant dimeric peptide components of the vaccine. The immune sera from rabbits immunized at 0, 4, and 16 weeks contained high titers of antibodies against the majority of the M peptides contained in the vaccine (Fig. 3). Antibody titers were determined for each of the 26 M peptides and Spa, a new protective antigen of group A streptococci (19). All preimmune titers were less than 200. Of the 81 immune serum titers determined (27 antigens times three rabbits), 69 (85%) showed fourfold or greater increases over the preimmune levels (Fig. 3). By the same criteria, the sera obtained from the rabbits immunized at 0, 4, and 8 weeks showed that 67 (83%) of the 81 immune titers showed fourfold or greater increases over preimmune levels (data not shown). Because there was no significant difference in the results obtained with the two immunization schedules, we chose to perform a detailed analysis of the immune sera from rabbits immunized at 0, 4, and 16 weeks.
FIG. 3.
Type-specific M protein antibodies evoked by the 26-valent vaccine in rabbits, as determined by ELISA. Three rabbits (represented by the three bars) were each immunized with 400 μg of the 26-valent vaccine at 0, 4, and 16 weeks. ELISAs were performed with preimmune and immune sera (18 weeks) and purified recombinant dimeric peptides copying each M protein fragment (and Spa) included in the vaccine. Antibody titers of <200 were observed for all preimmune sera when tested against each of the antigens. Two of the immune sera contained significant antibody levels (serum antibody titers that increased by fourfold or greater over preimmune levels) against 24 of the 27 vaccine antigens, while the third immune serum contained significant antibody levels against 21 of the 27 antigens.
Opsonic and bactericidal antibodies evoked by the 26-valent vaccine.
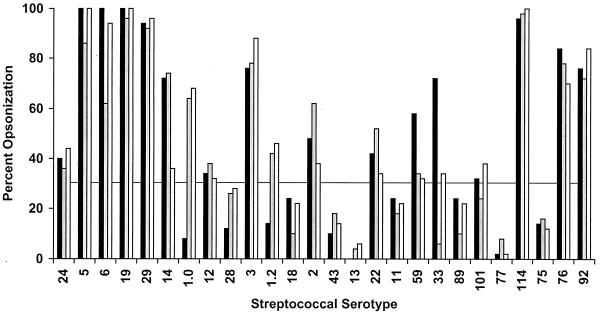
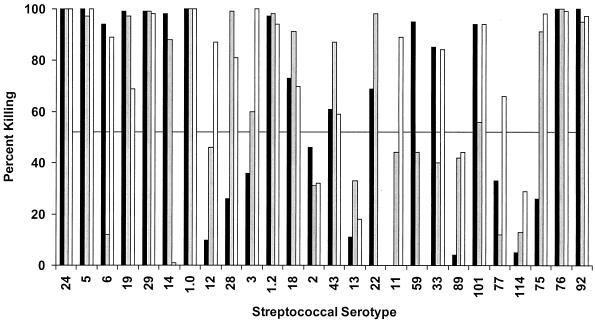
To determine the functional activity of the M protein antibodies evoked by the 26-valent vaccine, in vitro opsonization and bactericidal tests were performed by using each of the 26 serotypes of group A streptococci (Fig. 4 and 5). Opsonization assays were designed to determine the percentage of neutrophils that engulfed or were associated with streptococci after rotation in nonimmune blood that contained either preimmune or immune rabbit serum (Fig. 4). The preimmune sera from all three rabbits resulted in ≤10% opsonization of each of the 26 serotypes tested (data not shown), indicating that the donor blood used for these assays did not contain antibodies against the test organism and that each organism was fully resistant to opsonization in nonimmune blood. When 30% opsonization in the presence of immune serum (or three or more times the preimmune level) was used as a positive threshold result, 18 (69%) of the 26 serotypes were opsonized by at least one of three immune rabbit sera (Fig. 4).
FIG. 4.
Serum opsonic antibodies evoked in rabbits by the 26-valent vaccine. Immune sera from each of three rabbits (represented by the three bars) were tested in in vitro opsonization assays using the 26 different serotypes of group A streptococci. Percent opsonization is the percentage of neutrophils that had engulfed or were associated with streptococci after rotating for 45 min in whole, nonimmune human blood containing either preimmune or immune rabbit serum. The preimmune sera resulted in ≤10% opsonization with each organism. Thirty percent or greater opsonization (a threefold increase) in the presence of immune sera was considered a positive response.
FIG. 5.
Serum bactericidal antibodies evoked in rabbits by the 26-valent vaccine. Immune sera from each of three rabbits (represented by the three bars) were tested for bactericidal activity against the 26 different serotypes of group A streptococci. Growth of each test organism during 3 h of rotation in nonimmune human blood containing preimmune rabbit serum equaled or exceeded eight generations. Percent reduction in growth in the presence of immune serum is indicated as percent killing. A positive response was considered percent killing equal to or greater than 50%.
Bactericidal activity assays were also performed as an additional measurement of the potential protective efficacy of the 26-valent vaccine (Fig. 5). In these assays, each of the 26 serotypes of group A streptococci was rotated in nonimmune blood for 3 h in the presence of either preimmune or immune rabbit serum. In all of the experiments, the test mixture containing preimmune serum resulted in growth of the organisms for eight or more generations (data not shown), again indicating that the human blood did not contain opsonic antibodies against the test strains and that each organism was fully resistant to bactericidal killing in nonimmune blood. When a 50% reduction in growth (percent killing) after the 3-h rotation in immune serum compared to the preimmune serum was used as a positive threshold, bactericidal activity against 22 of the 26 serotypes tested was observed (Fig. 5). When the results of the opsonization and bactericidal activity assays were combined, 24 (92%) of the 26 serotypes tested were opsonized by the immune sera in one or both assays. Although the immune sera resulted in low levels of opsonization or bactericidal activity against type 13 and 89 streptococci, these two serotypes did not reach the positivity threshold in either assay.
Assays for tissue-cross-reactive antibodies evoked by the 26-valent vaccine.
To detect tissue-cross-reactive antibodies, indirect immunofluorescence assays were performed with all six immune rabbit sera and frozen sections of human heart, kidney, brain, or cartilage (15). None of the immune sera contained antibodies that cross-reacted with any of the human tissues tested, indicating that the vaccine did not contain potentially harmful autoimmune epitopes.
Bactericidal activity of the 26-valent antisera against type 4 streptococci.
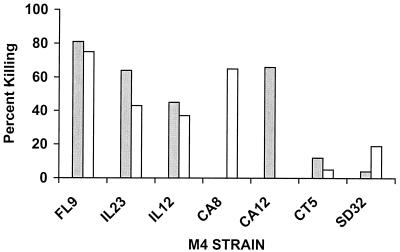
Type 4 streptococci are relatively common causes of uncomplicated pharyngitis and invasive infections. To our knowledge, a protective antigen has not been identified on the surface of this serotype. Purified recombinant type 4 M protein does not evoke opsonic antibodies (unpublished data). For this reason, an emm4 gene fragment was not included in the 26-valent vaccine. To determine whether any of the antibodies evoked by the 26-valent vaccine might be directed against cross-reactive opsonic epitopes on the surface of type 4 streptococci, we performed bactericidal activity assays with seven clinical isolates obtained from the ongoing U.S. Streptococcal Pharyngitis Surveillance Program (Fig. 6). Interestingly, bactericidal activity against five of the seven strains of type 4 streptococci was detected. The strains isolated from patients in Florida (FL9) and Illinois (IL23 and IL12) were opsonized by both of the 26-valent antisera tested. The two isolates from California (CA8 and CA12) were opsonized by one antiserum but not the other, and the strains from Connecticut (CT5) and South Dakota (SD32) were not opsonized by either antiserum (Fig. 6). The results suggest that the 26-valent vaccine may evoke antibodies that cross-react with protective epitopes on the surface of some strains of type 4 streptococci. In addition, the data indicate that type 4 streptococci may be a heterogeneous group of organisms that express different protective epitopes even though they all contain the type-specific emm4 gene.
FIG. 6.
Serum bactericidal antibodies against type 4 group A streptococci elicited in rabbits by the 26-valent vaccine. Two antisera (represented by the two bars) were tested for in vitro bactericidal activity against seven different clinical pharyngeal isolates of type 4 streptococci. One of the antisera was from a rabbit immunized at 0, 4, and 8 weeks (shaded bar), and the other was from a rabbit immunized at 0, 4, and 16 weeks (open bar). The M4 isolates were from the following five geographic locations: FL, Florida; IL, Illinois; CA, California; CT, Connecticut; SD, South Dakota. Percent killing was determined as described in the legend to Fig. 5.
DISCUSSION
The fundamental basis supporting the rationale for M protein-based group A streptococcal vaccines was, in large part, established by the seminal work of Rebecca Lancefield (22, 28-31). She first defined the type specificity of protective antibodies evoked by group A streptococci in 1919 (22). She later identified the type-specific substance as M protein in hot acid extracts of virulent organisms (28). Subsequent studies in the laboratories of E. H. Beachey (4, 5, 34) and V. A. Fischetti (23, 32) determined the amino acid sequences of several M proteins, which revealed the hypervariable structures of the amino-terminal regions that contained the type-specific, protective epitopes (2, 14, 17, 26, 27) defined by Lancefield decades earlier (22, 28). By focusing only on these defined regions of M proteins, the tissue-cross-reactive epitopes (1, 13, 15, 16, 35) could be excluded from multivalent vaccines. Recombinant technology has been used to construct multivalent vaccines containing four (18), six (12), and eight (21) M protein fragments linked in tandem. In the present study, we used the same techniques to produce a 26-valent vaccine.
The selection of M protein serotypes for inclusion in the expanded-valency vaccine was based on current epidemiologic studies of invasive disease and uncomplicated pharyngitis and historical information linking certain serotypes to the pathogenesis of ARF (9, 25, 38). The overall goal is to develop a vaccine that will have a significant impact on the total disease burden caused by group A streptococci in North America and western Europe. There is considerable overlap between the serotypes that cause invasive disease and those that are prevalent in a population and cause uncomplicated infections (25). Thus, approximately 80% of the serotypes identified in current epidemiologic studies are included in the 26-valent vaccine. In addition, certain serotypes, such as M19 and M24, that have historically been identified as rheumatogenic (9) are also included even though they are not present in the population today. This decision was based on the assumption that new serotypes could emerge under the immunologic pressure of a multivalent vaccine. If so, it would be desirable that the emerging serotypes have little or no rheumatogenic potential. Thus, the selection of serotypes was based on current data and a historical perspective that took into consideration projections about future epidemiologic trends that may occur under the influence of vaccine pressure.
In the present study, rabbits were immunized with three doses of 400 μg of the 26-valent vaccine. The vaccine contained a total of 31 peptides comprising 26 different M peptides, 1 Spa peptide, and 4 C-terminal peptides that were copies of the N-terminal components. Thus, each 400-μg dose contained the equivalent of approximately 12.9 μg of each component peptide. Based on weight, this is comparable to the adult formulation of recombinant hepatitis B vaccine, which contains up to 20 μg of protein per dose, or the acellular pertussis vaccine, which contains up to 25 μg of purified pertussis toxin per dose. Peptides included in the 26-valent vaccine represent only minimal fragments of M proteins that elicit opsonic antibodies. This approach, together with the fact that each component fusion protein serves as its own carrier, enhances the possibility that the majority of the antibodies elicited will be functionally active.
The immunogenicity of the 26-valent vaccine was assessed by ELISA, opsonization assays, and bactericidal activity tests. The ELISA was performed with individual recombinant M protein and Spa protein fragments included in the component fusion proteins of the vaccine. At least one of the three immunized rabbits developed significant levels of antibodies (a fourfold or greater titer increase) against 25 of the 26 type-specific antigens. Opsonization and indirect bactericidal activity assays were performed to determine the functional activity of the antisera by using each of the 26 different serotypes of group A streptococci. For the most part, there was a very good correlation among the results of all three assays. There was a direct correlation between the results of opsonization and bactericidal activity assays with 20 of the 26 serotypes tested. Interestingly, the majority of the serotypes that yielded inconsistent results were serum opacity factor-positive strains. For example, type 114 was strongly opsonized by all three immune rabbit sera yet the same sera resulted in little or no bactericidal activity. Conversely, type 75 streptococci were not opsonized by any of the three sera yet two of the antisera were strongly bactericidal against this serotype. The reasons for the occasional inconsistencies between the two assays is not clear, but we concluded that an overall assessment of the potential efficacy of M protein-based vaccines should be based on the results of both assays performed with the same immune sera.
The predicted potential efficacy of the 26-valent vaccine, as determined by the M types included in the vaccine and their frequency in the population, is reduced by the fact that a protective antigen of type 4 streptococci is not part of this construct. Type 4 organisms currently account for 3.7% of all invasive infections (Fig. 2) and 9.5% of all pharyngitis isolates in the ongoing U.S. surveillance program (B. Beall, personal communication). Studies are currently ongoing in our laboratories to identify the protective antigen(s) on the surface of type 4 streptococci. Of considerable interest was our finding that the 26-valent rabbit antisera showed bactericidal activity against some strains of type 4 streptococci. Cross-opsonic antibodies have recently been demonstrated in animals immunized with amino-terminal peptides of M proteins from strains of group A streptococci isolated from patients in the Northern Territory of Australia (10). A 20-amino-acid peptide from the amino terminus of one serotype of M protein evoked antibodies that opsonized a heterologous serotype. The cross-opsonic antibodies did not cross-react with the amino-terminal peptide of the heterologous M protein, indicating that the cross-opsonic antibodies may recognize epitopes in other regions of M or M-like proteins (10). In the present study, it is also possible that some of the antibodies evoked by amino-terminal peptides of M proteins cross-reacted with opsonic epitopes on serotypes not represented in the vaccine. Studies are currently in progress to determine the nonvaccine types that may be cross-opsonized and the vaccine peptides that may evoke cross-reactive antibodies. We are also conducting experiments to identify the serotypes that express Spa. If Spa is broadly distributed among multiple serotypes of group A streptococci, this may also enhance the cross-protective efficacy of the 26-valent vaccine. Results of these studies should provide a better understanding of the number of serotypes of group A streptococci that may be opsonized by antisera against the 26-valent vaccine.
In summary, we have shown that a highly complex 26-valent M protein-based vaccine is immunogenic in rabbits and evokes broadly opsonic antibodies against the majority of vaccine types. The vaccine was free of tissue-cross-reactive epitopes, as determined by indirect immunofluorescence assays. We believe that this vaccine has the potential to induce broadly protective antibody responses in humans that could have a significant impact on the overall disease burden caused by group A streptococci.
Acknowledgments
This work was support by National Institutes of Health grant AI-10085 and Merit Review funds from the Department of Veterans Affairs (J.B.D.).
We appreciate the expert technical assistance provided by Edna Chiang and Valerie Long and the expert secretarial assistance of Deborah Bueltemann in preparing the manuscript. We also appreciate the assistance of the members of the Active Bacterial Core Surveillance Program, part of the CDC Emerging Infections Program Network, specifically, Carolyn Wright for providing the calculations for the 1998 to 2000 surveillance data.
Editor: E. I. Tuomanen
REFERENCES
- 1.Baird, R. W., M. S. Bronze, W. Kraus, H. R. Hill, L. G. Veasey, and J. B. Dale. 1991. Epitopes of group A streptococcal M protein shared with antigens of articular cartilage and synovium. J. Immunol. 146:3132-3137. [PubMed] [Google Scholar]
- 2.Beachey, E. H., and J. M. Seyer. 1986. Protective and nonprotective epitopes of chemically synthesized peptides of the NH2-terminal region of type 6 streptococcal M protein. J. Immunol. 136:2287-2292. [PubMed] [Google Scholar]
- 3.Beachey, E. H., J. M. Seyer, and J. B. Dale. 1981. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature (London) 292:457-459. [DOI] [PubMed] [Google Scholar]
- 4.Beachey, E. H., J. M. Seyer, J. B. Dale, and D. L. Hasty. 1983. Repeating covalent structure and protective immunogenicity of native and synthetic polypeptide fragments of type 24 streptococcal M protein. J. Biol. Chem. 258:13250-13257. [PubMed] [Google Scholar]
- 5.Beachey, E. H., J. M. Seyer, and A. H. Kang. 1980. Primary structure of protective antigens of type 24 streptococcal M protein. J. Biol. Chem. 255:6284-6289. [PubMed] [Google Scholar]
- 6.Beachey, E. H., G. H. Stollerman, E. Y. Chiang, T. M. Chiang, J. M. Seyer, and A. H. Kang. 1977. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of the type 24 M antigen. J. Exp. Med. 145:1469-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beall, B., R. Facklam, T. Hoenes, and B. Schwartz. 1997. Survey of emm gene sequences and T-antigen types from systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J. Clin. Microbiol. 35:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beall, B., R. Facklam, and T. Thompson. 1995. Sequencing emm-specific polymerase chain reaction products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisno, A. L. 1980. The concept of rheumatogenic and nonrheumatogenic group A streptococci, p. 789-803. In S. E. Reed and J. B. Zabriskie (ed.), Streptococcal diseases and the immune response. Academic Press, Inc., New York, N.Y.
- 10.Brandt, E. R., K. S. Sriprakash, R. I. Hobb, W. A. Hayman, W. Zeng, M. R. Batzloff, D. C. Jackson, and M. F. Good. 2000. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nat. Med. 6:455-459. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale, J. B. 1999. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 17:193-200. [DOI] [PubMed] [Google Scholar]
- 13.Dale, J. B., and E. H. Beachey. 1985. Epitopes of streptococcal M proteins shared with cardiac myosin. J. Exp. Med. 162:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale, J. B., and E. H. Beachey. 1986. Localization of protective epitopes of the amino terminus of type 5 streptococcal M protein. J. Exp. Med. 163:1191-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale, J. B., and E. H. Beachey. 1985. Multiple heart-cross-reactive epitopes of streptococcal M proteins. J. Exp. Med. 161:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale, J. B., and E. H. Beachey. 1986. Sequence of myosin-cross-reactive epitopes of streptococcal M protein. J. Exp. Med. 164:1785-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale, J. B., and E. C. Chiang. 1995. Intranasal immunization with recombinant group A streptococcal M protein fragment fused to the B subunit of Escherichia coli labile toxin protects mice against systemic challenge infections. J. Infect. Dis. 171:1038-1041. [DOI] [PubMed] [Google Scholar]
- 18.Dale, J. B., E. Y. Chiang, and J. W. Lederer. 1993. Recombinant tetravalent group A streptococcal M protein vaccine. J. Immunol. 151:2188-2194. [PubMed] [Google Scholar]
- 19.Dale, J. B., E. Y. Chiang, S. Y. Liu, H. S. Courtney, and D. L. Hasty. 1999. New protective antigen of group A streptococci. J. Clin. Investig. 103:1261-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale, J. B., J. M. Seyer, and E. H. Beachey. 1983. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J. Exp. Med. 158:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale, J. B., M. Simmons, E. C. Chiang, and E. Y. Chiang. 1996. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 14:944-948. [DOI] [PubMed] [Google Scholar]
- 22.Dochez, A. R., O. T. Avery, and R. C. Lancefield. 1919. Studies on the biology of Streptococcus. J. Exp. Med. 30:179-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischetti, V. A., K. F. Jones, B. N. Manjula, and J. R. Scott. 1984. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification and comparison with streptococcal-derived M protein. J. Exp. Med. 159:1083-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopp, T. P., and K. R. Woods. 1983. A computer program for predicting protein antigenic determinants. Mol. Immunol. 20:483-489. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, D. R., D. L. Stevens, and E. L. Kaplan. 1992. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J. Infect. Dis. 166:374-382. [DOI] [PubMed] [Google Scholar]
- 26.Jones, K. F., and V. A. Fischetti. 1988. The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis of group A M6 streptococci. J. Exp. Med. 167:1114-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, K. F., B. N. Manjula, K. H. Johnston, S. K. Hollingshead, J. R. Scott, and V. A. Fischetti. 1985. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J. Exp. Med. 161:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancefield, R. C. 1928. The antigenic complex of Streptococcus haemolyticus. 1. Demonstration of type-specific substance in extracts of Streptococcus haemolyticus. J. Exp. Med. 47:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lancefield, R. C. 1962. Current knowledge of the type specific M antigens of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 30.Lancefield, R. C. 1957. Differentiation of group A streptococci with a common R antigen into three serologic types with special reference to the bactericidal test. J. Exp Med. 106:525-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancefield, R. C. 1959. Persistence of type-specific antibodies in man following infection with group A streptococci. J. Exp. Med. 110:271-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manjula, B. N., A. Seetharma-Acharya, S. M. Mische, T. Fairwell, and V. A. Fischetti. 1984. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J. Biol. Chem. 259:3686-3693. [PubMed] [Google Scholar]
- 33.McLellan, D. G., E. Y. Chiang, H. S. Courtney, D. L. Hasty, S. C. Wei, M. C. Hu, M. A. Walls, J. J. Bloom, and J. B. Dale. 2001. Spa contributes to the virulence of type 18 group A streptococci. Infect. Immun. 69:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, L., L. Gray, E. H. Beachey, and M. Kehoe. 1988. Antigenic variation among group A streptococcal M proteins: nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding type 1, 6 and 24 M proteins. J. Biol. Chem. 263:5668-5673. [PubMed] [Google Scholar]
- 35.Sargent, S. J., E. H. Beachey, C. E. Corbett, and J. B. Dale. 1987. Sequence of protective epitopes of streptococcal M proteins shared with cardiac sarcolemmal membranes. J. Immunol. 139:1285-1290. [PubMed] [Google Scholar]
- 36.Schuchat, A., T. Hilger, E. Zell, M. M. Farley, A. Reingold, L. Harrison, L. Lefkowitz, R. Danila, K. Stefonek, N. Barrett, D. Morse, and R. Pinner. 2001. Active bacterial core surveillance of the emerging infections program network. Emerg. Infect. Dis. 7:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens, D. L. 1999. The flesh-eating bacterium: what's next? J. Infect. Dis. 179:366-374. [DOI] [PubMed] [Google Scholar]
- 38.Stollerman, G. H. 1997. Rheumatic fever. Lancet 349:935-942. [DOI] [PubMed] [Google Scholar]
- 39.Veasey, L. G., L. Y. Tani, and H. R. Hill. 1994. Persistence of acute rheumatic fever in the intermountain area of the United States. J. Pediatr. 124:9-16. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. 1986. Community prevention and control of cardiovascular diseases. Report of a W. H. O. expert committee, p. 732. World Health Organization, Geneva, Switzerland. [PubMed]