Abstract
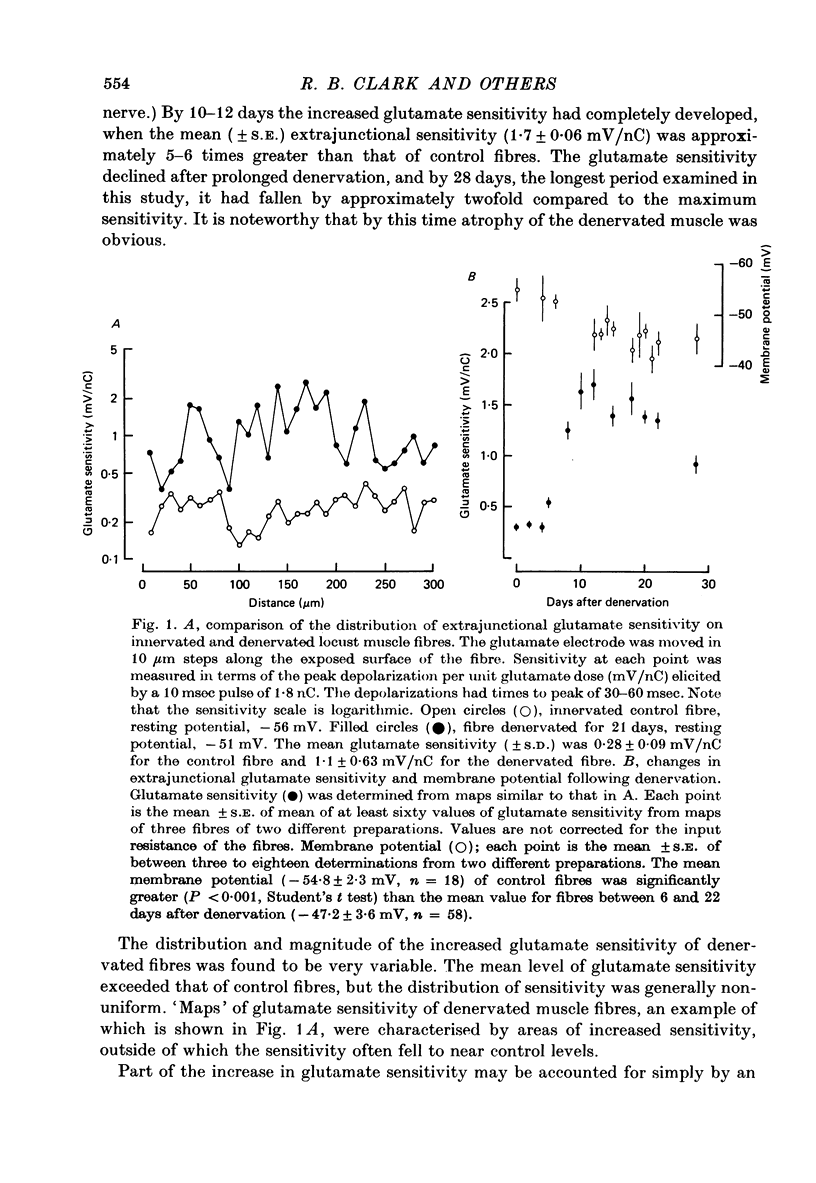
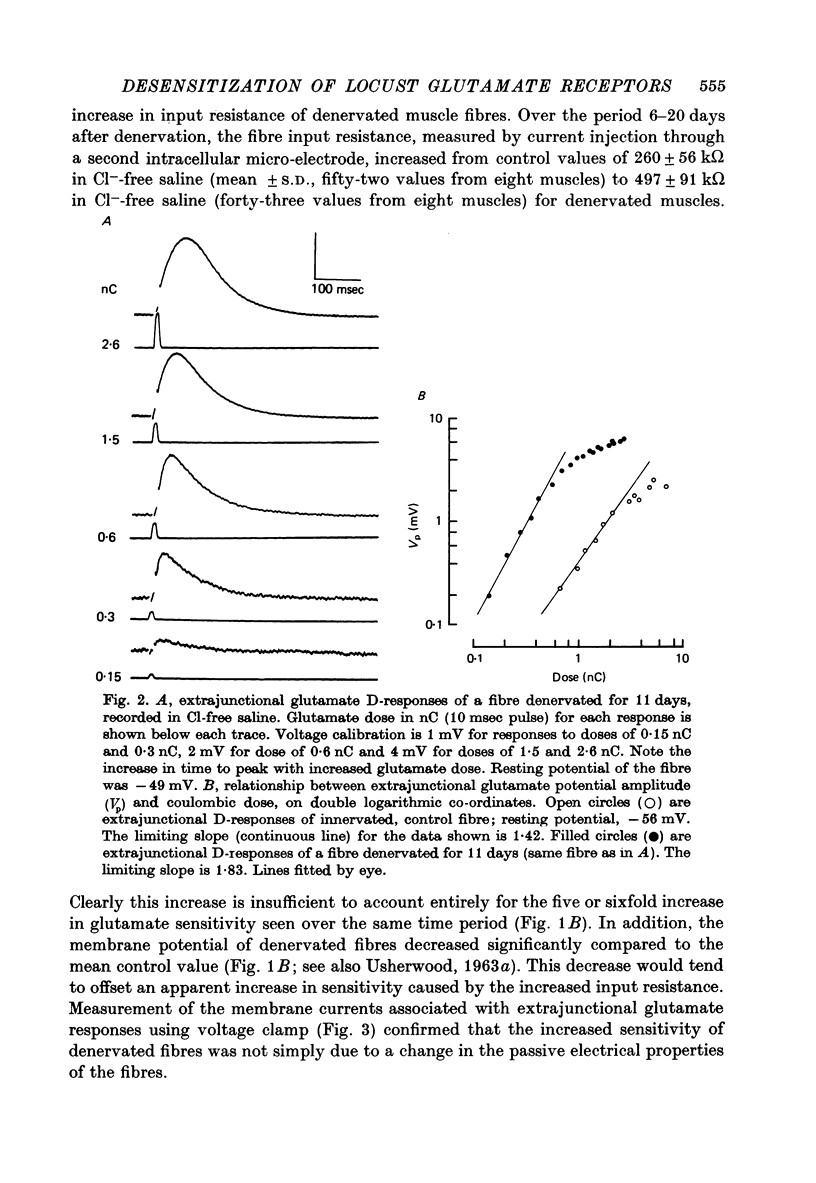
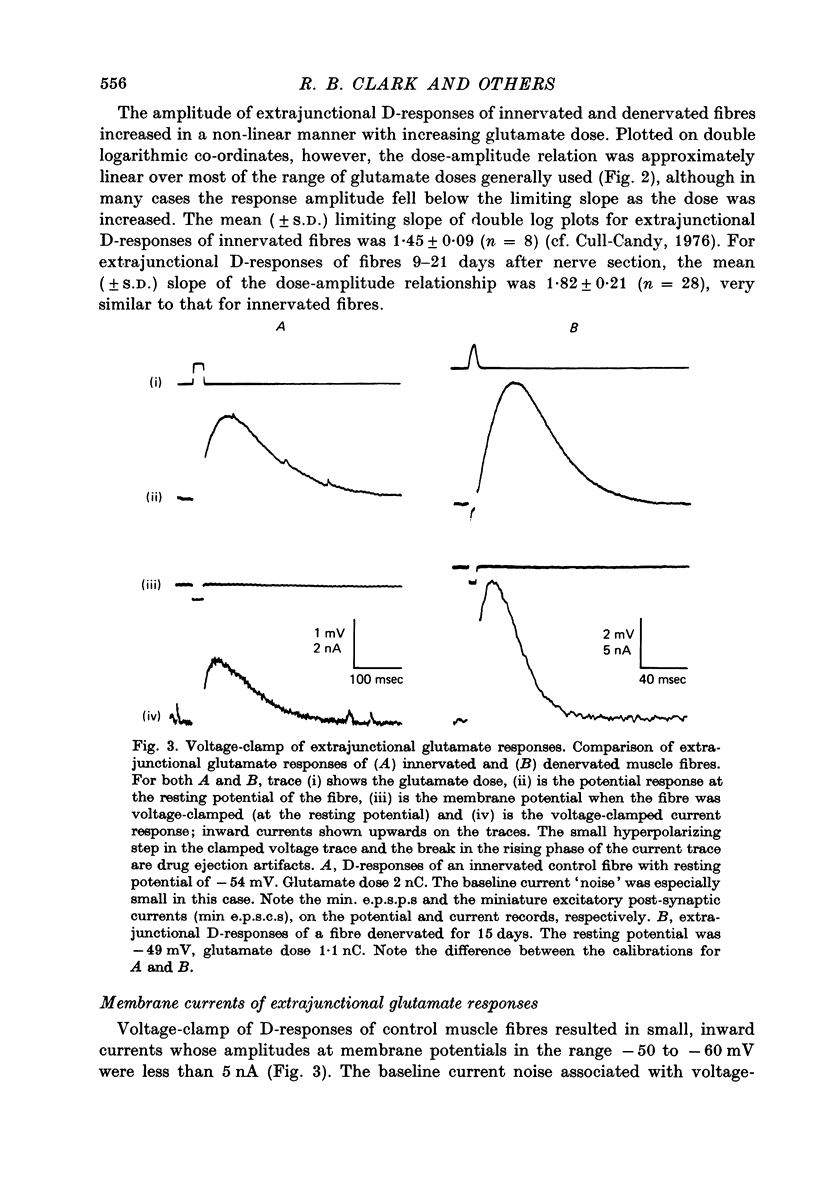
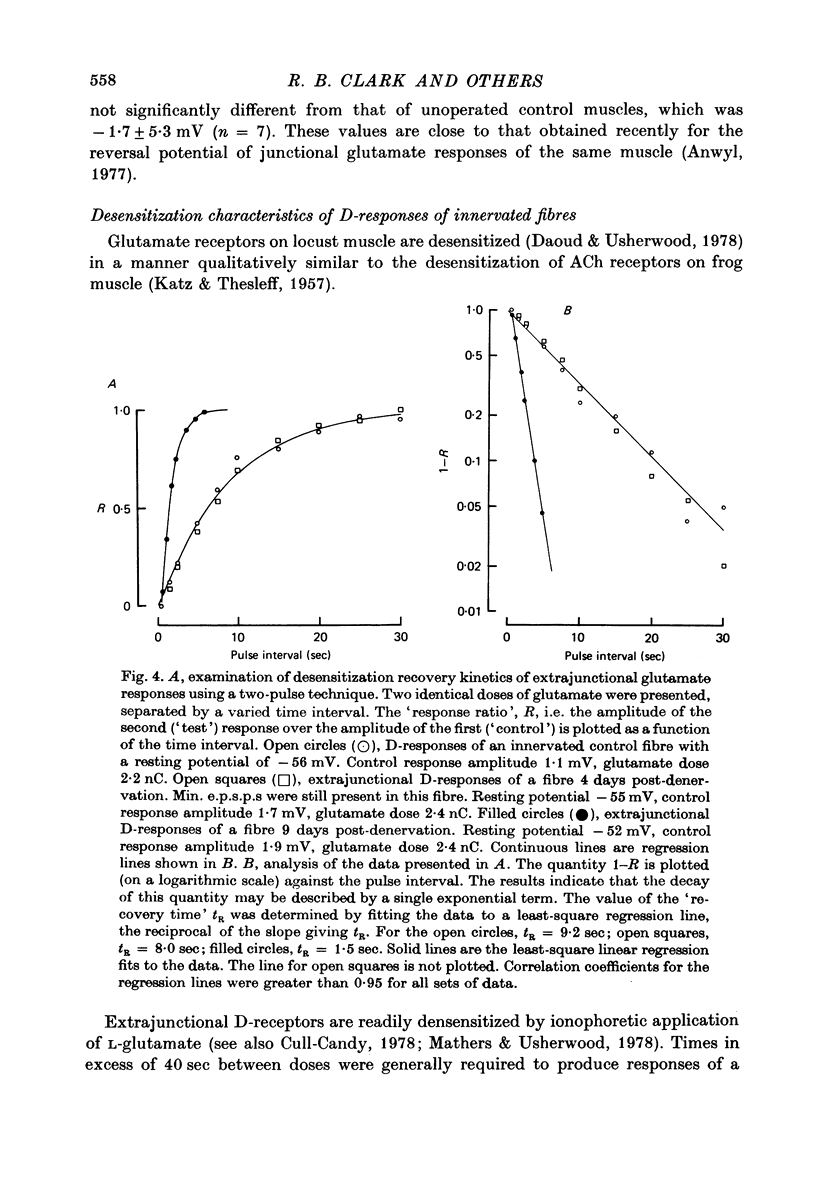
Depolarizations to L-glutamate, applied locally by microinophoresis to the extrajunctional membrane of locust extensor tibiae muscle fibres and measured either in current clamp or voltage clamp, increased in amplitude for equivalent doses of glutamate following chronic denervation of the muscle. 2. A two-pulse method was used to examine recovery from desentization of junctional and extrajunctional receptors. A 'response ratio', i.e. the amplitude of response to the second (test) of a pair of glutamate pulses over to the pulses. The 'response ratio' for extrajunctional depolarizations of innervated fibres increased exponentially with pulse interval, with a time constant of 15.6 +/- 4.7 sec (n = 11). Recovery of extrajunctional receptor populations from desensitization was accelerated after denervation. The recovery kinetics for responses from fibres 6-22 days after denervation were generally described by two exponential terms, with time constants in the range 0.5-10 sec which were inversely related to the glutamate sensitivity of the extrajunctional membrane. For junctional receptors on both innervated and denervated fibres the recovery kinetics were described by a single exponential with a time constant of 0.2-1 sec. 3. The results suggest that the increased extrajunctional glutamate sensitivity which occurs after denervation results from the 'appearance' of glutamate receptors with properties similar to those found at the post-junctional membrane on locust muscle fibres.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Permeability of the post-synaptic membrane of an excitatory glutamate synapse to sodium and potassium. J Physiol. 1977 Dec;273(2):367–388. doi: 10.1113/jphysiol.1977.sp012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Gration K. A., Usherwood P. N. Denervation-induced changes in extrajunctional glutamate responses of insect muscle [proceedings]. J Physiol. 1978 Mar;276:75P–75P. [PubMed] [Google Scholar]
- Cull-Candy S. G. Effect of denervation and local damage on extrajunctional L-glutamate receptors in locust muscle. Nature. 1975 Dec 11;258(5535):530–531. doi: 10.1038/258530a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Glutamate sensitivity and distribution of receptors along normal and denervated locust muscle fibres. J Physiol. 1978 Mar;276:165–181. doi: 10.1113/jphysiol.1978.sp012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G. Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J Physiol. 1976 Feb;255(2):449–464. doi: 10.1113/jphysiol.1976.sp011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usherwood P. N. Two populations of L-glutamate receptors on locust muscle fibres. Nat New Biol. 1973 Nov 14;246(150):62–64. doi: 10.1038/newbio246062a0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea T. J., Usherwood P. N. Effect of ibotenic acid on chloride permeability of insect muscle-fibres. Comp Gen Pharmacol. 1973 Dec;4(16):351–363. doi: 10.1016/0010-4035(73)90046-3. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Usherwood P. N. The sensitivity of locust skeletal muscle fibres to L-glutamate following denervation and injury. Comp Biochem Physiol C. 1978;60(1):7–10. doi: 10.1016/0306-4492(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Müller K. D. Analysis of cooperativity of drug-receptor interaction by quantitative iontophoresis at frog motor end plates. Cold Spring Harb Symp Quant Biol. 1976;40:187–192. doi: 10.1101/sqb.1976.040.01.020. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A., Zilber-Gachelin N. F. Further investigations on the effect of denervation and pH on the conductance change at the neuromuscular junction of the frog. Pflugers Arch. 1976 Jun 29;364(1):53–58. doi: 10.1007/BF01062911. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N., Cull-Candy S. G. Distribution of glutamate sensitivity on insect muscle fibres. Neuropharmacology. 1974 Jun;13(6):455–461. doi: 10.1016/0028-3908(74)90134-8. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N. Glutamate sensitivity of denervated insect muscle fibres. Nature. 1969 Jul 26;223(5204):411–413. doi: 10.1038/223411a0. [DOI] [PubMed] [Google Scholar]


