Abstract
Trichinella spiralis is an obligate parasite of animals that has an unusual intracellular life cycle. Investigation of parasitism at the cellular and molecular levels has been challenging because of a shortage of tools for in vitro cultivation of T. spiralis. We have found that T. spiralis larvae molt, ecdyse, develop to adulthood, and reproduce when they are inoculated onto cultured intestinal epithelial cells. Initially, larvae invade and migrate through cells in a monolayer (T. ManWarren, L. Gagliardo, J. Geyer, C. McVay, S. Pearce-Kelling, and J. Appleton, Infect. Immun. 65:4806-4812, 1997). During prolonged culture in Caco-2 epithelial cells, L1 larvae molted and ecdysed with efficiencies as high as 50%. Molting and ecdysis in vitro required entry of the parasite into cells; conditions that prevented entry into cells also prevented ecdysis. When larvae were inoculated at a low density and cultured for 5 to 9 days, as many as 50% of the larvae developed to adult stages. Low numbers of mature male worms with copulatory appendages were observed in these cultures. The majority of worms that survived for five or more days were unfertilized females. Low-density cultures supported development of female worms with embryos at rates of 4 to 5%. These results show that the intestinal life cycle of T. spiralis can be supported entirely by host epithelial cells. Our model should allow more detailed investigation of intracellular parasitism by T. spiralis.
Trichinella spiralis is an obligate parasite of animals that has an unusual intracellular life cycle. Infection occurs when a susceptible host ingests L1 larvae encysted in muscle. In the small intestine, T. spiralis molts four times in 30 to 40 h (2, 24). Little growth occurs during the four larval stages of T. spiralis, but a period of rapid growth occurs in adult stage females (2). Worms copulate within hours of the final ecdysis (39). Eggs hatch in utero, and female worms begin to release L1 larvae 4 days postinfection (20). Thus, the life cycle is completed in a single host.
Rats are natural hosts for T. spiralis and are important in the maintenance of this parasite in nature (25). Laboratory rodents have been invaluable for propagation of muscle stage larvae, adult worms, and newborn larvae for biochemical characterization. Furthermore, rodents have served as highly informative models for the study of parasitism and immunity against T. spiralis (reviewed by Bell [6]). Despite these advantages, investigation of parasitism by T. spiralis at the cellular and molecular levels has been challenging because of a shortage of tools for cultivation of the nematode in vitro.
When infectious larvae of T. spiralis are suspended in semisolid medium and inoculated onto monolayers of epithelial cells grown in vitro, the larvae invade cells, penetrate adjacent cells, and reside in the cytoplasm of the syncytia that they create (27). This mimics the location and activity of larvae in the intestinal epithelium of the animal host (36, 37). Recently, we reported that L1 larvae molt and ecdyse when they are inoculated into cultures of human colonic epithelial cell line Caco-2 (28). The aims of the present study were to extend these initial findings in order to further define the requirements for ecdysis and to establish culture conditions that support development, growth, and reproduction of T. spiralis.
MATERIALS AND METHODS
Cell culture.
Human colonic carcinoma cell line Caco-2 was obtained from the American Type Culture Collection (Rockville, Md.). Strain I of the Madin-Darby canine kidney (MDCK) cell line was a gift from William Young (University of Kentucky) (31). Cells were maintained in minimal essential medium (Earle's salts) (MEM) supplemented with sodium pyruvate, l-glutamine, nonessential amino acids (Life Technologies, Grand Island, N.Y.), and 10% fetal bovine serum (Gemini Bio-Products, Inc., Calabasas, Calif.) or in Dulbecco's MEM with the same supplements. Cells were harvested by trypsinization (0.5% trypsin, 0.65 mM EDTA) and passaged no more than 15 times before they were used in experiments.
Parasite.
T. spiralis (pig strain) infectious larvae were recovered from muscles of irradiated AO strain rats by digestion with 1% pepsin (12). The rats were housed in the James A. Baker Institute vivarium according to American Association for Accreditation of Laboratory Animal Care guidelines. The donor rats had been infected at least 28 days prior to collection. Adult worms were recovered from intestines of AO strain rats infected orally with L1 larvae. For in vitro experiments, L1 stage larvae were activated by using pig bile or gut contents as described by ManWarren et al. (27).
MAbs.
Rat monoclonal antibody (MAb) 18H (3) protects epithelial cells in vitro from infection with T. spiralis L1 larvae (28). The antibody binds to tyvelose-bearing N-glycans on L1 stage glycoproteins (15). Antibody was concentrated from ascites fluid (prepared in nude mice) or, for control immunoglobulin G from pooled normal rat sera, by (NH4)2SO4 precipitation. We have shown previously that this method yields very pure MAb from nude mouse ascites fluid (10). Protein concentrations were determined by measuring absorbance at 280 nm, using an extinction coefficient of 1.35.
Culture of parasites in vitro. (i) Molting and ecdysis.
Epithelial cells were grown to confluence in 12-well culture plates (Costar, Corning, Inc., Corning, N.Y.). Monolayers were overlaid with activated larvae suspended in Dulbecco's MEM supplemented with l-glutamine, nonessential amino acids, 15 mM HEPES, 10% fetal bovine serum, and 1.75% agarose (SeaPlaque low-temperature melting agarose; FMC Bioproducts, Rockland, Maine). Following incubation for 18 to 24 h (37°C, 8% CO2), larvae were recovered from cultures by adding 1 ml of 0.85% NaCl (37°C, 20 min), removing the agarose, and then washing the monolayers three times with saline. Monolayers with residual worms, as well as all parasites recovered by washing the agarose plug or the monolayer, were fixed with 2% formaldehyde and processed for fluorescent-antibody staining by using tyvelose-specific MAb 18H as previously described (27). Only L1 larvae display tyvelose on the body surface. Transition from one nematode larval stage to the next takes place in two steps. First, a larva synthesizes new cuticle or molts. Subsequently, the larva escapes the cuticle of the previous stage, a process called ecdysis or exsheathment. The percentage of larvae that were not fluorescent (i.e., had ecdysed) was determined by using an inverted microscope (Nikon Diaphot) equipped for epifluorescence (Opti-quip, Highland Mills, N.Y.).
(ii) Development and reproduction.
Cultures were established in 12- or 6-well culture plates or in petri dishes (diameter, 100 mm) as described above but were maintained for up to 11 days postinoculation. At various times, worms actively migrating in monolayers were counted in situ. These worms were parasitizing the epithelium and were considered successful parasites. Worms in agarose or in culture fluid were not counted as they did not occupy the epithelial habitat. For detailed microscopic examination of worms recovered from culture, parasites were fixed in 2% formaldehyde, cleared in 5% glycerol-70% ethanol, and mounted on glass slides in Glycergel mounting medium (DAKO Corporation, Carpinteria, Calif.).
Various treatments were tested to determine whether they enhanced or inhibited worm molting, ecdysis, and development. Media were supplemented with rat anti-tyvelose MAbs (described by McVay et al. [28]), human transforming growth factor β1 (TGF-β1), TGF-β2, antibodies specific for TGF-β (R & D Systems, Minneapolis, Minn.), ecdysone, 20-hydroxyecdysone (Sigma, St. Louis, Mo.), or rat intestinal contents (27).
Statistical analysis.
The numbers of larvae that ecdysed or were viable were estimated for three or four culture wells per treatment. Mean values for the treatment groups were compared by analysis of variance, and significant differences were identified by using Student's t test or Scheffé's mean separation test.
Images.
Photomicrography was performed with an Olympus AX70 microscope equipped with a Kodak DCS 460 digital camera. Images were prepared for printing with Adobe Photoshop 4.0. Photomicrographs were printed with a dye diffusion printer (NP-1600; Codonics, Middleburg Heights, Ohio).
RESULTS
Cells and agarose are required for ecdysis.
In preliminary experiments, we found that when L1 larvae were recovered from muscle by pepsin digestion, large numbers molted during incubation in liquid cell culture medium. Larvae in the process of ecdysis were observed only rarely under these conditions; occasionally larvae were observed trapped inside one or more sheaths. These observations are similar to those reported by other investigators (22, 23, 35). Subsequent treatment of pepsin-digested larvae with gut contents or bile (activation) did not promote ecdysis of larvae cultured in liquid medium (Table 1). In order to determine whether larvae could be induced to ecdyse by the presence of Caco-2 cells, we inoculated Caco-2 monolayers with activated larvae suspended in liquid medium. Larvae did not invade the monolayer and did not ecdyse (Table 1). In contrast, activated larvae suspended in semisolid medium invade cells and ecdyse (27, 28) (Table 1). Larvae that were suspended in agarose in the absence of cells did not ecdyse. These results indicate that ecdysis occurs only after the larvae enter an epithelial monolayer.
TABLE 1.
Ecdysis of T. spiralis larvae in vitro requires cells and semisolid medium but not serum
| Medium
|
No. of worms after 24 ha
|
||||||
|---|---|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
||||||
| Agarose | Liquid | Fetal bovine serum | Caco-2 cells | L1b | L2+c | L1b | L2+c |
| + | − | + | + | 52 ± 13 | 23 ± 5 | ||
| − | + | + | + | 120 ± 8 | 0 ± 0 | ||
| − | + | + | − | 114 ± 22 | 0 ± 0 | ||
| + | − | + | − | 61 ± 28 | 0 ± 0 | ||
| + | − | + | + | 93 ± 11 | 23 ± 12 | ||
| + | − | − | + | 96 ± 18 | 35 ± 9 | ||
Mean ± standard deviation based on three or four cultures per treatment.
First-stage larvae.
Second-, third-, or fourth-stage larvae.
We have reported previously that MDCK cells are susceptible to invasion by T. spiralis (27). Larval behavior in MDCK monolayers differs from the larval behavior observed in Caco-2 monolayers. Specifically, larvae spend more time on the surface and are more susceptible to antibody-mediated interference in MDCK monolayers than in Caco-2 monolayers (28). In order to determine whether these differences might extend to development, we compared the two cell lines in ecdysis assays. MDCK monolayers supported a much lower rate of ecdysis than Caco-2 monolayers supported (MDCK monolayers, 0.6% ± 0.6%; Caco-2 monolayers, 11% ± 3%; P < 0.0001). This suggests that prolonged occupation of cells by larvae is required for ecdysis or that MDCK cells are deficient in some factor required by the nematode for ecdysis.
Previous studies of T. spiralis development in vitro employed defined media supplemented with high concentrations of chicken embryo extract (7, 33). We tested the requirement for serum in ecdysis assays by using Caco-2 cells. Larvae ecdysed equally well in Caco-2 monolayers overlaid with serum-free medium and in Caco-2 monolayers overlaid with serum-supplemented medium (Table 1).
In an attempt to further mimic the intestinal environment, we added rat intestinal lumen contents (final concentration, 20, 4, or 0.8%; prepared as described by ManWarren et al. [27]) to agarose. Ecdysis was not improved significantly in cultures prepared with gut contents (ecdysis level in control, 20% ± 2%; ecdysis level in the presence of 20% gut contents, 25% ± 19%; ecdysis level in the presence of 4% gut contents, 28% ± 4%; ecdysis level in the presence of 0.8% gut contents, 18% ± 2.5%; P > 0.09 for all gut content groups compared with control, as determined by Scheffé's mean separation test).
20-Hydroxyecdysone is the major active molting hormone of insects. Ecdysone is the prohormone. Hitcho and Thorson (21) reported that supplementation of culture medium with ecdysteroid hormones significantly improved molting of T. spiralis. In our experiments, supplementation of culture media with either ecdysteroid hormone (0.1 to 10 μM) had no effect on ecdysis (data not shown).
TGF-β is a cytokine that is known to be influential in the intestine (reviewed by MacDonald and Monteleone [26]) and to bind specific receptors on the surface of the parasitic helminth Schistosoma mansoni (5). To test the effect of TGF-β on development of intestinal T. spiralis, we supplemented cultures with TGF-β1, TGF-β2 (2.5, 5, or 10 ng/ml), or anti-TGF-β (30 μg/ml). None of the treatments affected the number of larvae that ecdysed (data not shown).
Exclusion from cells by anti-tyvelose immunoglobulin G prevented ecdysis.
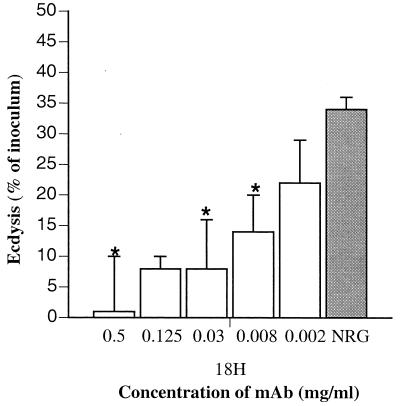
We have shown previously that antibodies specific for tyvelose prevent invasion of epithelial cells by T. spiralis L1 larvae in vivo and in vitro (10, 28). In Caco-2 cultures, high concentrations (>125 μg/ml) of MAb 18H prevent invasion entirely; lower concentrations cause larvae to become encumbered in the monolayer (28). When titrated for inhibition of ecdysis, MAb 18H was effective at a concentration as low as 8 μg/ml (Fig. 1). At this concentration the antibody encumbered larvae in the monolayer but did not prevent invasion. Thus, encumbrance or immobilization is sufficient to prevent ecdysis.
FIG. 1.
Anti-tyvelose MAb interferes with ecdysis by T. spiralis in Caco-2 cell cultures. MAb 18H was titrated in Caco-2 cell cultures inoculated with L1 larvae. Each antibody dilution was tested in triplicate (except 0.125 mg/ml, which was tested in duplicate). The control was 0.5 mg of normal rat globulin (NRG) per ml. The numbers of larvae that ecdysed during 18 to 24 h were determined as described in Materials and Methods and were expressed as percentages of the larvae inoculated. Data were analyzed by analysis of variance, and significant differences were determined by using Scheffé's test. Asterisks indicate that the mean was significantly different from the NRG control value (P < 0.05).
Survival and development of worms during prolonged culture.
The initial design of the ecdysis and development experiments was based on our experience with invasion assays. We chose an inoculum of 100 larvae per 3.8 cm2 (12-well culture plates; 26 larvae/cm2) based on the consistent efficiency of molting and ecdysis by larvae cultured at these densities. Between 1 and 9% (most often 3 to 4%) of the larvae survived and remained active in cell monolayers for 4 to 6 days (Table 2). The gender ratio was consistently biased towards female worms (Table 2). Fecund worms were rarely recovered from such cultures (data not shown).
TABLE 2.
Supplementation of culture medium with ecdysteroid hormones does not improve survival of worms with Caco-2 cells
| Treatment
|
% of viable worms aftera:
|
||||||
|---|---|---|---|---|---|---|---|
| 4 days
|
6 days
|
||||||
| Compound | Concn (M) | Male | Female | Total | Male | Female | Total |
| None | 0 ± 0 | 2 ± 1 | 3 ± 1 | 0 | 3 ± 2 | 3 ± 2 | |
| Ecdysone | 10−5 | 1 ± 1 | 8 ± 6 | 9 ± 7 | 0 | 3 ± 2 | 3 ± 2 |
| 10−6 | 0 | 5 ± 2 | 5 ± 2 | 0 | 4 ± 3 | 4 ± 2 | |
| 10−7 | 1 ± 1 | 5 ± 2 | 6 ± 3 | 0 | 1 ± 2 | 1 ± 2 | |
| 20-Hydroxyecdysone | 10−5 | 0 ± 0 | 5 ± 3 | 5 ± 3 | 0 | 4 ± 3 | 4 ± 3 |
| 10−6 | 1 ± 1 | 5 ± 1 | 6 ± 2 | 0 | 5 ± 2 | 5 ± 2 | |
| 10−7 | NDb | ND | ND | 0 ± 1 | 3 ± 3 | 4 ± 3 | |
Percentage of inoculum. The values are means ± standard deviations (n = 3). The inoculum was 108 larvae (4 days) or 97 larvae (6 days) per 3.8 cm2.
ND, not determined.
Supplementation of culture medium with ecdysteroid hormones (Table 2), with TGF-β, or with anti-TGF-β (data not shown) did not alter the survival or development of worms.
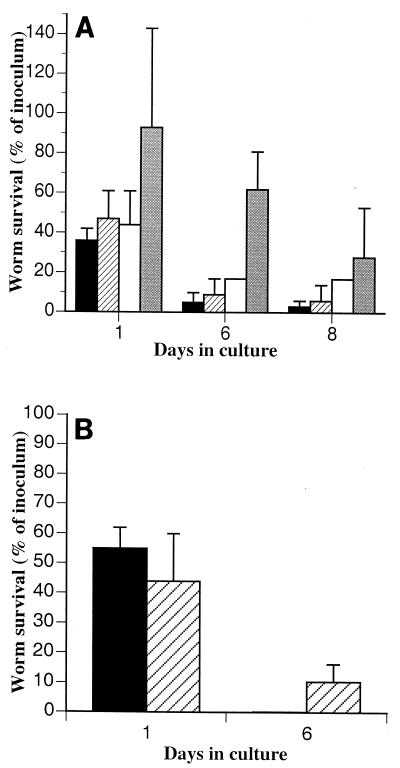
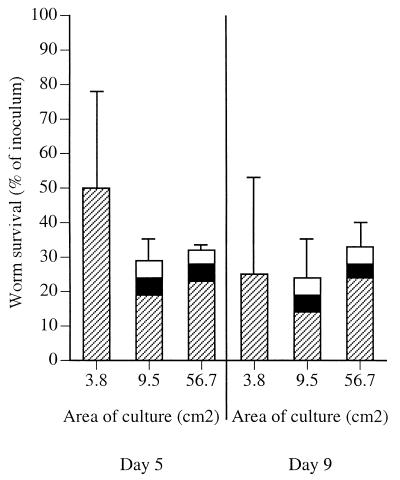
Reducing the size of the inoculum improved the efficiency with which worms survived and developed (Fig. 2A). When very few larvae were inoculated onto 3.8-cm2 surfaces (one or two larvae per cm2), the level of survival after 6 or 8 days in culture was more than 25%. In such low-density cultures male worms were rarely observed. When both sexes were present, the worms did not copulate. Similar results were obtained in 78.5-cm2 monolayers; that is, the survival rate improved in cultures with lower parasite densities compared with cultures with higher parasite densities (Fig. 2B). Maintaining a low density of parasites but using a larger monolayer (and thus more worms per culture) improved the fecundity (Fig. 3). During 5 days in culture, fecund females developed at rates of 4 to 5% in 9.1- and 78.5-cm2 monolayers. The numbers and fecundity of worms did not change between 5 and 8 days of culture.
FIG. 2.
In low-density cultures, fecundity increased with increasing number of worms. Larvae were inoculated at similar densities (average, 1.3 larvae/cm2) onto monolayers with different areas. Males (solid bars), nonfecund females (cross-hatched bars), and fecund females (open bars) were counted after 5 days of culture. The level of survival in each culture after 9 days was not significantly different from the level of survival after 5 days (data not shown).
FIG. 3.
Worm survival in Caco-2 monolayers improved as worm density decreased. (A) Larvae were inoculated onto 3.8-cm2 (12-well) monolayers at densities of 13 (solid bars), 6.6 (cross-hatched bars), 3 (open bars), and 1.6 (shaded bars) larvae per cm2 and cultured for 8 days. The mean numbers of worms counted in the monolayers after 1, 6, and 8 days were expressed as percentages of the inocula. Similar proportions of larvae invaded the monolayers and were counted after 1 day; however, survival after 6 days was inversely correlated with density. The standard deviation of the mean for 1.6 larvae/cm2 was high due to the low number of worms inoculated (six worms per culture). All but one of the worms counted were female. (B) Larvae were inoculated onto 78.5-cm2 (petri dish) monolayers at densities of 8.6 (solid bar) and 1.7 (cross-hatched bars) larvae per cm2 and cultured for 6 days. The numbers of worms that were active in the monolayers after 1 and 6 days were determined and expressed as percentages of the inocula. Similar proportions of larvae invaded the monolayers and were counted after 1 day; however, the survival of worms when the smaller inoculum was used was greater than the survival of worms when the larger inoculum was used. All of the worms observed in 6-day cultures were unfertilized females.
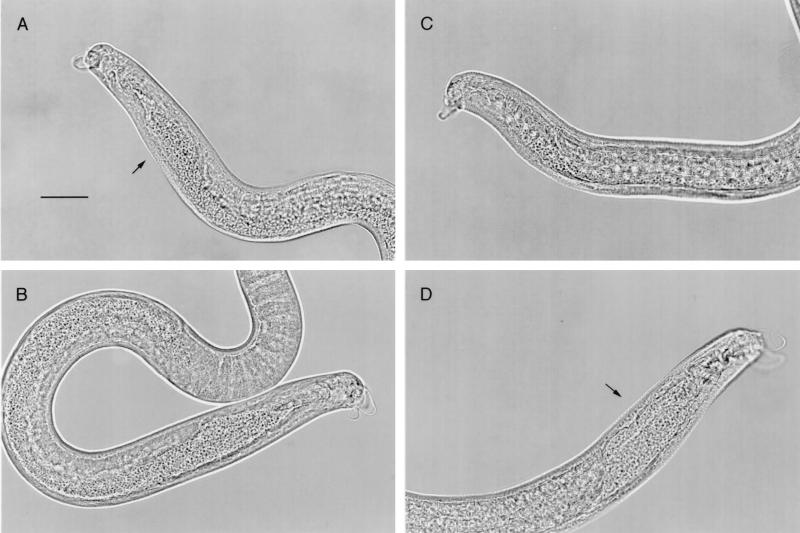
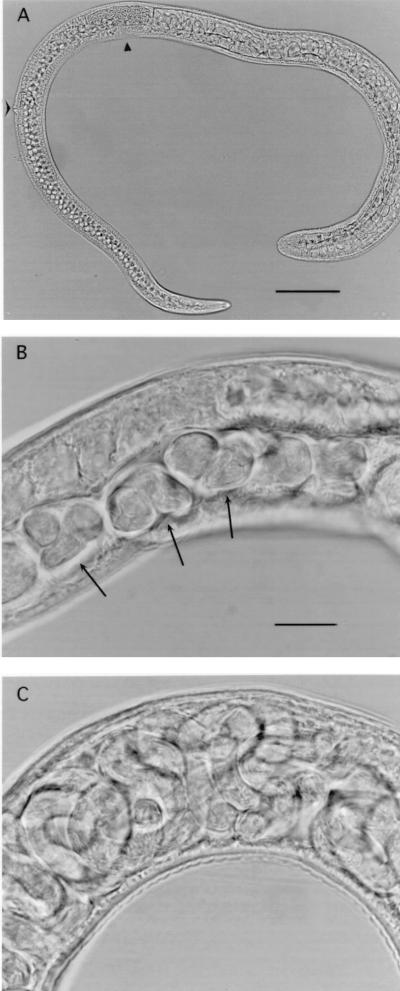
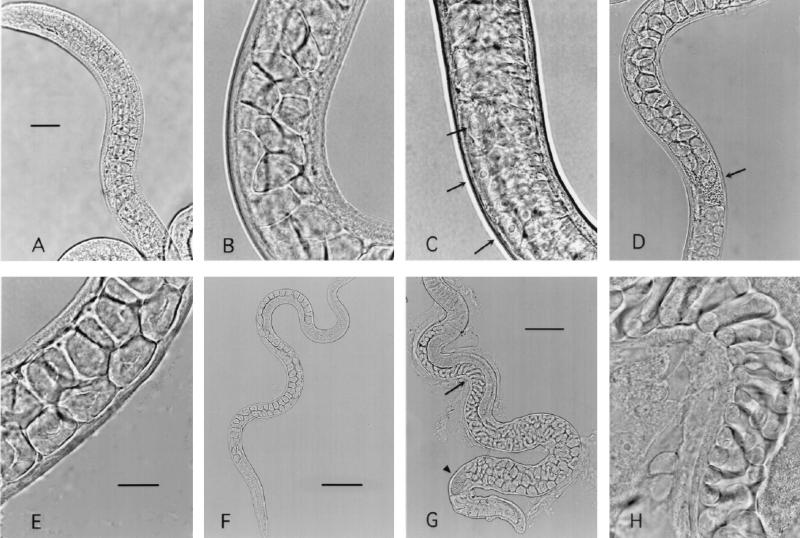
The appearance of male worms collected after 4 or 6 days in culture was similar to the appearance of worms collected 3 days postinfection from rats (Fig. 4). Cultured worms had the vacuolated copulatory appendages, sperm-filled testes, and seminal vesicles typical of T. spiralis adult males (8, 38). The appearance of female worms was comparable to the appearance of adult females recovered from rats (Fig. 5 and 6). Some females bore embryos as early as the fourth day of culture (Fig. 6E to H). Some worms were misshapen or had abnormal cuticles, while others appeared to be normal in terms of size and form.
FIG. 4.
Male T. spiralis worms recovered from Caco-2 cultures (26 larvae/cm2) or from infected rats. (A) Male worm collected after 3 days in culture. Copulatory appendages are prominent. The seminal vesicle (arrow) is filled with sperm. Bar = 30 μm. Objective, ×40. (B) Male worm collected after 6 days in culture. The appearance is similar to the appearance of the worm in panel A. Objective, ×40. (C) Male adult stage worm collected from the intestine of a rat 30 h postinfection. Although copulatory appendages are prominent, very few sperm are evident. Objective, ×40. (D) Male worm collected 5 days postinfection. Copulatory appendages are prominent. The seminal vesicle (arrow) is filled with sperm. Objective, ×40.
FIG. 5.
Female T. spiralis worms recovered from infected rats. (A) Female worm recovered from the intestine of a rat 30 h following infection with larvae. The vaginal opening (arrow head) and sperm in the seminal receptacle (triangle) are visible. Objective, ×20. Bar = 100 mm. (B) Embryos (arrows) in uterus of worm recovered 4 days postinfection. Objective, ×100. Bar = 20 μm. (C) Larvae in uterus of worm recovered 5 days postinfection. Objective, ×100.
FIG. 6.
Female T. spiralis worms recovered from Caco-2 cultures inoculated with 26 larvae/cm2. (A) Female worm collected after 3 days in culture with Caco-2 cells. Objective, ×40. Bar = 20 μm. (B) Ovary of female worm collected after 4 days in culture with Caco-2 cells and 10−6 M 20-hydroxyecdysone. Objective, ×100. (C) Ovary of female worm collected after 4 days in culture with Caco-2 cells and 10−5 M 20-hydroxyecdysone. Ova have prominent nuclei (arrows). Objective, ×100. (D) Uterus containing fertilized ova in worm shown in panel C. The arrow indicates sperm at the base of the uterus. Objective, ×40. (E) Embryos in uterus of worm shown in panel C. Objective, ×100. Bar = 10 μm. (F) Female worm collected after 4 days in culture with Caco-2 cells and 10−5 M 20-hydroxyecdysone. Objective, ×10. Bar = 100 μm. (G) Female worm collected after 6 days in culture with Caco-2 cells and 10−5 M 20-hydroxyecdysone. The arrow indicates the area enlarged in panel H. The arrowhead indicates sperm in the base of the uterus. Objective, ×20. Bar = 50 μm. (H) Enlarged view of larvae in uterus of worm shown in panel G. Objective, ×100.
DISCUSSION
The developmental parameters of T. spiralis in rodents are well established. Larvae molt four times in 30 to 40 h following infection (2, 24). The first molt is initiated 8 to 14 h postinfection. Worms copulate soon after the final molt and ecdysis (18), and female worms begin to release L1 larvae during the fourth and fifth days of the life cycle (20). We found that 10 to 50% of larvae ecdysed at least once during 18 h in culture with Caco-2 cells. We did not evaluate larval stages between L2 and L4. Our data show that T. spiralis larvae must occupy the host epithelium in order to ecdyse. Ecdysis was prevented under conditions that prevented larvae from entering cells or that encumbered larvae in the monolayer (e.g., suspension of larvae in liquid medium, inoculation onto resistant cells, or treatment with specific antibodies). Empty sheaths were found embedded in monolayers, indicating that ecdysis occurred in the epithelium in vitro as it does in vivo (9). Occupation of cells may stimulate larvae to produce the protease(s) necessary to digest the cephalic end of the sheath. In addition, the cells may provide the physical support necessary for the worm to shed the old cuticle, as suggested by Despommier (14).
Several studies aimed at identifying conditions that support T. spiralis development in vitro have been described (7, 22, 23, 29, 33, 35). The most successful method was described by Berntzen (7) and later confirmed by Sakamoto (33). Only Berntzen quantified his results. Unfortunately, interpretation of the data is compromised by confusion over the developmental stage of the muscle larvae used to start the cultures. Nevertheless, Berntzen reported that in his culture system 75% of the worms recovered developed to adult stages. The method used to prepare larvae for culture was similar to our method. Larvae were freed from muscle by pepsin digestion and were activated by treatment with trypsin, pancreatin, and sodium tauroglycocholate. The larvae were cultured in a defined medium supplemented with chicken embryo extract and 0.01 M cysteine and then maintained with a continuous medium flow in the presence of 85% N2, 5% CO2, and 10% O2. Ecdysis was facilitated by transferring parasites to a desheathing apparatus. Gravid females were not enumerated; however, newborn larvae were observed in cultures after 10 days. Although these conditions provide insight into the developmental requirements of T. spiralis, we sought to develop a model that would more closely mimic the intestinal environment of the parasite.
It has been suggested that molting animals, specifically arthropods and nematodes, may be more closely related than previously thought (1). 20-Hydroxyecdysone is the main molting hormone in insects. Nematodes synthesize ecdysteroid hormones (11, 17, 30), and the concentrations are high in the reproductive systems of Dirofilaria immitis and Ascaris suum (11). Experiments designed to test the influence of exogenous ecdysteroid hormones on the development and reproduction of nematodes have revealed a variety of effects. Hormone concentrations between 10−5 and 10−8 M promoted molting in T. spiralis (although ecdysis was not described) (21), A. suum (16), and D. immitis (34). Exogenous ecdysone stimulated microfilarial release in Brugia pahangi, meiotic reinitiation in the oocytes of D. immitis (4), and egg laying in Nippostrongylus brasiliensis (19). In contrast, culture of Heligmosomoides polygyrus adult females in the presence of concentrations of ecdysone similar to the concentration detectable in the body of the nematode (2.2 × 10−11 M) (13) did not increase egg output (32). In our experiments, exogenous ecdysteroid hormones did not significantly affect ecdysis or development of T. spiralis.
Following experimental infection of rodents, T. spiralis larvae and adults are often found in intestinal epithelial cells at the crypt-villus junction (18). The physical organization of this site is quite similar to the physical organization of a monolayer of epithelial cells. Both habitats allow worms to migrate through large numbers of cells. The density of the worms in the epithelium of the intestine must be very low as the surface area is vast. We found that by reducing parasite density we enhanced worm development and copulation in vitro. The survival rate of worms cultured in Caco-2 cells at low densities ranged from 26 to 50%. When rats are experimentally infected with T. spiralis, on average only 50% of the larvae delivered to an animal can be recovered from the intestinal tract. Nevertheless, our culture model is not optimal as only 5% of the worms developed into fecund females. The majority of the surviving worms were unfertilized females, and the factor limiting fecundity appeared to be male worm survival in the monolayer. If we did not observe male worms in monolayers at the end of the experiment, we did not observe fecund females. The males that were present appeared to be fully developed; however, in experiments in which we evaluated all of the worms recoverable from culture, we often found more males in the agarose than in the monolayer, suggesting that male worms were less able to maintain their position in the epithelium. Improving survival of male worms is critical to enhancing fecundity in this model system.
Studies performed with laboratory rodents have shown that T. spiralis females become fecund at a time of active host immune defense, approximately 5 to 7 days postinfection (reviewed by Bell [6]). In vitro culture allows investigation of parasitism and nematode reproduction free of the influence of most innate immune responses and all adaptive immune responses. Furthermore, introduction of immune mediators into defined cultures permits further testing of the specific effects of host defense.
Acknowledgments
We thank A. Wenski-Roberts and D. Beiting for assistance with photomicrography. D. Beiting, S. Bliss, M. Duffy, and F. Romaris provided helpful comments on the manuscript.
This research was supported by grant AI 14490 from the National Institute of Allergy and Infectious Diseases.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aguinaldo, A. M. A., J. M. Turbeville, L. S. Linford, M. C. Rivera, J. R. Garey, R. A. Raff, and J. A. Lake. 1997. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387:489-493. [DOI] [PubMed] [Google Scholar]
- 2.Ali Khan, Z. 1966. The postembryonic development of Trichinella spiralis with special reference to ecdysis. J. Parasitol. 52:248-259. [Google Scholar]
- 3.Appleton, J. A., L. R. Schain, and D. D. McGregor. 1988. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology 65:487-492. [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, G. C., J. G. Mercer, H. H. Rees, and R. E. Howells. 1991. The effect of ecdysteroids on the microfilarial production of Brugia pahangi and the control of meiotic reinitiation in the oocytes of Dirofilaria immitis. Parasitol. Res. 77:65-71. [DOI] [PubMed] [Google Scholar]
- 5.Beall, M. J., and E. J. Pearce. 2001. Human transforming growth factor-beta activates a receptor serine/threonine kinase from the intravascular parasite Schistosoma mansoni. J. Biol. Chem. 276:31613-31619. [DOI] [PubMed] [Google Scholar]
- 6.Bell, R. G. 1998. The generation and expression of immunity to Trichinella spiralis in laboratory rodents. Adv. Parasitol. 41:149-217. [DOI] [PubMed] [Google Scholar]
- 7.Berntzen, A. K. 1965. Comparative growth and development of Trichinella spiralis in vitro and in vivo, with a redescription of the life cycle. Exp. Parasitol. 16:74-106. [DOI] [PubMed] [Google Scholar]
- 8.Burnham, J. C., and D. D. Despommier. 1984. Development of the male genitalia of Trichinella spiralis during the enteral phase of infection in the mouse: an SEM study. J. Parasitol. 70:310-311. [PubMed] [Google Scholar]
- 9.Capo, V., D. D. Despommier, and D. S. Silberstein. 1984. The site of ecdysis of the L1 larva of Trichinella spiralis. J. Parasitol. 70:992-994. [PubMed] [Google Scholar]
- 10.Carlisle, M. S., D. D. McGregor, and J. A. Appleton. 1990. The role of mucus in antibody-mediated rapid expulsion of Trichinella spiralis in suckling rats. Immunology 70:126-132. [PMC free article] [PubMed] [Google Scholar]
- 11.Cleator, M., C. J. Delves, R. E. Howells, and H. H. Rees. 1987. Identity and tissue localization of free and conjugated ecdysteroids in adults of Dirofilaria immitis and Ascaris suum. Mol. Biochem. Parasitol. 25:93-105. [DOI] [PubMed] [Google Scholar]
- 12.Crum, E. D., D. D. Despommier, and D. D. McGregor. 1977. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology 33:787-795. [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis, R. D. 1976. Insect morphogenetic hormones and developmental mechanisms in the nematode Nematospiroides dubius. Comp. Biochem. Physiol. A 53:53-56. [DOI] [PubMed] [Google Scholar]
- 14.Despommier, D. D. 1983. Biology, p. 75-151. In W. C. Campbell (ed.), Trichinella and trichinosis. Plenum Press, New York, N.Y.
- 15.Ellis, L. A., A. J. Reason, H. R. Morris, A. Dell, R. Iglesias, F. M. Ubeira, and J. A. Appleton. 1994. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology 4:585-592. [DOI] [PubMed] [Google Scholar]
- 16.Fleming, M. W. 1985. Ascaris suum: role of ecdysteroids in molting. Exp. Parasitol. 60:207-210. [DOI] [PubMed] [Google Scholar]
- 17.Fleming, M. W. 1993. Ecdysteroids during development in the ovine parasitic nematode Haemonchus contortus. Comp. Biochem. Physiol. B 104:653-655. [DOI] [PubMed] [Google Scholar]
- 18.Gardiner, C. H. 1976. Habitat and reproductive behavior of Trichinella spiralis. J. Parasitol. 62:865-870. [PubMed] [Google Scholar]
- 19.Goudey-Perriere, F., B. F. Simo, J. Maccario, C. Perriere, and P. Gayral. 1992. Effects of ecdysteroids on reproductive physiology of Nippostrongylus brasiliensis (Nematoda) in vivo. Comp. Biochem. Physiol. C 103:105-109. [DOI] [PubMed] [Google Scholar]
- 20.Harley, J. P., and V. Gallicchio. 1971. Trichinella spiralis: migration of larvae in the rat. Exp. Parasitol. 30:11-21. [DOI] [PubMed] [Google Scholar]
- 21.Hitcho, P. J., and R. E. Thorson. 1971. Possible molting and maturation controls in Trichinella spiralis. J. Parasitol. 57:787-793. [PubMed] [Google Scholar]
- 22.Kim, C. W. 1961. The cultivation of Trichinella spiralis in vitro. Am. J. Trop. Med. Hyg. 10:742-747. [DOI] [PubMed] [Google Scholar]
- 23.Kim, C. W. 1962. Further study on the in vitro cultivation of Trichinella spiralis. Am. J. Trop. Med. Hyg. 11:491-496. [DOI] [PubMed] [Google Scholar]
- 24.Kozek, W. J. 1971. The molting pattern in Trichinella spiralis. I. A light microscope study. J. Parasitol. 57:1015-1028. [PubMed] [Google Scholar]
- 25.Leiby, D. A., C. H. Duffy, K. D. Murrell, and G. A. Schad. 1990. Trichinella spiralis in an agricultural ecosystem: transmission in the rat population. J. Parasitol. 76:360-364. [PubMed] [Google Scholar]
- 26.MacDonald, T. T., and G. Monteleone. 2001. IL-12 and Th1 immune responses in human Peyer's patches. Trends Immunol. 22:244-247. [DOI] [PubMed] [Google Scholar]
- 27.ManWarren, T., L. Gagliardo, J. Geyer, C. McVay, S. Pearce-Kelling, and J. Appleton. 1997. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect. Immun. 65:4806-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McVay, C. S., P. Bracken, L. F. Gagliardo, and J. Appleton. 2000. Antibodies to tyvelose exhibit multiple modes of interference with the epithelial niche of Trichinella spiralis. Infect. Immun. 68:1912-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meerovitch, E. 1965. Studies on the in vitro axenic development of Trichinella spiralis. I. Basic culture techniques, pattern of development, and the effects of the gaseous phase. Can. J. Zool. 43:69-79. [DOI] [PubMed] [Google Scholar]
- 30.Nembo, B., P. Duie, M. Garcia, P. Breton, P. Gayral, P. Porcheron, and F. Goudey-Perriere. 1993. Levels of ecdysteroid-like material in adults of Nippostrongylus brasiliensis during the intestinal phase. J. Helminthol. 67:305-315. [DOI] [PubMed] [Google Scholar]
- 31.Nichols, G. E., J. C. Lovejoy, C. A. Borgman, J. M. Sanders, and W. W. Young, Jr. 1986. Isolation and characterization of two types of MDCK epithelial cell clones based on glycosphingolipid pattern. Biochim. Biophys. Acta 887:1-12. [DOI] [PubMed] [Google Scholar]
- 32.Richardson, T. L., and B. M. MacKinnon. 1990. Heligmosomoides polygyrus: effect of exogenous steroid hormones on egg output in vitro. J. Helminthol. 64:123-132. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto, T. 1979. Development and behaviour of adult and larval Trichinella spiralis cultured in vitro. Mem. Fac. Agric. Kagoshima Univ. 15: 107-114. [Google Scholar]
- 34.Warbrick, E. V., G. C. Barker, H. H. Rees, and R. E. Howells. 1993. The effect of invertebrate hormones and potential hormone inhibitors on the third larval moult of the filarial nematode, Dirofilaria immitis, in vitro. Parasitology 107:459-463. [DOI] [PubMed] [Google Scholar]
- 35.Weller, T. H. 1943. The development of the larvae of Trichinella spiralis in roller tube tissue cultures. Am. J. Pathol. 19:503-515. [PMC free article] [PubMed] [Google Scholar]
- 36.Wright, K. A. 1979. Trichinella spiralis: an intracellular parasite in the intestinal phase. J. Parasitol. 65:441-445. [PubMed] [Google Scholar]
- 37.Wright, K. A., E. Weidman, and H. Hong. 1987. The distribution of cells killed by Trichinella spiralis in the mucosal epithelium of two strains of mice. J. Parasitol. 73:935-939. [PubMed] [Google Scholar]
- 38.Wu, L.-Y. 1955. Studies on Trichinella spiralis. I. Male and female reproductive systems. J. Parasitol. 41:40-47. [PubMed] [Google Scholar]
- 39.Wu, L. Y., and A. A. Kingscote. 1957. Studies on Trichinella spiralis. II. Times of final molt, spermatozoa formation, ovulation, and insemination. Can. J. Zool. 35:207-211. [Google Scholar]