Abstract
This study was conducted to evaluate the immunogenicity of the Brucella abortus lumazine synthase (BLS) gene cloned into the pcDNA3 plasmid, which is driven by the cytomegalovirus promoter. Injection of plasmid DNA carrying the BLS gene (pcDNA-BLS) into BALB/c mice elicited both humoral and cellular immune responses. Antibodies to the encoded BLS included immunoglobulin G1 (IgG1) IgG2a, IgG2b, IgG3, and IgM isotypes. Animals injected with pcDNA-BLS exhibited a dominance of IgG2a over IgG1. In addition, spleen cells from vaccinated animals produced interleukin-2 and gamma interferon but not IL-10 or IL-4 after in vitro stimulation with recombinant BLS (rBLS), suggesting the induction of a Th1 response. Protection was evaluated by comparing the levels of infection in the spleens of vaccinated mice challenged with B. abortus 544. Immunization with pcDNA-BLS- reduced the bacterial burden relative to those in the control groups. Mice immunized with rBLS produced a significant humoral response but did not show a specific cellular response or any protection from challenge. Altogether, these data suggest that pcDNA-BLS is a good immunogen for the production of humoral and cell-mediated responses in mice and is a candidate for use in future studies of vaccination against brucellosis.
Brucella abortus is a gram-negative, facultative, intracellular bacterium that infects both cattle and humans, causing abortion and infertility in the former and undulant fever, endocarditis, arthritis, and osteomyelitis in the latter (35).
In cattle, variable protective efficacy against brucellosis is obtained by vaccination with live attenuated B. abortus S19 (smooth) or strain RB51 (rough). Although the mechanisms of protection that are induced by attenuated strains are unknown, it is generally accepted that immunity to Brucella is due to antibody- and cell-mediated mechanisms (2, 5, 18). Th1 immune responses, characterized by production of gamma interferon (IFN-γ), are associated with protective immunity to Brucella. Zhan and colleagues (36, 37) have found that infection of mice with live B. abortus S19 induced a high percentage of IFN-γ-producing Th1 cells, while injection of Brucella protein extracts induced interleukin-4 (IL-4) producing Th2 cells. However, attenuated vaccines are far from being ideal, as they can cause disease in humans and abortion when administered to pregnant cattle. Moreover, because B. abortus S19 induces antibodies to smooth lipopolysaccharide (LPS), it is difficult to differentiate vaccinated animals from naturally infected animals (3, 25). Therefore, the development of better vaccines is necessary for disease control.
Immunization with plasmid DNA, consisting of a bacterial plasmid that includes a viral promoter and the gene of interest, represents a promising method in vaccine research. Plasmid DNA vaccination can protect against many viral and protozoal diseases in animal models (23, 26, 32). The effect against bacterial infections is less well documented. For tuberculosis, independent studies with mice have demonstrated the protective efficacy of the injection of DNA encoding the Ag 85 protein antigen (16, 22) and heat shock protein 65 (30). Also, Kurar and Splitter (20) showed that DNA vaccination with the B. abortus ribosomal L7/L12 gene elicits humoral and cellular immune responses and partial protection. Thus, plasmid DNA vaccination may be a successful alternative method for conferring protection against Brucella. In addition, a genetic vaccine, by inducing an immune response to a single protein, would make possible the development of diagnostic tests that could differentiate vaccinated animals from infected animals.
Previous reports have shown that an 18-kDa cytoplasmic protein of Brucella can be used for the serological diagnosis of human and animal brucellosis (3, 4, 13). Moreover, it has been demonstrated that this 18-kDa protein is an enzyme with lumazine synthase activity (14). Other authors have shown that fractions from Brucella melitensis and B. abortus, which encode proteins with similar molecular weights, induced T-cell responses, lymphocyte proliferation, IFN-γ secretion, and delayed-type hypersensitivity in different species (9, 28). In this study, we examined whether the injection of plasmid DNA carrying the Brucella lumazine synthase (BLS) gene (pcDNA-BLS) could induce antibody formation and cellular immune responses in mice and compared these responses with the ones elicited by recombinant BLS (rBLS). The protective efficacies of pcDNA-BLS and rBLS against Brucella infection were also assessed.
MATERIALS AND METHODS
Animals.
Four- to 6-week-old female BALB/c mice (obtained from Instituto Nacional de Tecnología Agropecuaria, CICV, Castelar, Argentina) were acclimated and randomly distributed into experimental groups. The mice were kept in conventional animal facilities and received water and food ad libitum.
Bacteria.
Escherichia coli strains BL21(DE3) and JM109 were used as hosts during the cloning experiments and for the propagation of plasmids. The bacterial strains were routinely grown at 37°C in Luria-Bertani broth or agar supplemented, when required, with 100 μg of ampicillin per ml. B. abortus S19 (live attenuated vaccine) and 544 (virulent strain) were cultured in tryptose-soy agar supplemented with yeast extract (Merck, Buenos Aires, Argentina).
Cloning of the gene encoding BLS and expression of the protein.
The BLS gene was cloned in pET11b vector (Novagen, Madison, Wis.), as reported previously (14), with the sequence information previously described (15). The BLS protein was successfully expressed as inclusion bodies in competent cells of E. coli strain BL21(DE3) (Stratagene, La Jolla, Calif.). The inclusion bodies were solubilized in 50 mM Tris-8 M urea (pH 8.0) and refolded by dialysis against phosphate-buffered saline (PBS) containing 1 mM dithiothreitol. This preparation was purified in a MonoQ column in a fast-performance liquid chromatography apparatus (Pharmacia, Uppsala, Sweden). The purity was assessed by silver staining and has been previously reported (7, 14). The protein preparation contained less than 0.05 endotoxin unit per mg of protein, as assessed by a limulus amebocyte lysate analysis kit (Sigma, St Louis, Mo.).
Cloning of the BLS gene in a pcDNA3 vector for DNA vaccination.
The BLS gene, including the consensus sequence described by Kozak (19), was cloned in pcDNA3 (Invitrogen, Carlsbad, Calif., or R & D Systems, Abingdon, United Kingdom) as described previously (33). pcDNA3 was sequenced across the gene insert, and expression of BLS was confirmed in vitro by transient transfection of COS-7 cells. The plasmid was amplified in E. coli JM109 (Promega, Madison, Wis.) and isolated with Mega Prep plasmid isolation columns (Qiagen, Dorking, United Kingdom). The plasmid preparation contained less than 0.05 endotoxin unit per 100 μg of DNA, as assessed by a Limulus amebocyte lysate analysis kit.
Immunizations.
The mice were anesthetized with methoxyfuorane (Metofan; Mallinckrodt) and immunized intramuscularly with 100 μg of pcDNA-BLS in 100 μl of saline (50 μl of the solution was injected into each quadriceps muscle). The control mice were injected with the expression vector alone (pcDNA3). Each mouse was injected on days 0, 15, 30, and 45. Each mouse in another group was injected with 10 μg of rBLS in 100 μl of saline under the same schedule. Sera were obtained at 15, 30, 45, and 60 days after the first immunization. The mice used as the positive control group in protection experiments were vaccinated intraperitoneally on day 0 with 5 × 104 CFU of B. abortus S19 in 0.2 ml of PBS.
ELISAs.
Serum reactivity against BLS was determined by indirect enzyme-linked immunosorbent assay (ELISA) as described previously (33). Briefly, polystyrene plates (Maxisorp; Nunc, Roskilde, Denmark) that were sensitized with rBLS were incubated with serial dilutions of the sera. After the plates were washed, a sheep anti-mouse immunoglobulin-horseradish peroxidase conjugate (Sigma) was added and the reaction was developed with orthophenylenediamine-H2O2. The conditions were the same for isotype determination of the antibodies, except that sheep anti-mouse immunoglobulin M (IgM)-, IgG1-, IgG2a-, IgG2b-, and IgG3-specific antibodies (Sigma) were used and were detected by incubation with anti-sheep IgG-horseradish peroxidase conjugate. The cutoff value for the assay was calculated as the mean specific optical density plus 3 standard deviations (SD) for 20 sera from nonimmunized mice assayed at dilutions of 1:100. The titer of each serum was calculated as the last serum dilution yielding a specific optical density higher than the cutoff value.
Stimulation of spleen cells.
Spleen cell suspensions from immunized and control mice were prepared in RPMI 1640 (Gibco) supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum. The cells were cultured at 4 × 106/ml in duplicate with rBLS (5 μg/ml) or concanavalin A (ConA; 2.5 μg/ml) (Sigma). The cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 24 h for cytokine gene expression or 48 h for cytokine secretion.
Detection of cytokine mRNA by semiquantitative RT-PCR.
Reverse transcriptase (RT) PCR was performed as previously described (8, 34). PCR products were separated on agarose gels, transferred by Southern blot hybridization to a Nytran nylon membrane (Schleicher & Schuell, Keene, N.H.), probed with digoxigenin-labeled oligonucleotide probes, and visualized with a chemiluminescence detection system (Genius kit; Boehringer Mannheim, Indianapolis, Ind.). All cytokine amplicon levels were normalized for the amount of mRNA carrying β-actin, a housekeeping gene, that was detected in the same sample. The chemiluminescence signals were quantified with 1D Image Analysis software (Kodak Digital Science; Eastman Kodak Co., Rochester, N.Y.). The results were expressed in terms of the increase in the mRNA levels of stimulated cells over that of cells cultured in the absence of antigen. An increase of greater than two fold was considered to be indicative of an up-regulation of the investigated cytokine gene.
Cytokine ELISAs.
The levels of IL-4, IL-10, and IFN-γ in culture supernatants were measured by sandwich ELISAs with paired cytokine-specific monoclonal antibodies according to the manufacturer's instructions (Pharmingen, San Diego, Calif.).
Protection experiments.
The mice were challenged intraperitoneally with 1.12 × 105 CFU of B. abortus 544 30 days after the last booster injection. The mice were killed by cervical dislocation 30 days after being challenged, and their spleens were removed aseptically and weighed. Each spleen was homogenized in a stomacher bag, serially diluted, plated on tryptic soy agar, and incubated for 4 days at 37°C with 5% CO2. The number of CFU per spleen was counted, and the results were represented as the mean log CFU ± SD per group. To evaluate protection, two independent experiments were conducted.
Statistical analysis of the data.
The CFU data were normalized transformation and evaluated by one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test (InStat; GraphPad, San Diego, Calif.). The Kruskal-Wallis test and one-way ANOVA were used to analyze antibody response and cellular response, respectively.
RESULTS
pcDNA-BLS and rBLS immunizations induce a vigorous humoral response.
The titers of anti-BLS antibodies were measured by ELISA of sera from mice immunized with pcDNA-BLS, with the expression vector alone (pcDNA3), or with rBLS. Immunization with pcDNA-BLS elicited a humoral immune response that was detectable 15 days after the first immunization (titer range, 100 to 200) and increased steadily after each injection to reach a maximum (titer range, 51,200 to 102,400) at day 60 postvaccination (end of the experiment) (Fig. 1A). Animals injected with rBLS also exhibited an antibody response that was detectable 15 days after the first injection (titer range, 1,600 to 6,400) but increased very little with successive immunizations. None of the animals inoculated with pcDNA3 showed specific anti-BLS antibodies (data not shown).
FIG. 1.
Characterization of antibody responses to rBLS after immunization with pcDNA-BLS or rBLS. Mice (five per group) were immunized with pcDNA-BLS or rBLS and bled retroorbitally. The titers of antibodies were evaluated by ELISA. Data are representative of two separate experiments. (A) Kinetics of the anti-BLS humoral response in mice immunized with 100 μg of pcDNA-BLS (solid lines) or 10 μg of rBLS (dashed lines). The squares represent the mean titers± SD (error bars) of antibodies in five animals. The arrows indicate the days of inoculation. PI, postimmunization. (B) Antibody isotype profiles of mice immunized with pcDNA-BLS or rBLS. IgG1 (filled bars) and IgG2a (open bars) antibody titers of mice immunized with pcDNA-BLS or rBLS were studied by ELISA 60 days after the immunization. Each bar represents the mean titers ± SD of antibodies in five animals. ★, significantly different from titer of IgG2a in mice immunized with rBLS (P < 0.01). P values were calculated by the Kruskal-Wallis test.
The mice vaccinated with pcDNA-BLS or rBLS developed specific antibodies of all the isotypes assayed (IgM, IgG1, IgG2a, IgG2b, and IgG3). In those animals injected with pcDNA-BLS, IgG2a-specific antibody titers (range, 25,600 to 51,200) were higher than IgG1-specific antibody titers (range, 6,400 to 12,800) (IgG2a/IgG1 ratio = 2.8) (Fig. 1B) while the levels of IgG2b-, IgG3-, and IgM-specific antibodies were low (data not shown). After rBLS immunization, in contrast, the animals had higher IgG1-specific antibody titers (range, 51,200 to 102,400) than IgG2a-specific antibody titers (range, 800 to 12,800) and the IgG1/IgG2a ratio was 0.06. The subclass profile shown in Fig. 1B was constant during the whole immunization schedule, indicating that multiple injections caused no alteration of the type of response (data not shown). As LPS is a strong immunogen and a frequent contaminant in plasmids as well as in recombinant proteins produced in E. coli, we performed ELISAs on polystyrene plates coated with E. coli LPS to rule out the possibility of the antibody being directed against LPS. All the sera tested negative against E. coli LPS (data not shown).
pcDNA-BLS immunization induces a Th1 cellular response.
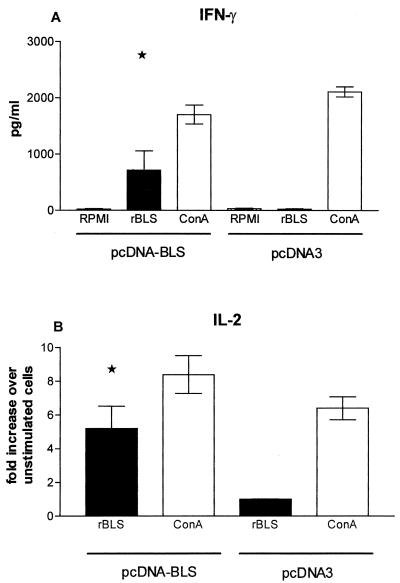
Cytokine gene expression and secretion were assessed by semiquantitative RT-PCR or ELISA of supernatants of spleen cells from rBLS-, pcDNA-BLS-, or pcDNA3-immunized mice. BLS significantly induced the production of IFN-γ in cells from pcDNA-BLS-immunized mice (P < 0.05) (Fig. 2A). Additionally, rBLS induced a significant up-regulation of the expression of the IL-2 gene (Fig. 2B) (P < 0.05) in cells from pcDNA-BLS-immunized mice. Up-regulation of IL-4 or IL-10 (gene and protein production) could not be detected under the same conditions (data not shown). rBLS did not induce the gene transcription of any of the studied cytokines in spleen cells from rBLS- or pcDNA3-immunized mice (Fig. 2 and data not shown). ConA induced the expression of the cytokine genes and the production of the corresponding proteins in all groups (Fig. 2 and data not shown). Taken together, our results indicated that immunization with pcDNA-BLS induces a specific Th1 response in mice.
FIG. 2.
(A) Concentrations of rBLS-induced IFN-γ in cells from mice immunized with pcDNA-BLS. Spleen cells (4 × 106/ml) from pcDNA3- and pcDNA-BLS-immunized mice were stimulated with RPMI 1640, rBLS (5 μg/ml), or ConA (2.5 μg/ml) for 48 h. Levels of IFN-γ in the cell supernatants were quantified by antibody capture ELISA. Each bar represents the geometric mean ± SD (error bar) of the responses in spleen cells from five individual mice, with experiment run in duplicate. ★, significantly different from results for RPMI 1640-stimulated cells of mice immunized with pcDNA-BLS (P < 0.05). The data are representative of two separate experiments. (B) rBLS-induced IL-2 mRNA expression in cells from mice immunized with pcDNA-BLS. Spleen cells (4 × 106/ml) from pcDNA3- and pcDNA-BLS-immunized mice were stimulated with RPMI 1640, rBLS (5 μg/ml), or ConA (2.5 μg/ml) for 24 h. The induced mRNA levels of IL-2 were determined by RT-PCR. The responses are shown in terms of the increases (n-fold) in the IL-2 levels in stimulate cells over that in unstimulated cells. All values were normalized with respect to β-actin mRNA levels. Each bar represents the geometric mean ± SD of the responses in spleen cells from five individual mice, with experiments run in duplicate. ★, significantly different from the rBLS-induced IL-2 levels in cells of mice immunized with pcDNA3 (P < 0.05). The data are representative of two separate experiments.
pcDNA-BLS immunization induces protection against Brucella infection.
Protection experiments were carried out by challenging vaccinated and control mice by intraperitoneal injection of B. abortus 544, and the levels of infection were evaluated by measuring the CFU in the spleens. Two independent protection experiments demonstrated that mice given pcDNA-BLS had a significantly higher degree of protection (1.65 log increase in protection) than did controls receiving PBS (P < 0.01). To compare the extents to which mice could be protected, we included the best vaccine available (live B. abortus S19; log protection, 2.27). No reduction in the number of CFU was seen in animals injected with rBLS or pcDNA3 compared to the number in control animals (Table 1). This result indicated that pcDNA-BLS afforded a significant degree of protection against Brucella infection.
TABLE 1.
Protection against B. abortus in mice immunized with a DNA vaccine coding for BLS
| Expt | Vaccinea | Log CFU of Brucella/spleen (Mean ± SD) | Log protection | Significanceb |
|---|---|---|---|---|
| 1 | None (PBS) | 4.39 ± 0.36 | 0 | |
| B. abortus S19 | 2.35 ± 0.30 | 2.04 | P < 0.01 | |
| pcDNA3 | 4.38 ± 0.18 | 0.01 | P > 0.05 | |
| rBLS | 4.36 ± 0.19 | 0.02 | P > 0.05 | |
| pcDNA-BLS | 3.14 ± 0.62 | 1.25 | P < 0.01 | |
| 2 | None (PBS) | 5.48 ± 0.13 | 0 | |
| B. abortus S19 | 3.21 ± 0.13 | 2.27 | P < 0.01 | |
| pcDNA3 | 5.28 ± 0.13 | 0.01 | P > 0.05 | |
| rBLS | 5.11 ± 0.41 | 0.37 | P > 0.05 | |
| pcDNA-BLS | 3.83 ± 0.48 | 1.65 | P < 0.01 |
Vaccines were injected into groups of six to eight mice.
Statistical differences from the values of the negative (PBS-inoculated) controls were estimated by ANOVA and Dunnett's post hoc test.
DISCUSSION
The study of the immunogenicities of antigens and their use in combination with new systems of immunization is very important for the development of better vaccines. New strategies are necessary to prevent brucellosis while avoiding the disadvantages of the currently used live vaccines.
The improvement of the methods for cloning and purifying proteins has led to the use of purified recombinant proteins as acellular vaccines in experimental trials. These preparations, as well as synthetic peptides, are more convenient to use than attenuated vaccines but are not able to confer a high degree of protection or induce a strong immune response (24, 29). The reduced effectiveness of the acellular vaccines might be related to inadequate processing and presentation of the antigen. Immunization with plasmid DNA coding for the antigen of interest represents a novel and promising method in vaccine research and development. A number of studies have demonstrated that after naked DNA immunization, the antigen is naturally processed and presented on major histocompatibility complex class I and class II molecules, inducing cellular and humoral immune responses (11, 27, 31).
The objective of this study was to investigate the immunogenicities of B. abortus BLS as a purified recombinant protein and the BLS gene carried in a plasmid DNA vaccine. Previous reports have shown the usefulness of BLS in the serological diagnosis of human and animal brucellosis (3, 4, 13) and have characterized its biological activity (14). In this study, we demonstrated that immunization with rBLS in the absence of adjuvant induced high titers of antibodies, which indicates that this protein is very immunogenic. Three-dimensional analysis has revealed that BLS forms a pentameric structure (7). Interestingly, polymeric, virus-like protein particles have been shown to be highly immunogenic (12). Thus, the polymeric arrangement of BLS could explain the high humoral response elicited when mice were immunized with rBLS (Fig. 1).
Injection of plasmid DNA containing the sequence encoding BLS into BALB/c mice elicited specific humoral and cellular immune responses. Several previously described DNA vaccines (21, 26) induced antibody titers lower than that induced by the pcDNA-BLS employed in this work. This difference should be attributed to the polymeric structure of BLS. As shown by Aberle and colleagues (1), immunization with a DNA vaccine encoding a viral protein that produces a secreted subviral polymeric particle induces a high humoral response while immunization with a DNA vaccine encoding a secreted dimeric protein or a nonsecreted protein induces a low humoral response.
In brucellosis, protection is thought to be dependent upon cell-mediated immunity (2, 18) and, under some conditions, upon antibodies specific to membrane proteins (6, 17). As with immunity to other intracellular bacteria, immunity to B. abortus depends on antigen-specific T-cell-mediated activation of macrophages, which are the major effectors mediating the killing of the bacterium. Th1-induced cytokines, like IFN-γ, play an important role in the activation of macrophages and in resistance in vivo and in vitro (10, 36). Cells from pcDNA-BLS-immunized mice stimulated in vitro with rBLS produced IL-2 and IFN-γ but did not produce IL-4 or IL-10, indicating that immunization with the pcDNA-BLS plasmid induces a Th1 cellular response. The predominance of IgG2a over IgG1 also supported this conclusion. It is interesting that although immunization with rBLS or pcDNA-BLS elicited a specific humoral response, only the latter induced a specific cellular response and conferred protection, which confirms the importance of the cellular response in preventing brucellosis. The reasons for the discrepancies between rBLS immunization and pcDNA-BLS immunization are unknown, but differences in the processing of BLS after inoculation or synthesis in vivo could play a role. Intracellular expression of plasmid-encoded proteins also may lead to the proper processing and presentation of critical epitopes.
Although immunization with pcDNA-BLS did not fully protect animals from B. abortus infection, bacterial replication in these animals was significantly lower than that in control animals. Animals vaccinated with pcDNA-BLS showed a log protection of 1.65. While no protection was seen in animals injected with rBLS or pcDNA3, the DNA vaccine induced considerable protection. This is a quite promising result and is in agreement with that of Kurar and Splitter (20), who used a plasmid DNA coding for the ribosomal L7/L12 protein to immunize BALB/c mice against B. abortus.
In conclusion, we have shown that inoculation of plasmid DNA containing the BLS gene leads to in vivo synthesis of the native antigen, elicits both antibody and Th1-cell-mediated immune responses, and confers protection against B. abortus challenge. Further studies are required to improve the efficacy of DNA immunization and to better understand the mechanisms of protection.
Acknowledgments
This work was supported by grant PIP 0764 from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and grant PICT 0084 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and SECYT (UNICEN). C.A.V. and J.C. are recipients of a fellowship from CONICET. S.E. is a member of C.I.C. (Buenos Aires). G.H.G., F.A.G., C.A.F., and M.S. are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Editor: R. N. Moore
REFERENCES
- 1.Aberle J. H., S. W. Aberle, S. L. Allison, K. Stiasny, M. Ecker, C. W. Mandl, R. Berger, and F. X. Heinz. 1999. DNA immunization model study with constructs expressing the tick-borne encephalitis virus envelope protein E in different physical forms. J. Immunol. 163:6756-6761. [PubMed] [Google Scholar]
- 2.Araya, L. N., and A. J. Winter. 1990. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect. Immun. 58:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi, P. C., G. H. Giambartolomei, F. A. Goldbaum, L. F. Abdón, C. A. Velikovsky, R. Kittelberger, and C. A. Fossati. 1996. Humoral immune response against lipopolysaccharide and cytoplasmic proteins of Brucella abortus in cattle vaccinated with B. abortus S19 or experimentally infected with Yersinia enterocolitica serotype 0:9. Clin. Diagn. Lab. Immunol. 3:472-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldi, P. C., M. M. Wanke, M. E. Loza, N. Monachesi, and C. A. Fossati. 1997. Diagnosis of canine brucellosis by detection of IgG antibodies against an 18 kDa cytoplasmic protein of Brucella spp. Vet. Microbiol. 57:273-281. [DOI] [PubMed] [Google Scholar]
- 5.Bascoul, S., A. Cannat, M. F. Huguet, and A. Serre. 1978. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology 35:213-221. [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden, R. A., A. Cloeckaert, M. Zygmunt, and G. Dubray. 1995. Outer membrane protein and rough lipopolysaccharide-specific monoclonal antibodies protect mice against Brucella ovis. J. Med. Microbiol. 43:344-347. [DOI] [PubMed] [Google Scholar]
- 7.Braden, B. C., C. A. Velikovsky, A. A. Cauerhff, I. Polikarpov, and F. A. Goldbaum. 2000. Divergence in macromolecular assembly: X-ray crystallographic structure analysis of lumazine synthase from Brucella abortus. J. Mol. Biol. 297:1031-1036. [DOI] [PubMed] [Google Scholar]
- 8.Chirgwin, J. J., A. E. Przbyla, R. J. MacDonald, and W. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294-5299. [DOI] [PubMed] [Google Scholar]
- 9.Denoel, P. A., T. K. O. Vo, V. E. Weynants, A. Tibor, D. Gilson, M. S. Zygmunt, J. N. Limet, and J. J. Letesson. 1997. Identification of the major T-cell antigens present in the Brucella melitensis B115 protein preparation, Brucellergene OCB. J. Med. Microbiol. 46:801-806. [DOI] [PubMed] [Google Scholar]
- 10.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 12.Gilbert, S. C., M. Plebanski, S. J. Harris, C. E. Allsopp, R. Thomas, G. T. Layton, and A. V. Hill. 1997. A protein particle vaccine containing multiple malaria epitopes. Nat. Biotechnol. 15:1280-1284. [DOI] [PubMed] [Google Scholar]
- 13.Goldbaum, F. A., J. Leoni, J. C. Wallach, and C. A. Fossati. 1993. Characterization of an 18-kilodalton Brucella cytoplasmic protein which appears to be a serological marker of active infection of both human and bovine brucellosis. J. Clin. Microbiol. 31:2141-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldbaum, F. A., C. A. Velikovsky, P. C. Baldi, S. Mortl, A. Bacher, and C. A. Fossati. 1999. The 18 kDa cytoplasmic protein of Brucella spp., an antigen useful for diagnosis, is a lumazine synthase. J. Med. Microbiol. 48:833-839. [DOI] [PubMed] [Google Scholar]
- 15.Hemmen, F., V. Weynants, T. Scarcez, J.-J. Letesson, and E. Saman. 1995. Cloning and sequence analysis of a newly identified Brucella abortus gene and serological evaluation of the 17-kilodalton antigen that it encodes. Clin. Diagn. Lab. Immunol. 2:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. De Souza, A. Drowart, E. Lozes, P. Vandembussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 17.Jacques, I., A. Cloeckaert, J. N. Limet, and G. Dubray. 1992. Protection conferred on mice by combinations of monoclonal antibodies directed against outer-membrane proteins or smooth lipopolysaccharide of Brucella. J. Med. Microbiol. 37:100-103. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez de Bagüés, M. P., P. H. Elzer, S. M. Jones, J. M. Blasco, F. M. Enright, G. G. Schurig, and A. J. Winter. 1994. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect. Immun. 62:4990-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak, M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurar, E., and G. A. Splitter. 1997. Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine 15:1851-1857. [DOI] [PubMed] [Google Scholar]
- 21.Laylor, R., N. Porakishvili, J. B. De Souza, J. H. Playfair, P. J. Delves, and T. Lund. 1999. DNA vaccination favors memory rather than effector B cell responses. Clin. Exp. Immunol. 117:106-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozes, E. J., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vandembussche, J. P. Van Vooren, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the complex of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 23.Manickan, E. R., J. D. Rouse, Z. Yu, W. S. Wire, and B. T. Rouse. 1995. Genetic immunization against herpes simplex virus. J. Immunol. 155:259-268. [PubMed] [Google Scholar]
- 24.Oliveira, S.C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 25.Olsen, S. C., D. Evans, S. G. Hennager, N. F. Cheville, and M. G. Stevens. 1996. Serologic responses of Brucella abortus strain 19 calfhood-vaccinated cattle following adult vaccination with strain RB51. J. Vet. Diagn. Invest. 8:451-454. [DOI] [PubMed] [Google Scholar]
- 26.Peet, N. M., J. A. McKeating, B. Ramos, T. Klonisch, J. B. De Souza, P. J. Delves, and T. Lund. 1997. Comparison of nucleic acid and protein immunization for induction of antibodies specific for HIV-1 gp120. Clin. Exp. Immunol. 109:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertmer, T. M., M. D. Eisenbraun, D. McCabe, S. K. Prayaga, D. H. Fuller, and J. R. Haynes. 1995. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine 13:1427-1430. [DOI] [PubMed] [Google Scholar]
- 28.Stevens, M. G., S. C. Olsen, and G. W. Pugh, Jr. 1994. Lymphocyte proliferation in response to Brucella abortus 2308 or RB51 antigens in mice infected with strain 2308, RB51, or 19. Infect. Immun. 62:4659-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabatabai, L. B., and G. W. Pugh, Jr. 1994. Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 30.Tascon, R. E., M. J. Colston, S. Ragno, E. Stovropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]
- 31.Tighe, H., M. Corr, M. Roman, and E. Raz. 1998. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol. Today 19:89-97. [DOI] [PubMed] [Google Scholar]
- 32.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. Dewitt, A. Friedman, L. A. Hawe, K. R. Leander, D. Martinez, H. C. Perry, J. W. Shiver, D. L. Montgomery, and M. A. Liu. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 33.Velikovsky, C. A., J. Cassataro, M. Sanchez, L. Fainboim, C. A. Fossati, and M. Spitz. 2000. Single-shot plasmid DNA intrasplenic immunization for the production of monoclonal antibodies. Persistent expression of DNA. J. Immunol. Methods 244:1-7. [DOI] [PubMed] [Google Scholar]
- 34.Wynn, T. A., I. Eltoum, A. W. Cheever, F. A. Lewis, W. C. Gause, and A. Sher. 1993. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J. Immunol. 151:1430.. [PubMed] [Google Scholar]
- 35.Young, E. J. 1983. Human brucellosis. Rev. Infect. Dis. 5:821-842. [DOI] [PubMed] [Google Scholar]
- 36.Zhan, Y., and C. Cheers. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan, Y., A. Kelso, and C. Cheers. 1993. Cytokine production in the murine response to Brucella infection or immunization with antigenic extracts. Immunology 80:458-464. [PMC free article] [PubMed] [Google Scholar]