Abstract
Neisseria gonorrhoeae is capable of utilizing a variety of iron sources in vitro, including human transferrin, human lactoferrin, hemoglobin, hemoglobin-haptoglobin complexes, heme, and heterologous siderophores. Transferrin has been implicated as a critical iron store for N. gonorrhoeae in the human male urethra. The demonstration that gonococci can infect the lower genital tracts of estradiol-treated BALB/c mice in the absence of human transferrin, however, suggests that other usable iron sources are present in the murine genital tract. Here we demonstrate that gonococcal transferrin and hemoglobin receptor mutants are not attenuated in mice, thereby ruling out transferrin and hemoglobin as essential for murine infection. An increased frequency of phase variants with the hemoglobin receptor “on” (Hg+) occurred in ca. 50% of infected mice; this increase was temporally associated with an influx of neutrophils and detectable levels of hemoglobin in the vagina, suggesting that the presence of hemoglobin in inflammatory exudates selects for Hg+ phase variants during infection. We also demonstrate that commensal lactobacilli support the growth of N. gonorrhoeae in vitro unless an iron chelator is added to the medium. We hypothesize that commensal lactobacilli may enhance growth of gonococci in vivo by promoting the solubilization of iron on mucosal surfaces through the production of metabolic intermediates. Finally, transferrin-binding lipoprotein (TbpB) was detected on gonococci in vaginal smears, suggesting that although gonococci replicate within the genital tracts of mice, they may be sufficiently iron-stressed to express iron-repressible proteins. In summary, these studies support the potential role of nontransferrin, nonhemoglobin iron sources during gonococcal infection of the female genital tract.
Neisseria gonorrhoeae is a human-specific, sexually transmitted pathogen that most commonly infects the lower urogenital tract, namely, the urethra of males and the endocervix and/or urethra of females of reproductive age. The rectum, pharynx, and conjunctiva may also be colonized. Ascension to the upper reproductive tract occurs in both sexes, and dissemination to the bloodstream with hematogenous spread to the joints and skin occurs in 0.5 to 3% of lower genital tract infections (25, 26).
Growth of N. gonorrhoeae in different body sites, like those of most microbial pathogens, is challenged by the sequestration of extracellular iron by host glycoproteins and iron-containing compounds. The major iron-binding protein in serum is transferrin. Heme bound to hemoglobin and to hemoglobin-haptoglobin complexes is also present in the bloodstream due to spontaneous lysis of erythrocytes. Free heme may also be present; however, the majority is rapidly bound to hemopexin upon release. Lactoferrin is the most abundant iron-binding glycoprotein in mucosal secretions, although lower concentrations of transferrin are also present. The female genital tract, unlike other mucosal surfaces, is cyclically enriched for iron during menses in the form of heme, hemoglobin, and hemoglobin-haptoglobin complexes. Nonsterile mucosal sites such as the rectum, pharynx, vagina, and endocervix contain iron bound to siderophores produced by commensal flora. Finally, heme and ferritin, the major intracellular storage form of iron, are also present on mucosal surfaces due to the constant sloughing of epithelial cells or following tissue damage (reviewed in references 22 and 53).
The pathogenic neisseriae are classic examples of bacteria that have met the challenge of iron limitation in vivo by evolving specific surface receptors for host iron-binding proteins. Both N. meningitidis and N. gonorrhoeae obtain iron from transferrin through the expression of a two-component outer membrane receptor (Tbp) (1, 15, 27, 36). Similarly, both pathogens can utilize heme bound to hemoglobin or to hemoglobin-haptoglobin complexes through the expression of the hemoglobin receptor, HpuAB (9, 10, 37). Meningococci make an additional hemoglobin receptor, HmbR (54, 55). Neisseria spp. can also utilize free heme as an iron source, but not heme bound to hemopexin (17, 19, 43). Only approximately 50% of gonococcal strains can utilize lactoferrin as an iron source (44), a finding that suggests that lactoferrin is not an essential source of iron for this pathogen despite its abundance on mucosal surfaces. In addition to stripping iron from host transport proteins, gonococci can also scavenge iron from siderophores produced by other bacteria (8, 43, 48) via the expression of specific surface receptors. FetA is the best-characterized siderophore receptor in N. gonorrhoeae; FetA binds to ferric enterobactin and is necessary for growth in the presence of ferric enterobactin as a sole iron source (8).
The neisserial transferrin, lactoferrin, and hemoglobin (Hpu) receptors are the products of the tbpBA, lbpBA, and hpuAB operons, respectively (reviewed in reference 53). Acquisition of iron from transferrin and lactoferrin by the Tbp and Lbp receptors is restricted to that of humans (35). In contrast, the Hpu receptor has a much broader host range, and hemoglobin from a variety of mammalian sources can be utilized as an iron source (55). All three receptors require the TonB-ExbB-ExbD inner membrane complex to energize transport through the outer membrane (4, 56), although a second, less efficient TonB-like system may perform a similar function (18). All three receptors are repressed on iron-supplemented media via regulation by a ferric uptake regulator (Fur) homologue (3, 58). Regulation of the hpuAB operon is further complicated by the fact that it undergoes phase-variable expression (10, 38).
The relevance of different host iron stores to the site of neisserial infection has been tested in a variety of infection models. It has long been known that experimental meningococcal septicemia and nasopharyngeal colonization of mice can be enhanced by the coadministration of iron dextran (24, 50, 51), human transferrin, human lactoferrin (52), or hemoglobin (7). Similarly, growth of N. gonorrhoeae in the peritoneums of adult mice and dissemination into the bloodstream required the administration of hemoglobin (13). These studies demonstrate that iron is critical for systemic infection and that transferrin and hemoglobin can fulfill this nutritional requirement. The capacity of N. meningitidis to utilize hemoglobin during systemic infection is further supported by the reduced recovery of a meningococcal hmbR mutant from the bloodstreams of infant rats (54). Recently, meningococcal tonB, exbB, and exbD mutants were identified as attenuated in causing septicemia in infant rats by using signature-tagged mutagenesis (57). This finding suggests that one or more TonB-dependent receptors may be critical for survival of N. meningitidis in the bloodstream. With regard to genital tract infection, the importance of the gonococcal transferrin receptor during urethritis is strongly supported by human volunteer studies in which a genetically engineered Tbp mutant of a naturally lactoferrin receptor-deficient gonococcal strain was noninfectious compared to an equivalent dose of the parent strain (16). Recently, however, we reported long-term recovery of N. gonorrhoeae from the genital tracts of estradiol-treated BALB/c mice in the absence of exogenously administered iron (29). High numbers of gonococci were isolated from vaginal swabs, and quantitative cultures evaluated over time suggested that replication of gonococci occurred in vivo. Here we investigate the iron sources utilized by N. gonorrhoeae during experimental murine genital tract infection as a means to identify iron stores other than transferrin and lactoferrin that may play a role during gonococcal infection of women.
MATERIALS AND METHODS
Bacterial strains.
N. gonorrhoeae strain FA1090 (porB1a, AHU (an auxotype for arginine, hypoxanthine, and uracil), serum resistant) was originally isolated from a female with disseminated gonococcal infection and has been extensively tested in male volunteers (12). Strain FA1090 is naturally deficient in expressing the components of the Lbp receptor. Strain FA6916 is a ΔtbpB-tbpA deletion mutant of FA1090, which is unable to utilize human transferrin as an iron source (16). FA6982 (kindly provided by P. Frederick Sparling, University of North Carolina) is an Hpu receptor mutant of strain FA1090 containing an omega (Ω) cassette within the hpuA structural gene. FA6982 expresses neither HpuA nor HpuB due to the polar nature of the insertion (10). Lactobacillus jensenii (ATCC 25258) was obtained from the American Type Culture Collection (Manassas, Va.). Commensal vaginal isolates from BALB/c mice were tentatively identified as lactobacilli based on Gram stain characteristics and the ability to grow anaerobically and on lactobacillus MRS agar. Strains LB10A and LB11 were identified as Lactobacillus murinus by 16S ribosome sequence analysis (Laboratory for Molecular Typing, Ithaca, N.Y.), and were isolated from mice that were concurrently infected with N. gonorrhoeae. All lactobacillus isolates from mice were phenotypically negative for H2O2 production by the qualitative chromogenic agar assay of McGroarty et al. (42).
Bacterial culture conditions.
N. gonorrhoeae was cultured in 7% CO2 at 37°C on GC agar (GC medium base) containing Kellogg's supplement I (32) and 12 μM Fe(NO3)3. GC agar with antibiotic selection (GC-vancomycin, colistin, nystatin, trimethoprim sulfate [VCNTS]) was used to isolate N. gonorrhoeae from murine vaginal mucus as described previously (29). Gonococci that were capable of utilizing hemoglobin as a sole iron source (Hg+ variants) were isolated on GC-Hg agar, which consisted of GC-VCNTS agar without Fe(NO3)3 to which desferoxamine mesylate (DF) (final concentration, 50 μM) and human hemoglobin (final concentration, 0.2 mg/ml) (Sigma, St. Louis, Mo.) were added. Lactobacilli were cultured on Lactobacillus MRS agar in 5% CO2 at 37°C. Heart infusion agar (HIA) was used in lactobacillus coculture experiments and to isolate murine commensal flora. All bacterial culture media were from Difco (Becton Dickinson, Sparks, Md.).
Experimental infection model.
Groups of female BALB/c mice (4 to 6 weeks old; National Cancer Institute, Bethesda, Md.) were treated with 17-β-estradiol to promote long-term susceptibility to N. gonorrhoeae as described previously (29), with the modification that mice were treated with only streptomycin sulfate (0.24 mg intraperitoneally, twice daily) and trimethoprim sulfate (0.04 g/100 ml of drinking water) to inhibit the overgrowth of commensal flora that occurs under the influence of estradiol. Intravaginal inoculation of mice with N. gonorrhoeae and monitoring of infection were as described previously (29). Briefly, mice were inoculated intravaginally with ∼106 CFU of piliated FA1090, FA6916 (ΔtbpB-tbpA), or FA6982 (hpuA::Ω) suspended in phosphate-buffered saline (PBS). Vaginal mucus was collected daily for 10 days with a sterile swab, and samples of undiluted and diluted swab contents were plated on GC-VCNTS agar. A small portion of sample was also cultured on HIA and lactobacillus MRS agar to monitor the presence of facultatively anaerobic commensal flora. The percentage of polymorphonuclear leukocytes (PMNs) among 100 vaginal cells was determined by microscopic examination of stained vaginal smears. Animal experiments were conducted at the Uniformed Services University of the Health Sciences, a facility fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, under a protocol that was approved by the University's Institutional Animal Care and Use Committee.
Analysis of vaginal isolates for frequency of Hg+ variants.
In some experiments, the frequency of gonococcal Hg+ variants among the inoculum and vaginal isolates was determined by culturing equal volumes of undiluted and diluted samples of inoculum or vaginal swab suspensions on GC-VCNTS agar and on GC-Hg agar (also with VCNTS selection). The ratio of the number of CFU isolated on GC-Hg agar to the number of CFU isolated on GC agar was determined to obtain the frequency of Hg+ variants in each population. To confirm that the hpuAB operon was in frame in Hg+ variants, a 640-bp region corresponding to the 5′ end of hpuA was PCR amplified from lysates prepared directly from primary colony isolates by using 5′-CGCCCCATAAAACAGTTGACAT and 5′-AAGAGGTCGATTTCGCCGTTAG as the forward and reverse primers, respectively. The resultant PCR product was gel purified and sequenced directly with 5′-ATGGAAATGCCGGATGTGTTT as the sequencing primer. Nucleotide sequencing and primer synthesis were performed by the Uniformed Services University of the Health Sciences BioInstrumentation Center.
Hemoglobin enzyme-linked immunosorbent assay.
The concentration of hemoglobin in vaginal washes was determined by a capture enzyme-linked immunosorbent assay method as follows. Vaginal mucus was collected by gently pipetting 30 μl of PBS in and out of the mouse vagina two times with a Pipetteman. The recovered fluid was added to a polypropylene microcentrifuge tube containing 2 μl of 5% bovine serum albumin, which served as a carrier protein. Samples were collected 1 h after a vaginal swab was taken for culture on days 1, 3, 5, 7, and 9 following bacterial inoculation. Samples were further diluted to 150 μl and were ultrasonically irradiated for 2 min (model 250/450 Sonifier [Branson Ultrasonics Corporation, Danbury, Conn.]; output control, 7; cycle dial, 90%) to release hemoglobin from erythrocytes. Wells were coated with 100 μl of goat anti-human hemoglobin antibody (2 ng/ml; Bethyl Laboratories, Montgomery, Tex.). The plates were washed, and wells were blocked with 3% bovine serum albumin-PBS with 0.05% Tween 20. One hundred microliters of vaginal wash sample or mouse hemoglobin standards was dispensed, and plates were incubated overnight at 4°C. After washing, 100 μl of goat anti-human hemoglobin antibody conjugated to horseradish peroxidases was added to all wells and incubated for 1 h at room temperature. The color reaction was developed by addition of 200 μl of o-phenylenediamine substrate (Sigma, St. Louis, Mo.) with 0.01% H2O2 in citrate buffer, and the mixture was incubated 20 min at room temperature in the dark. Optical density was measured at 450 nm with a SpectraMAX 250 multiwell spectrophotometer (Molecular Devices, Sunnyvale, Calif.).
Coculture experiments.
The ability of lactobacilli to support the growth of N. gonorrhoeae strain FA1090 in vitro was tested using an agar overlay assay. Briefly, lactobacilli were grown overnight on MRS agar and then suspended in sterile saline to an optical density of 0.3 at 600 nm. Fifty microliters of each lactobacillus suspension to be tested was inoculated onto HIA and HIA containing 50 μM DF. The inoculum was allowed to dry, and the plates were incubated overnight in 5% CO2 at 37°C. The following day, HIA containing 50 μM DF was prepared, and before solidification, N. gonorrhoeae strain FA1090 was added to a concentration of ∼6 × 106 CFU/ml. Eight milliliters of the inoculated agar suspension was poured over the HIA base upon which lactobacilli had been cultured overnight. Plates were incubated as described above and examined for growth of N. gonorrhoeae after 24 and 48 h of incubation. To test whether the addition of hemoglobin increases the plating efficiency of HIA for N. gonorrhoeae, single colonies of Hg+ or Hg− variants were cultured on GC-Hg and regular GC agar, respectively. Offspring from each colony was suspended in saline to a concentration of ca. 108 CFU/ml, and equal volumes of serial dilutions were cultured on GC agar, GC-Hg agar, HIA, and HIA containing 0.2 mg of hemoglobin per ml in triplicate. A suspension of FA6982 (hpuA::Ω) was tested in parallel as a control.
Indirect fluorescent-antibody staining.
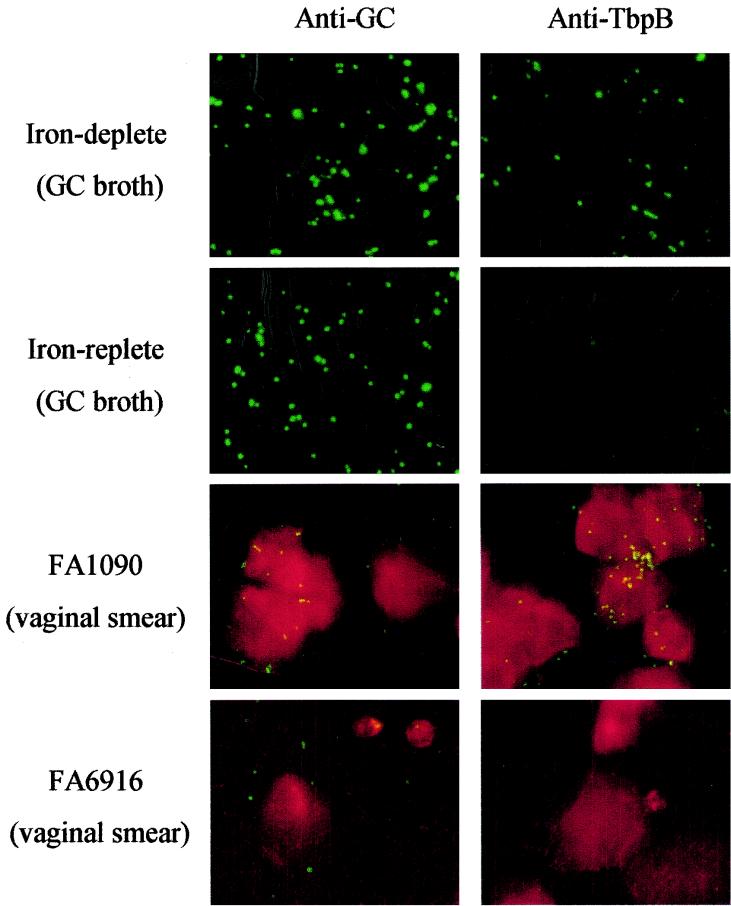
Expression of TbpB during experimental infection of mice was detected by indirect immunofluorescence staining of vaginal smears with rabbit polyclonal serum against purified TbpB as the primary antibody (a generous gift of Fred Sparling and Christopher Thomas, University of North Carolina). Briefly, vaginal specimens were collected with a sterile swab and smeared in duplicate onto glass slides (Electron Microscopy Sciences, Ft. Washington, Pa.). The samples were fixed in 100% methanol at −20°C before staining. Samples of strain FA1090 cultured in vitro under iron-replete and iron-limited conditions as described by Chen et al. (9) were included as controls in each experiment. Slides were incubated for 1 h at room temperature with PBS containing 0.1% immunoglobulin-free bovine serum albumin (Sigma) to block nonspecific binding. Samples were then incubated with polyclonal anti-TbpB (1:1,000) or polyclonal rabbit serum raised against whole N. gonorrhoeae strain FA1090 (anti-GC) (1:2,500) for 1 h at room temperature. Goat anti-rabbit immunoglobulin G (whole molecule, fluorescein isothiocyanate conjugate) (Sigma) was used as the secondary antibody (30 min, room temperature), and samples were rinsed three times with PBS containing 0.05% Tween 20 after incubation with primary and secondary antibodies. Evans Blue (Sigma) was used to counterstain vaginal epithelial cells by diluting a 0.5% (wt/vol) stock 1:300 in the secondary antibody solution. The average number of gonococci recognized by anti-TbpB serum compared to that recognized by anti-GC serum was based on the number of fluorescent gonococci seen in each of five fields at a magnification of ×40 under a fluorescence microscope (Olympus BX60; wavelength spectrum. 560 to 650 nm). All slides were examined by two independent viewers.
Statistical analysis.
The duration of recovery and number of gonococci isolated at selected time points were compared between experimental groups by using the two-tailed unpaired t test at a 95% confidence interval (GraphPad Software, San Diego, Calif.).
RESULTS
Ability of gonococcal transferrin and hemoglobin receptor mutants to cause experimental infection of mice.
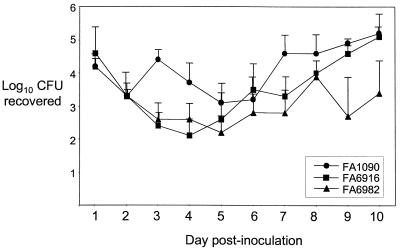
N. gonorrhoeae can persist in the genital tracts of estradiol-treated mice in the absence of exogenously administered iron such as human transferrin for an average of 12 days in a 14-day experiment (29) and for as long as 40 days postinoculation (A. N. Bordner and A. E. Jerse, unpublished data). This result is in contrast to evidence that transferrin is critical for gonococcal survival in the human male urethra, based on the inability of a transferrin receptor mutant to infect male volunteers (16). In light of this apparent inconsistency, we considered the possibility that another ligand for the transferrin receptor may exist in vivo. We also considered the possibility that murine hemoglobin may be responsible for proliferation of gonococci in the mouse genital tract, since both murine and human hemoglobins can be utilized as an iron source via the Hpu receptor (54). To test these hypotheses, mice were inoculated intravaginally with 106 CFU of wild-type FA1090, FA6916 (ΔtbpB-tbpA), or FA6982 (hpuA::Ω), and infection was monitored over a period of 10 days. No significant difference was found between the average duration of recovery of the wild-type strain (6.9 days [range, 0 to 10 days]) and that of the Tbp mutant (8.9 days [range, 3 to 10 days] or the Hpu mutant (8.9 days [range, 4 to 10 days]) (P = 0.277 and P = 0.512, respectively). The number of bacteria recovered from individual mice in each group ranged from 10 to 106 CFU or greater per vaginal swab suspension, with an initial decline in recovery followed by a 1- to 4-log10-unit increase over time. There was no reproducible difference in the average number of viable gonococci recovered from daily vaginal swabs (Fig. 1). The demonstration that the Tbp mutant showed no difference from the wild-type strain with regard to infectivity in mice is consistent with the host restriction of the Tbp receptor. The fact that the Hpu mutant also retained infectivity for mice strongly suggests that hemoglobin is not responsible for long-term growth and survival of N. gonorrhoeae in the murine genital tract.
FIG. 1.
Recovery of wild-type N. gonorrhoeae, Tbp mutant FA6916, and Hpu mutant FA6982 from estradiol-treated mice over time. The average log10 CFU recovered in 100 μl of vaginal swab suspension was determined for mice inoculated with FA1090 (n = 5), FA6916 (n = 5), and FA6982 (n = 8) and plotted over time. Standard errors are shown. No significant difference in the number of gonococci recovered each day was detected except for day 3, on which significantly fewer gonococci were recovered from mice infected with the Tbp mutant (P = 0.002) and the Hpu mutant (P = 0.009). This difference, however, was not reproduced in a repeat experiment.
Frequency of Hg+ variants among vaginal isolates.
As shown in Fig. 1, the number of CFU recovered at each time point following inoculation varied between mice, and as previously observed (29), the number of CFU recovered from a single mouse often fluctuated between 1 and 4 log units over the course of infection. We hypothesized that the higher numbers of gonococci isolated from some mice, or from a single mouse on days of increased recovery, were due to the presence of hemoglobin in the vaginal lumen. To investigate this possibility, we took advantage of the fact that expression of the hpuAB operon is phase variable, switching from “off” (Hg−) to “on” (Hg+) at an in vitro rate of 10−3 to 10−4 per cell per generation in strain FA1090 (9). We predicted that if hemoglobin was responsible for increased replication of gonococci in vivo, a high frequency of Hg+ isolates would be present in samples from mice with high numbers of gonococci. Mice were inoculated with wild-type FA1090 or mutant FA6916 (ΔtbpB-tbpA), and the frequency of Hg+ variants in the inoculum and among vaginal isolates over time was determined. As shown in Table 1, a 1,000-fold or greater increase in the frequency of Hg+ variants was detected among vaginal isolates from three of eight mice infected with FA1090 (mice 2, 5, and 6), with the periods of increased frequency lasting 3 to 9 days. Transient 10- to 100-fold increases were detected in three of the remaining five mice (mice 1, 4, and 7). A prolonged increase (10-fold to greater than 1,000-fold) in Hg+ variants was detected in two of six mice infected with FA6916 (mice 11 and 14); 10-fold increases in Hg+ frequency were detected in two of the remaining four mice (mice 12 and 13), lasting 1 to 7 days.
TABLE 1.
Frequency of Hg+ variants isolated from mice infected with FA1090 or tbp mutant FA6916 during experimental murine infection
| Strain and mouse no. | Frequency of Hg+ variantsa among vaginal isolates on day:
|
|||||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | |
| FA1090 | ||||||
| 1 | <0.0067 | —b | — | <0.0014 | 0.0030c | 0.0010 |
| 2 | 0.0007 | <0.0006 | 0.40c | 0.21c | 0.0141c | 0.0007 |
| 3 | 0.0009 | <0.0001 | <0.0083 | 0.0002 | — | 0.0007 |
| 4 | — | — | 0.0200d | 0.0004 | <0.0014 | — |
| 5 | <0.0022 | <0.0005 | 0.35e | 0.27e | — | — |
| 6 | — | 0.26e | 0.76e | 0.62e | 1.18e | 1.00e |
| 7 | <0.0002 | 0.0007 | 0.0006 | <0.005 | 0.0067c | — |
| 8 | <0.0005 | 0.0016c | — | — | 0.0004 | — |
| FA6916 | ||||||
| 9 | <0.0067 | — | — | <0.0090 | <0.0050 | — |
| 10 | 0.0007 | 0.0002 | — | — | — | — |
| 11 | 0.0004 | <0.0010 | 0.0130c | 0.20e | <0.0200 | NDf |
| 12 | 0.0042c | 0.0028c | 0.0028c | 0.0080c | 0.0067c | ND |
| 13 | <0.0008 | 0.0005 | 0.0028c | 0.0001 | 0.0013 | ND |
| 14 | <0.0001 | 0.86e | 0.16d | 0.0818d | 0.72e | ND |
Combined data from two experiments are shown, the frequencies of Hg+ variants in the bacterial inocula were 0.0002 (FA1090, mice 1 to 8), 0.001 (FA6916, mice 9 and 10), and 0.0002 (FA6916, mice 11 to 14).
—, total counts were <100 CFU/100 μl of vaginal suspension.
The frequency of Hg+ variants was 10- to 100-fold greater than that of the inoculum.
The frequency of Hg+ variants was 100- to 1000-fold greater than that of the inoculum.
The frequency of Hg+ variants was more than 1,000-fold greater than that of the inoculum.
ND, not determined.
Phase-variable expression of hpuAB is hypothesized to occur via DNA strand slippage within the hpuA gene during replication, resulting in transcripts that are in or out of frame due to the loss or addition of a single guanine in the hpuA reading frame (10). To confirm that Hg+ isolates had undergone phase variation of the hpuA gene, the hpuA nucleotide sequence was determined for two to three Hg+ colonies from mice with a high frequency of Hg+ variants and for an Hg− colony from a mouse with no significant increase in Hg+ isolates during the course of infection (control lysate). As predicted, the nucleotide sequence obtained from the control lysate contained 9 guanines in the phase-variable region of hpuA, resulting in a stop codon 67 bases downstream from the ATG start site. In contrast, the PCR-amplified hpuA fragment from the Hg+ variants contained 10 guanines in the phase-variable region and was in frame (Fig. 2). Sequences were identical to the sequence of hpuA published previously (10).
FIG. 2.
Comparison of the hpuA nucleotide and predicted amino acid sequence in Hg+ and Hg− vaginal isolates from BALB/c mice. The 5′ end of the hpuA gene was PCR amplified directly from colonies isolated from one mouse with a frequency of Hg+ isolates similar to that of the inoculum (A) and from two mice with significantly increased frequencies of Hg+ variants (B). The region of the nucleotide sequence of the resultant PCR products containing the polyguanine stretch that is responsible for phase variation is shown (nucleotides 39 to 82 downstream of the start site).
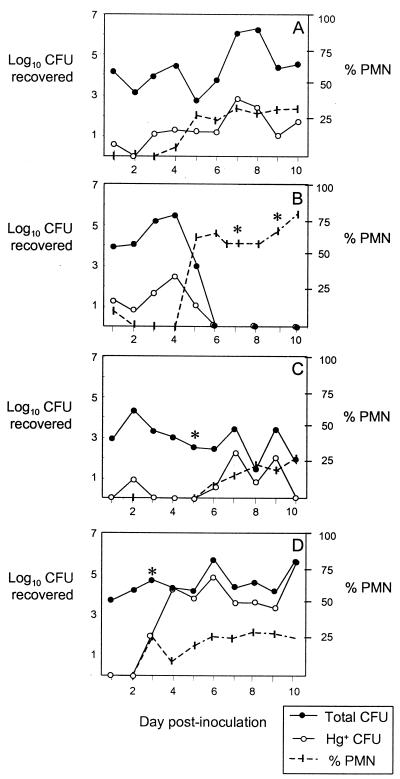
We did not detect a difference between the average numbers of gonococci recovered from mice that demonstrated an increased frequency of Hg+ variants in vivo and from mice with frequencies similar to that of the inoculum in any of three experiments. The lack of a significant difference in the daily recovery of gonococci from mice infected with wild-type FA1090 versus Hpu mutant FA6982 (Fig. 1) is consistent with this observation. These findings suggest that little or no selective advantage was conferred by the apparent selection for Hg+ variants in vivo. To further investigate if the increased frequency of Hg+ variants in some samples was due to selection by the presence of hemoglobin, we performed an additional experiment in which we also measured the concentration of hemoglobin in vaginal washes collected every other day during the course of infection. As before, mice were inoculated with FA1090 (n = 5) or FA6916 (n = 5), and the frequency of Hg+ variants among vaginal isolates was determined. Stained vaginal smears were also prepared and examined for the presence of PMNs to see if there was an association between inflammation and the frequency of Hg+ variants. Similar to what was found in the previous experiments, 5 of 10 mice demonstrated a 10-fold (3 mice) or 100- to 1,000-fold (2 mice) increase in Hg+ variants over that of the inoculum during the course of infection. All five of these mice had >10% PMNs on vaginal smears (range, 12 to 78%) at one or more time points following inoculation. Interestingly, the influx of PMNs occurred shortly before or on the day on which the increase in Hg+ variants was first detected. Hemoglobin was detected in vaginal washes from three of these five mice. The time point at which hemoglobin was detected consistently coincided with both the influx of PMNs into the vagina and the day on which a higher frequency of Hg+ variants was first observed. Comparisons of the isolation of Hg+ variants, the percentage of vaginal PMNs, and the detection of hemoglobin in vaginal washes from four mice with respect to time are shown in Fig. 3. We conclude from this analysis that hemoglobin selects for Hg+ variants during experimental murine infection and that its presence is associated with inflammation. Consistent with this conclusion is the fact that no PMNs were seen in vaginal smears from the five mice that showed no increased frequency of Hg+ variants during infection; similarly, no hemoglobin was detected in vaginal washes from these mice.
FIG. 3.
Comparison of changes in the frequency of Hg+ variants, the percentage of PMNs among vaginal cells, and the detection of hemoglobin in vaginal washes over time. Results are shown for two mice with a 100-fold increase (A and B; days 5 to 6 and 5, respectively) and two mice with a 100- to 1,000-fold increase (C and D; days 7 to 10 and 5 to 10, respectively) in Hg+ variants during the course of infection. Mice were inoculated with 106 CFU of FA1090 (C) or FA6916 (A, B, and D); the frequencies of Hg+ variants in the inocula were 0.0003 (FA1090) and 0.0002 (FA6916). The log10 CFU recovered from 100 μl of vaginal suspension on GC agar (total CFU) versus GC-Hg agar is shown on the left y axis. The daily percentage of PMNs detected among 100 vaginal cells is shown on the right y axis. Asterisks indicate time points at which hemoglobin was detected in vaginal washes (for panel B, >1,500 and 877 ng/ml on days 7 and 9, respectively; for panels C and D, 200 ng/ml on day 3 [the sensitivity of detection was 15 ng/ml]).
Support of gonococcal growth by commensal lactobacilli.
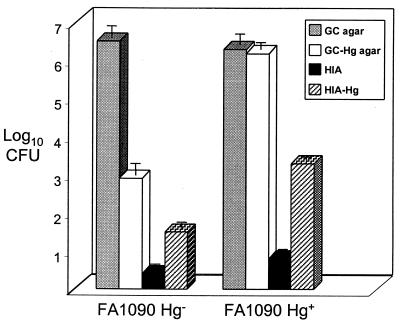
As previously noted, we did not observe a growth advantage in mice with detectable hemoglobin in the vaginal lumen. The isolation of high numbers of gonococci from mice with no evidence of selection for Hg+ variants suggested that other sources of iron might promote proliferation of N. gonorrhoeae in the genital tract. In our experience with experimental murine infection, we routinely observe that mice with commensal lactobacilli usually also have high numbers of vaginal gonococci. We therefore hypothesized that lactobacilli may enhance growth of N. gonorrhoeae in vivo. To test this hypothesis, we isolated several commensal vaginal isolates from BALB/c mice that appeared to be Lactobacillus spp. based on the criteria described in Materials and Methods. Two isolates of different colony morphology (LB10A and LB11) were definitively identified as different strains of L. murinus. We then tested whether L. murinus could support growth of N. gonorrhoeae strain FA1090 on HIA in the presence or absence of an iron chelator (DF). We chose HIA because strain FA1090 grows poorly on HIA, in part due to insufficient iron. As illustrated in Fig. 4, ca. 105 fewer CFU of FA1090 were recovered on HIA versus GC agar; the addition of hemoglobin increased the recovery of the FA1090 Hg− and Hg+ variants on HIA by ca. 1 and > 3 log10 units, respectively. There was no difference in plating efficiency on HIA and HIA-Hg for the Hpu mutant FA6982. It should be noted that the addition of hemoglobin did not completely support growth of Hg+ FA1090 to the level which occurs on iron-supplemented GC agar, suggesting that HIA lacks other nutrients that are necessary for optimum growth of N. gonorrhoeae.
FIG. 4.
Plating efficiency of FA1090 on GC agar, GC-Hg agar (containing 50 μM DF), HIA, and HIA-Hg (no chelator added). The plating efficiencies for bacterial suspensions containing predominantly Hg− or Hg+ phase variants were compared to show that the addition of hemoglobin to the agar increased recovery in a predictable manner. The number of CFU recovered from a suspension of growth derived from a single Hg− colony differed by ca. 4 log units on GC agar versus GC-Hg agar, as expected based on the rate of hpuAB phase variation in this strain. In contrast, similar numbers of CFU of a Hg+ variant were isolated on GC and GC-Hg agar.
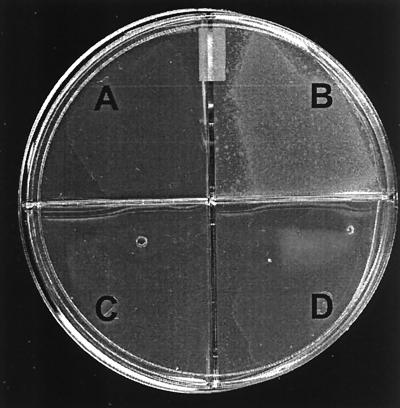
L. murinus supported growth of N. gonorrhoeae on unsupplemented HIA in the overlay assay described in Materials and Methods. Faint growth of gonococci (confirmed by oxidase test and Gram stain) could be seen overlying L. murinus on plates containing HIA base without DF after 24 h of incubation. Luxurious growth of N. gonorrhoeae was detected on top of the lactobacilli after an additional 24 h of incubation (Fig. 5). L. murinus did not support growth of N. gonorrhoeae on plates containing HIA plus DF as the base agar. The degrees of lactobacillus growth on base agar with and without DF were similar. L. jensenii, an H2O2-producing human strain, did not support growth of FA1090 in either the presence or absence of chelator. This result was expected, since H2O2-producing lactobacilli have been shown by others to inhibit N. gonorrhoeae (49, 60). None of the lactobacilli isolated from mice produced H2O2 as measured by a qualitative assay. From these data we conclude that commensal lactobacilli may increase the availability of iron to N. gonorrhoeae during infection of females, although the exact mechanism by which this occurs is not known.
FIG. 5.
Agar overlay assay illustrating the capacity of L. murinus to support growth of FA1090 on HIA unless an iron chelator (DF) is added to the base agar. FA1090 suspended in HIA with DF was overlaid on L. murinus cultured on HIA-DF (A), L. murinus cultured on HIA (B), HIA alone (no lactobacilli) (C), or L. jensenii strain 25258 (H2O2 producing) cultured on HIA (D). Growth of FA1090 occurred in quadrant B only. Lactobacilli present on the base agar are faintly visible in quadrants A and D.
Expression of transferrin-binding protein B in vivo.
Iron limitation is a major stimulus of gene expression in the pathogenic neisseriae (58). Based on the fact that transcription of the tbpBA operon is repressed by iron supplementation in vitro, we chose to explore the microenvironment of the lower murine genital tract with respect to iron availability indirectly, by staining for TbpB-expressing gonococci in vaginal smears in an indirect fluorescent-antibody technique. Vaginal smears from mice infected for 1 or 6 days with either FA1090 or the Tbp mutant FA6916 were incubated with either anti-TbpB serum or serum raised against whole N. gonorrhoeae (anti-GC) followed by fluorescein-tagged secondary antibody. The average numbers of gonococci detected by each serum were enumerated and compared. As shown in Table 2 and Fig. 6, the anti-TbpB and anti-GC sera detected similar numbers of gonococci in smears from the majority (six of eight) of mice infected with wild-type FA1090. Different numbers of gonococci were recognized by anti-GC and anti-TbpB sera in smears from two mice (mice 1 and 3); these results may be explained by an unequal distribution of gonococci on the slides. In contrast to gonococci recovered from mice infected with FA1090, no binding of the anti-TbpB antibodies to gonococci in vaginal smears from mice infected with mutant FA6916 was observed (Fig. 6; Table 2). Although other regulatory stimuli may influence transcription of the tbpBA operon (47), these results suggest that although N. gonorrhoeae can replicate in the lower genital tracts of mice, iron may be sufficiently limited to induce the expression of iron-repressed virulence factors during infection.
TABLE 2.
Detection of gonococci in vaginal smears from mice infected with N. gonorrhoeae strain FA1090 or tbp mutant FA6916 with anti-TbpB or anti-GC serum
| Mouse no. (inoculum) | Daya | Avg no. of fluorescent gonococci/ ×40 field (SD) with:
|
|
|---|---|---|---|
| Anti-GC | Anti-TbpB | ||
| 1 (FA1090) | 1 | 63 (3) | 13 (2) |
| 2 (FA1090) | 1 | 3 (1) | 2 (1) |
| 3 (FA1090) | 1 | 17 (4) | 71 (21) |
| 4 (FA1090) | 1 | 28 (10) | 25 (12) |
| 5 (FA1090) | 6 | 46 (27) | 66 (24) |
| 6 (FA1090) | 6 | >300 | >300 |
| 7 (FA6916) | 6 | 12 (4) | 0 |
| 8 (FA6916) | 6 | >100 | 0 |
| —b (FA1090), iron replete | >150 | 0 | |
| — (FA1090), iron limited | >150 | >150 | |
Day postinoculation.
—, in vitro growth conditions.
FIG. 6.
Vaginal smears from mice infected with FA1090 or FA6916 incubated with anti-TbpB antiserum or antiserum raised against whole gonococci (anti-GC) followed by a fluorescein isothiocyanate-conjugated secondary antibody. FA1090 grown in GC broth under iron-replete or iron-limited conditions was included as a control for each experiment.
DISCUSSION
N. gonorrhoeae is a highly human-adapted pathogen, a characteristic that is reflected in part by the human specificity of gonococcal transferrin and lactoferrin receptors (35). In light of the demonstration that the gonococcal transferrin receptor is critical for urethral infection in male volunteers (16), the capacity of N. gonorrhoeae to cause long-term colonization of the lower genital tracts of mice in the absence of human transferrin is perhaps surprising. Here we explored this apparent inconsistency to identify sources of iron other than lactoferrin or transferrin that may promote growth of N. gonorrhoeae in the female genital tract.
The human female lower genital tract differs from the male urethra with regard to potential available iron sources in several critical ways. For example, the lower pH of the female genital tract may promote the reduction of ferric iron to the more soluble ferrous state, as well as facilitate iron release from host iron-binding glycoproteins (39). The passage of hemoglobin during menses dramatically creates an iron-rich environment in which N. gonorrhoeae presumably can flourish. Lactoferrin levels also cyclically fluctuate, with the highest levels occurring in endometrial tissue during the secretory phase of the cycle (39) and in the vaginal mucus just after menses (11). Finally, unlike the region of the male urethra that is colonized by N. gonorrhoeae, an abundance of commensal flora reside in the vaginal and endocervical mucosa. These microbes may supply N. gonorrhoeae with iron in the form of loaded siderophores. Additionally, commensal flora may produce metabolic intermediates that can form complexes with iron that have been shown to support growth of N. gonorrhoeae in vitro (43).
The murine genital tract naturally lacks human transferrin and human lactoferrin, and it is not cyclically exposed to high concentrations of hemoglobin since mice do not menstruate. The female mouse genital tract, however, is similar to that of human females with respect to oxygen tension, cervical pH, commensal flora, and hormonally driven changes in mucus and histology (5, 14). One or more of these factors may be responsible for the iron stores that N. gonorrhoeae utilizes during murine infection. For example, it is likely that that the high rate of epithelial cell proliferation and death that occurs under the influence of estrogen may enrich the mucosa with iron from intracellular stores. While this hypothesis is particularly relevant to gonococcal infection of estradiol-treated mice, there may be parallels with human infection, since several studies have found that infected women have a higher positive culture rate during the proliferative (high-estrogen) stage than during the secretory stage of the menstrual cycle (28, 30, 34, 40).
The presence of commensal vaginal flora may also play an important role in providing usable iron to N. gonorrhoeae during experimental infection of mice. Although mice are treated with antibiotics to reduce the commensal flora load in our model, some mice remain colonized with facultatively anaerobic flora, most commonly gram-positive cocci and lactobacilli. Here we provide in vitro evidence that lactobacilli, in particular, may enhance growth of N. gonorrhoeae in vivo. The ability to prevent the observed enhancement of growth in vitro with an iron chelator suggests that lactobacilli may create an iron-rich environment. Lactobacilli are unusual among microbes in that they do not require iron for growth. The exact mechanism by which lactobacilli support growth of N. gonorrhoeae in vitro is presently unclear; however, it is conceivable that the production of acids as part of their fermentative pathway increases the solubility of iron. Other metabolic intermediates produced by lactobacilli may also form complexes with iron which N. gonorrhoeae might then utilize (43). In vivo, the reduced pH resulting from acid production by lactobacilli may cause the release of iron from murine transferrin and lactoferrin. An additional way by which lactobacilli might enhance gonococcal infection is via the production of lactate, which when used as a carbohydrate source in vitro increases the resistance of gonococci to complement-mediated lysis via induction of higher levels of lipooligosaccharide (20, 45) and, in particular, of a lipooligosaccharide species that can be posttranslationally sialylated (41). It should be noted that we generally do not isolate L. murinus from estradiol-treated mice until 4 to 5 days after implantation of the estradiol pellet; this emergence of lactobacilli often parallels the increased recovery of N. gonorrhoeae that occurs during the course of experimental murine infection. An association between lactobacillus colonization and increased recovery of N. gonorrhoeae during the course of infection is not found for all mice, however, suggesting that other factors may also play a role. Hormonally driven fluctuations in other commensal flora, including obligate anaerobes for which we did not culture, may also enhance growth of N. gonorrhoeae through the production of siderophores or certain nutrients.
Lactobacilli are the predominant facultatively anaerobic flora of the vagina and endocervix (23, 46). Hydrogen peroxide-producing lactobacilli are considered healthy genital flora due to an inverse association between colonization with H2O2-producing lactobacilli and bacterial vaginosis (33) and the ability of H2O2-producing lactobacilli to inhibit a variety of sexually transmitted pathogens in vitro, including N. gonorrhoeae (49, 60). Clinical surveys comparing the types of vaginal and endocervical flora with the incidence of gonorrhea further support the ability of H2O2-producing lactobacilli to protect against gonorrhea (2, 49). Non-H2O2-producing lactobacillus species also reside in the female genital tract, however, and our demonstration that non-H2O2-producing lactobacilli support growth of N. gonorrhoeae in vitro suggests that some lactobacilli may actually play a negative role in combating gonococcal infection.
Although the hemoglobin receptor was not critical for murine genital tract infection, we did detect an increased frequency of Hg+ phase variants during infection in some mice. A temporal relationship between the influx of PMNs into the vagina, the detection of hemoglobin, and the isolation of Hg+ variants at an increased frequency suggests that hemoglobin accompanies inflammatory exudates into the vaginal lumen. Although one might predict that the presence of hemoglobin would increase the overall recovery of N. gonorrhoeae, we did not find this association. It is possible that any growth advantage conferred to the gonococcus by hemoglobin may be balanced by the killing of gonococci by components of the inflammatory response. The association between the host response and the presence of hemoglobin is consistent with the detection by Genco et al. (21) of heme in the form of hemoglobin in transudate fluid within mouse subcutaneous chambers. It is important to note, however, that in contrast to what we have found in lower genital tract infection of mice, proliferation of gonococci in subcutaneous chambers appeared to rely on the capacity to utilize hemoglobin as an iron source. This difference likely reflects the different iron stores that are available to N. gonorrhoeae in these two body sites.
At this time, we do not know if the presence of hemoglobin and the influx of PMNs seen in some mice occurred in response to gonococcal infection or if it was due to microhemorrhage produced by our culturing technique. Previously we showed that an increase in PMNs occurred in 80% of infected mice compared to placebo-treated controls, which were also swabbed daily (29), suggesting that the onset of PMN infiltration in this model was in response to bacterial infection. Studies designed to minimize trauma to the mucosa during the collection of vaginal samples are under way to resolve this issue.
Finally, the detection of TbpB on gonococci within vaginal smears from infected mice suggests that although there is adequate iron for growth of gonococci within the lower genital tracts of estradiol-treated mice, it may be sufficiently limited to induce an iron-stressed phenotype. Numerous gonococcal proteins appear to be expressed under conditions of iron limitation (58). Many of these products may be virulence factors, based on the demonstration that iron-starved meningococci (6) and gonococci (31) display increased virulence in animal models. The evidence that an iron-stressed phenotype is induced during experimental murine infection supports the use of estradiol-treated mice as a tool for studying the expression of other iron-regulated genes in vivo, as well as for testing candidate vaccines directed against iron-repressed surface molecules.
Acknowledgments
We thank Fred Sparling and Christopher Thomas for providing the hpuA mutant and anti-TbpB serum.
This work was supported by Public Health Service grants RO1-AI4372053 (to A.E.J), HUD-B 98 SP MD 074 (to K.M.), and PO1 AI45967 (to A.E.J., T.R.M., and K.M.) from the National Institute of Allergy and Infectious Diseases.
Editor: V. J. DiRita
REFERENCES
- 1.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonio, M. A., S. E. Hawes, and S. L. Hillier. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180:1950-1956. [DOI] [PubMed] [Google Scholar]
- 3.Berish, S. A., S. Subbarao, C. Y. Chen, D. L. Trees, and S. A. Morse. 1993. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect. Immun. 61:4599-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas, G. D., J. E. Anderson, and P. F. Sparling. 1997. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB and exbD genes. Mol. Microbiol. 24:169-179. [DOI] [PubMed] [Google Scholar]
- 5.Braude, A. I., L. B. Corbeil, S. Levine, J. Ito, and J. A. McCutchan. 1978. Possible influence of cyclic menstrual changes on resistance to the gonococcus, p. 328-337. In G. F. Brooks, E. C. Gotschlich, K. K. Holmes, W. D. Sawyer, and F. E. Young (ed.), Immunobiology of Neisseria gonorrhoeae. American Society for Microbiology, Washington, D.C.
- 6.Brener, D., I. W. DeVoe, and B. E. Holbein. 1981. Increased virulence of Neisseria meningitidis after iron-limited growth at low pH. Infect. Immun. 33:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodeur, B. R., P. S. Tsang, J. Hamel, Y. Larose, and S. Montplaisir. 1986. Mouse models of infection for Neisseria meningitidis B,2b and Haemophilus influenzae type b diseases. Can. J. Microbiol. 32:3-37. [DOI] [PubMed] [Google Scholar]
- 8.Carson, S. D. B., P. E. Klebba, S. M. C. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, C.-J., P. F. Sparling, L. A. Lewis, D. W. Dyer, and C. Elkins. 1996. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect. Immun. 64:5008-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, C.-J., C. Elkins, and P. F. Sparling. 1998. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect. Immun. 66:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, M. S., B. E. Britigan, M. French, and K. Bean. 1987. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation and implications for infection with Neisseria gonorrhoeae. Am. J. Obstet. Gynecol. 157:1122-1125. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 13.Corbeil, L. B., A. C. Wunderlich, R. R. Corbeil, J. A. McCutchan, J. I. Ito, and A. I. Braude. 1979. Disseminated gonococcal infection in mice. Infect. Immun. 26:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbeil, L. B., A. Chatterjee, L. Foresman, and J. A. Westfall. 1985. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell 17:53-68. [DOI] [PubMed] [Google Scholar]
- 15.Cornelissen, C. N., G. D. Biswas, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 17.Desai, P. J., R. Nzeribe, and C. A. Genco. 1995. Binding and accumulation of hemin in Neisseria gonorrhoeae. Infect. Immun. 63:4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai, P. J., E. Garges, and C. A. Genco. 2000. Pathogenic neisseriae can use hemoglobin, transferrin, and lactoferrin independently of the tonB locus. J. Bacteriol. 182:5586-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer, D. W., E. P. West, and P. F. Sparling. 1987. Effects of serum carrier proteins on the growth of pathogenic neisseriae with heme-bound iron. Infect. Immun. 55:2171-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao, L., N. J. Parson, A. Curry, J. A. Cole, and H. Smith. 1998. Lactate causes changes in gonococci including increased lipopolysaccharide synthesis during short-term incubation in media containing glucose. FEMS Microbiol. Lett. 169:309-316. [DOI] [PubMed] [Google Scholar]
- 21.Genco, C. A., C.-Y. Chen, R. J. Arko, D. R. Kapczynski, and S. A. Morse. 1991. Isolation and characterization of a mutant of Neisseria gonorrhoeae that is defective in the uptake of iron from transferrin and haemoglobin and is avirulent in mouse subcutaneous chambers. J. Gen. Microbiol. 137:1313-1321. [DOI] [PubMed] [Google Scholar]
- 22.Genco, C. A., and P. J. Desai. 1996. Iron acquisition in the pathogenic Neisseria. Trends Microbiol. 4:179-184. [DOI] [PubMed] [Google Scholar]
- 23.Gorbach, L. L., K. B. Menda, H. Thadepalli, and L. Keith. 1973. Anaerobic microflora of the cervix in healthy women. Am. J. Obstet. Gynecol. 8:1053-1055. [DOI] [PubMed] [Google Scholar]
- 24.Holbein, B. E. 1981. Difference in virulence for mice between disease and carrier strains of Neisseria meningitidis. Can. J. Microbiol. 27:738-741. [DOI] [PubMed] [Google Scholar]
- 25.Holmes, K. K., G. W. Counts, and H. N. Beaty. 1971. Disseminated gonococcal infection. Ann. Intern. Med. 74:979-993. [DOI] [PubMed] [Google Scholar]
- 26.Hook, E. W., and H. H. Handsfield. 1999. Gonococcal infection in the adult, p. 451-472. In K. K. Holmes, P. A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill Companies, Inc., New York, N.Y.
- 27.Irwin, S. W., N. Averil, C. Y. Cheng, and A. B. Schryvers. 1993. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes tbpA and tbpB from Neisseria meningitidis. Mol. Microbiol. 8:1125-1133. [DOI] [PubMed] [Google Scholar]
- 28.James, J. F., and J. Swanson. 1978. Color/opacity colonial variants of Neisseria gonorrhoeae and their relationship to the menstrual cycle, p. 338-343. In G. F. Brooks, E. C. Gotschlich, K. K. Holmes, W. D. Sawyer, and F. E. Young (ed.), Immunobiology of Neisseria gonorrhoeae. American Society for Microbiology, Washington, D.C.
- 29.Jerse, A. E. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 67:5699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. W., K. K. Holmes, P. A. Kvale, C. W. Halverson, and W. P. Hirsch. 1969. An evaluation of gonorrhea case finding in the chronically infected female. Am. J. Epidemiol. 90:438-448. [DOI] [PubMed] [Google Scholar]
- 31.Keevil, C. W., D. B. Davies, B. J. Spillane, and E. Mahenthiralingam. 1989. Influence of iron-limited and replete continuous culture on the physiology and virulence of Neisseria gonorrhoeae. J. Gen. Microbiol. 135:851-863. [DOI] [PubMed] [Google Scholar]
- 32.Kellogg, D. S., W. L. Peacock, W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klebanoff, S. J., S. L. Hillier, D. A. Eschenbach, and A. M. Waltersdorph. 1991. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J. Infect. Dis. 164:94-100. [DOI] [PubMed] [Google Scholar]
- 34.Koch, M. L. 1947. A study of cervical cultures taken in cases of acute gonorrhea with special reference to the phases of the menstrual cycle. Am. J. Obstet. Gynecol. 54:861-866. [DOI] [PubMed] [Google Scholar]
- 35.Lee, B. C., and A. B. Schryvers. 1988. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol. Microbiol. 2:827-829. [DOI] [PubMed] [Google Scholar]
- 36.Legrain, M., V. Mazarin, S. W. Irwin, B. Bouchon, M.-R. Quentin-Millet, E. Jacobs, and A. B. Schryvers. 1993. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130:81-90. [DOI] [PubMed] [Google Scholar]
- 37.Lewis, L. A., E. Gray, Y. P. Wang, B. A. Roe, and D. W. Dyer. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol. Microbiol. 23:737-749. [DOI] [PubMed] [Google Scholar]
- 38.Lewis, L. A., M. Gipson, K. Hartman, T. Ownbey, J. Vaughn, and D. W. Dyer. 1999. Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol. Microbiol. 32:977-989. [DOI] [PubMed] [Google Scholar]
- 39.Masson, P. L., J. F. Heremans, and J. Ferin. 1968. Presence of an iron-binding protein (lactoferrin) in the genital tract of the human female. I. Its immunohistochemical localization in the endometrium. Fertil. Steril. 19:679-689. [DOI] [PubMed] [Google Scholar]
- 40.McCormack, W. M., R. J. Stumacher, K. Johnson, A. Donner, and R. Rychwalski. 1977. Clinical spectrum of gonococcal infection in women. Lancet ii:1182-1185. [DOI] [PubMed]
- 41.McGee, D. J., and R. F. Rest. 1996. Regulation of gonococcal sialyltransferase, lipooligosaccharide, and serum resistance by glucose, pyruvate, and lactate. Infect. Immun. 64:4630-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGroarty, J. A., L. Tomeczek, D. G. Pond, G. Reid, and A. W. Bruce. 1992. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J. Infect. Dis. 165:1142-1144. [DOI] [PubMed] [Google Scholar]
- 43.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mickelsen, P. A., E. Blackman, and P. F. Sparling. 1982. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. 35:915-920. [DOI] [PMC free article] [PubMed]
- 45.Parsons, N. J., J. P. Emond, M. Goldner, J. Bramley, H. Crooke, J. A. Cole, and H. Smith. 1996. Lactate enhancement of sialylation of gonococcal lipopolysaccharide and of induction of serum resistance by CMP-NANA is not due to direct activation of the sialyltransferase: metabolic events are involved. Microb. Pathog. 21:193-204. [DOI] [PubMed] [Google Scholar]
- 46.Redondo-Lopez, V., R. L. Cook, and J. G. Sobel. 1990. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev. Infect. Dis. 12:856-872. [DOI] [PubMed] [Google Scholar]
- 47.Ronpirin, C., A. E. Jerse, and C. N. Cornelissen. 2001. Gonococcal genes encoding transferrin-binding proteins A and B are arranged in a bicistronic operon but are subject to differential expression. Infect. Immun. 69:6336-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutz, J. M., T. Abdullah, S. P. Singh, V. I. Kalve, and P. E. Klebba. 1991. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J. Bacteriol. 173:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saigh, J. H., C. C. Sanders, and W. E. Sanders. 1978. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect. Immun. 19:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salit, I. E., E. Van Melle, and L. Tomalty. 1984. Experimental meningococcal infection in neonatal animals: models for mucosal invasiveness. Can. J. Microbiol. 30:1022-1029. [DOI] [PubMed] [Google Scholar]
- 51.Salit, I. E., and L. Tomalty. 1986. A neonatal mouse model of meningococcal disease. Clin. Investig. Med. 9:119-123. [PubMed] [Google Scholar]
- 52.Schryvers, A. B., and G. C. Gonzalez. 1989. Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infect. Immun. 57:2425-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 54.Stojiljkovic, I., V. Hwa, L. Saint Martin, P. O'Gaora, Z. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 55.Stojiljkovic, I., J. Larson, V. Hwa, S. Anic, and M. So. 1996. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J. Bacteriol. 178:4670-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stojiljkovic, I., and N. Srinivasan. 1997. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J. Bacteriol. 179:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, Y.-H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 58.Thomas, C. E., and P. F. Sparling. 1996. Isolation and analysis of a fur mutant Neisseria gonorrhoeae. J. Bacteriol. 178:4224-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West, S. E. H., and P. F. Sparling. 1987. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J. Bacteriol. 169:3414-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng, H., T. M. Alcorn, and M. S. Cohen. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J. Infect. Dis. 170:1209-1215. [DOI] [PubMed] [Google Scholar]