Abstract
The members of the genus Brucella are gram-negative, facultatively intracellular bacterial pathogens that cause brucellosis in many animal species and humans. Although live, attenuated vaccines are available to protect several animal species from the disease, there is no safe and effective vaccine for human use. Here we report that a bacterium that is closely related to Brucella species, Ochrobactrum anthropi, can be used as a vaccine vector for the delivery of Brucella antigens to mice, leading to the elicitation of protective immunity against brucellosis. Brucella abortus Cu,Zn superoxide dismutase (SOD), a protective Brucella antigen, was expressed in large amounts in O. anthropi strain 49237 by use of the broad-host-range plasmid pBBR1MCS. Neither O. anthropi strain 49237 nor the recombinant O. anthropi strain 49237SOD, expressing B. abortus Cu,Zn SOD, provided protection against virulent Brucella infection in mice. Analysis of immune responses indicated that strains 49237 and 49237SOD stimulated a mix of Th1 and Th2 type responses in the mice. After the immune response was switched to a Th1-biased response by addition of oligonucleotides containing unmethylated CpG motifs, both O. anthropi strain 49237 and the recombinant O. anthropi strain 49237SOD induced protection in mice. However, the protection conferred by strain 49237SOD was significantly better than that induced by the parental strain, 49237.
Brucella species are gram-negative, facultatively intracellular bacterial pathogens that can cause chronic infections in several terrestrial and marine mammals. Brucellosis in humans is primarily a zoonotic disease; infection is acquired either through consumption of contaminated dairy products or by coming in contact with infected animal secretions (1). In general, cell-mediated immune (CMI) responses play a major role in protection against brucellosis, although antibodies to O-polysaccharide (O antigen) of smooth lipopolysaccharide (LPS) appear to be protective in some host species (3, 4). Live, attenuated vaccines that can stimulate strong CMI responses are very effective against brucellosis. Attenuated strains such as Brucella melitensis Rev1 and Brucella abortus S19 and RB51 are being used to control brucellosis in domestic animals. However, there is no safe, effective vaccine available for human use; the vaccine strains used for animals are considered too virulent or unsafe for humans. Vaccines that will be noninfectious to humans but effective in stimulating a broad protective immune response in humans as well as in several domestic and wild animal species are needed to control brucellosis. To develop the next generation of Brucella vaccines, several research groups are pursuing different strategies, including development of subunit vaccines (30, 31), utilization of vaccinia virus as a vector (41), overexpression of protective homologous antigens (44), and creation of attenuated strains through deletion of specific genes (8, 16, 17). In this study, we examined if Ochrobactrum anthropi, a bacterium that is very closely related to Brucella, can be used as a vaccine or vaccine vector for brucellosis. O. anthropi is a gram-negative, rod-shaped, strictly aerobic, nonpigmented, oxidase-producing, non-lactose-fermenting bacillus that is motile by means of peritrichous flagella (22, 38, 40). There are at least 56 strains, and they are rarely pathogenic to humans. The close relationship between O. anthropi and Brucella has been clearly demonstrated through DNA-rRNA hybridization (14), PCR (33), delayed-type hypersensitivity reactivity of Brucella-infected animals (14), double gel immunodiffusion (39), and Western blot analysis (11, 39). For instance, DNA-rRNA hybridization has shown that Brucella was the nearest rRNA neighbor to O. anthropi (14). Unpublished results from our laboratory indicate that mouse sera obtained after infection or vaccination with B. abortus strain 2308 or RB51 recognize many O. anthropi antigens.
O. anthropi strain 47237 was originally isolated from soil and, unlike the majority of O. anthropi strains, is sensitive to almost all the common antibiotics (Y. He, R. Vemulapalli, and G. G. Schurig, unpublished data). O. anthropi strain 47237 does not appear to carry a plasmid, and it can be easily transformed with the broad-host-range plasmid pBBR1MCS, allowing the expression of Brucella antigens (this study). Brucella Cu,Zn superoxide dismutase (SOD) is a protective antigen (5, 31, 37). Escherichia coli expressing Brucella Cu,Zn SOD induced significant protection in mice against infection with the virulent B. abortus strain 2308 (31). Mice immunized with purified Brucella Cu,Zn SOD (5) or SOD synthetic peptides (37) developed significant protection against infection with the virulent strain 2308. Vaccination of mice with Brucella RB51 overexpressing homologous Cu,Zn SOD also stimulated enhanced protection (44). Since O. anthropi has many antigens that cross-react with Brucella, we hypothesized that intact O. anthropi strain 49237 alone or expressing Brucella Cu,Zn SOD would provide protection against virulent Brucella infection. In this study we demonstrated that O. anthropi or recombinant O. anthropi expressing SOD does not protect mice against Brucella challenge. Nevertheless, if the immune response is switched to a Th1 type by coadministration of a CpG adjuvant, significant protection against Brucella infection is achieved.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
O. anthropi strain 49237 was purchased from the American Type Culture Collection, Manassas, Va. B. abortus strains 2308 and RB51 were from our culture collection. All bacteria were grown in tryptic soy broth or on tryptic soy agar (TSA) plates. Chloramphenicol at a concentration of 30 μg/ml was added to the broth or agar during culture of bacteria containing the broad-host-range plasmid pBBR1MCS (26).
Reagents, antigens, and antisera.
A phosphothioate-modified oligonucleotide containing the CpG motif was custom synthesized by Genosys Biotechnologies, Inc. (The Woodlands, Tex.). The sequence of the oligonucleotide was 5′-TCC ATG ACG TTC CTG ATG CT-3′ (boldfaced letters represent the active motif). Brucella Cu,Zn SOD was purified by ion-exchange chromatography as reported previously (7, 44). Goat anti-B. abortus RB51 and goat anti-Cu,Zn SOD sera were available in our laboratory. E. coli strain DH5α (GIBCO BRL, Gaithersburg, Md.) was used for cloning the necessary plasmid constructs. Live B. abortus or O. anthropi strains were heat inactivated by incubating sealed tubes with bacteria in a 65°C water bath for 30 min.
Construction of recombinant O. anthropi strain 49237SOD.
A plasmid designated pBBSOD was previously constructed in our laboratory (44). This plasmid was constructed by subcloning a 1.1-kb DNA fragment of B. abortus containing the sodC gene (encoding Cu,Zn SOD) along with its promoter sequence into pBBR1MCS (26). Plasmids pBBSOD and pBBR1MCS were electroporated into the electrocompetent O. anthropi strain 49237 according to a protocol described previously for Brucella (29). Colonies of the O. anthropi strain 49237 containing pBBSOD or pBBR1MCS (designated O. anthropi strain 49237SOD or 49237pBB, respectively) were selected from TSA plates containing chloramphenicol at a concentration of 30 μg/ml.
Protection experiment with mice.
To determine whether O. anthropi 49237 or O. anthropi 49237SOD could stimulate protection against virulent Brucella infection in mice, four different protection experiments were performed. In experiment 1, groups of eight mice were inoculated intraperitoneally (i.p.) with either saline (negative control), B. abortus strain RB51 (2 × 108 CFU/mouse; positive control), recombinant B. abortus strain RB51SOD (2 × 108 CFU/mouse; positive control), O. anthropi 49237 (5 × 108 CFU/mouse), O. anthropi 49237pBB (5 × 108 CFU/mouse), or O. anthropi 49237SOD (5 × 108 CFU/mouse). At 2, 4, and 6 weeks after inoculation, three mice from each group were bled retroorbitally, and sera were obtained and stored at −40°C until use in an enzyme-linked immunosorbent assay (ELISA) or Western blot analysis. Six weeks after inoculation, five mice from each group were challenged i.p. with 2 × 104 CFU of virulent B. abortus 2308/mouse. These mice were killed 2 weeks later, spleens were collected and homogenized, and dilutions were plated to determine the numbers of Brucella CFU per spleen (37). The remaining three unchallenged mice from each group were killed 6 to 8 weeks postinoculation, and spleen cells were collected for in vitro cell culture work. Experiment 2 was carried out to determine whether multiple injections were required for protection. Groups of five mice were immunized i.p. with either saline, B. abortus strain RB51, or O. anthropi 49237SOD at the doses described for experiment 1. Two weeks later, mice inoculated with O. anthropi 49237SOD were reimmunized with O. anthropi 49237SOD (5 × 108 CFU/mouse). Mice of all the groups were challenged at 6 weeks after the first immunization. To determine whether different doses influenced the results of protection, a third experiment was carried out. Four groups of five mice each were inoculated with O. anthropi 49237 at four different doses: 5 × 108, 5 × 107, 5 × 106, and 5 × 105 CFU/mouse. An additional group of five mice was inoculated i.p. with saline as the negative control. All mice in experiments 2 and 3 were challenged and killed according to the protocol of experiment 1. In experiment 4, synthetic CpG-containing oligodeoxynucleotides (CpG-ODN) were administered as an immunostimulatory adjuvant. Groups of eight mice were inoculated i.p. with O. anthropi strain 49237 alone (5 × 108 CFU/mouse), strain 49237 (5 × 108 CFU/mouse) with the CpG adjuvant, strain 49237SOD alone (5 × 108 CFU/mouse), or strain 49237SOD (5 × 108 CFU/mouse) with the CpG adjuvant. The CpG adjuvant was inoculated i.p. at a dose of 10 nmol at 4 h before inoculation and again at the time of inoculation of the bacterial strains. As controls, three groups of mice were inoculated with either saline alone, CpG-ODN alone, or E. coli DH5α (106 CFU/mouse) with CpG-ODN. Mice were bled at 2, 4, and 6 weeks after inoculation. At 6 weeks after inoculation, five mice from each group were challenged with the virulent Brucella strain 2308; 2 weeks later, they were killed for a protection study as described above. The remaining three unchallenged mice were used for CMI response detection.
Serological tests.
A colony immunoblot assay and Western blotting were performed according to the procedures published previously (44). An indirect ELISA was performed to measure the isotypes of specific anti-Brucella Cu,Zn SOD antibodies in the sera of mice. Brucella Cu,Zn SOD purified by ion-exchange chromatography (44) was adsorbed to wells of polystyrene plates (Maxisorp; Nunc) at a concentration of 1 μg of SOD/well in 50 μl of bicarbonate buffer (pH 9.6). After overnight incubation at 4°C, plates were blocked with 2% bovine serum albumin in phosphate-buffered saline (pH 7.4) for 2 h at room temperature. Mouse serum samples at a 1:100 dilution were added to wells in duplicate and incubated for 3 h at room temperature. Plates were washed three times with phosphate-buffered saline containing 0.05% Tween 20. Isotype-specific goat anti-mouse horseradish peroxidase-conjugated antisera (ICN Pharmaceuticals, Inc.) were added for 30 min at room temperature, plates were washed for three times, and 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added and incubated in the dark for 20 min. The reaction was stopped by addition of 100 μl of 0.18 M sulfuric acid/well, and the absorbance of the developed color was measured at 450 nm.
Splenocyte cultures and quantitation of IFN-γ and IL-4.
Mice were killed by CO2 asphyxiation, and their spleens were removed under aseptic conditions. Single spleen cell suspensions were prepared from the spleens according to the standard procedures (42, 43). Red blood cells were lysed with ACK solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2 EDTA [pH 7.3]). Splenocytes were cultured in 96-well flat-bottom plates at a concentration of 5 × 105 viable cells/well in the presence of heat-inactivated RB51, O. anthropi strain 49237, or 49237SOD bacteria equivalent to 106 CFU with either 1 μg of purified SOD, 1 μg of concanavalin A, or no additives (unstimulated control). RPMI 1640 medium (GIBCO BRL) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal calf serum, and 50 μM penicillin-streptomycin was used for culturing the splenocytes. The cells were cultured for 5 days, and the plates were centrifuged at 250 × g for 10 min. The clear culture supernatants were transferred to a new 96-well plate and stored at −70°C until an ELISA was performed to determine gamma interferon (IFN-γ) concentrations (42). For detection of interleukin-4 (IL-4) in culture supernatants, cells were cultured with the different stimulants for 4 days and then 50 ng of phorbol myristate acetate (PMA) per ml and 1 μM ionophore were added (35). Supernatants were collected 16 h after stimulation with PMA-ionophore. IFN-γ and IL-4 levels in culture supernatants were determined by sandwich ELISAs as previously described using purified recombinant mouse IFN-γ or IL-4 as the standard (42). All assays were performed in triplicate. The concentration of IFN-γ or IL-4 in the culture supernatants was calculated by using a linear-regression equation obtained from the absorbance values of the standards.
Survival of O. anthropi strain 49237 and O. anthropi strain 49237SOD in mice.
Six-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were used. Groups of 15 mice each were inoculated i.p. with approximately 4 × 108 CFU of O. anthropi strain 49237, O. anthropi strain 49237SOD, or strain 49237SOD plus CpG adjuvant. The exact CFU of bacteria injected were determined retrospectively by plating serial dilutions of the bacterial suspensions used for infection on TSA plates or chloramphenicol-containing TSA plates (44). The CpG adjuvant was inoculated at a dose of 10 nmol at 4 h before inoculation and again at the time of injection with strain 49237SOD. At 5, 8, and 14 days postinoculation, five mice from each group were euthanatized by CO2 asphyxiation. Their spleens were collected, and bacterial CFU per individual spleen were determined by plating the serial dilutions of the spleen homogenates on TSA plates as well as on chloramphenicol-containing TSA plates.
Statistical analysis.
The CFU data in the protection study were analyzed by performing analysis of variance with an unequal-variance model. The mixed-model procedure of SAS Institute Inc. (Cary, N.C.) was used. Single degree-of-freedom contrasts were used to test specific hypotheses of interest. The Student t test was performed in the bacterial clearance study and the Ig isotype study using O. anthropi strain 49237SOD with or without CpG-ODN.
RESULTS
Expression of Brucella Cu,Zn SOD in O. anthropi 49237SOD.
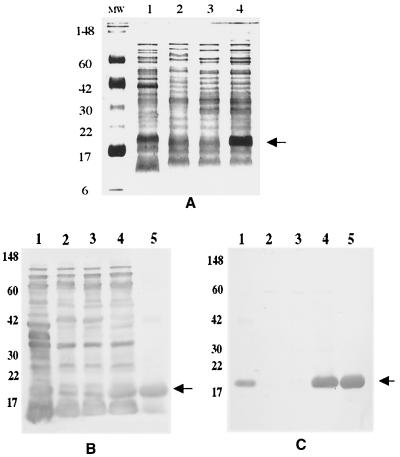
Expression of Brucella Cu,Zn SOD in strain 49237SOD was confirmed by Coomassie blue staining (Fig. 1A) and Western blot analyses using goat anti-RB51 sera (Fig. 1B) and mouse anti-SOD sera (Fig. 1C). As shown in Fig. 1C, O. anthropi strain 49237SOD expressed large amounts of Brucella Cu,Zn SOD, while O. anthropi strain 49237 alone did not express any detectable Cu,Zn SOD that cross-reacted with Brucella SOD-specific antibodies. Since the O antigen of Brucella LPS induces protective antibodies in the mouse model (4), we tested for possible cross-reactivity between the O antigens of O. anthropi and Brucella by using monoclonal antibody Bru38, which is specific to Brucella O antigen (34). O. anthropi strains 49237, 49237pBB, and 49237pBBSOD and Brucella strain RB51 did not react with the monoclonal antibody, while the Brucella smooth strain 2308 reacted strongly with the monoclonal antibody (data not shown) in Western blots, as expected (34). Western blot analysis using polyclonal antibodies against Brucella strain 2308 LPS also indicated that O. anthropi 49237 or the recombinant O. anthropi 49237SOD did not contain reactive O antigens (data not shown).
FIG. 1.
Detection of Brucella Cu,Zn SOD expression in O. anthropi strain 49237SOD by Coomassie blue staining of a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (A) and by Western blot analysis with a goat anti-RB51 serum (B) and a mouse anti-SOD serum (C). In all panels, lanes 1 to 4 contain B. abortus strain RB51, O. anthropi strain 49237, O. anthropi strain 49237pBB, and O. anthropi strain 49237pBBSOD, respectively. In panels B and C, lane 5 contains purified B. abortus Cu,Zn SOD. Arrows indicate the Cu,Zn SOD band.
O. anthropi strains 49237 and 49237SOD failed to stimulate a protective response against virulent Brucella infection.
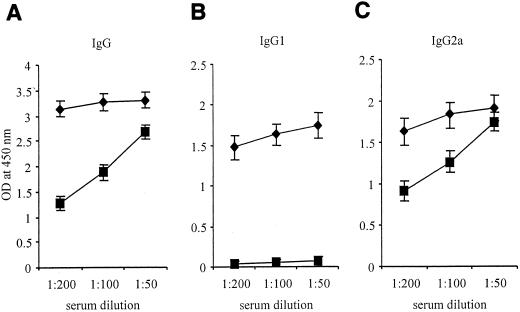
In experiment 1, none of the mice immunized with O. anthropi strains were protected against B. abortus strain 2308 challenge (P > 0.05) (Table 1). As expected, mice immunized with B. abortus strain RB51 or B. abortus strain RB51SOD demonstrated 1.5 and 3.0 log10 units of protection, respectively, (P < 0.01 in comparison to mice immunized with saline alone) (Table 1). In experiment 2, mice were immunized twice with O. anthropi 49237SOD (5 × 108 CFU/mouse); the booster inoculation was given 2 weeks after the first immunization. However, no protection against B. abortus 2308 challenge was observed for these mice (data not shown). Serological studies indicated that mice immunized with the O. anthropi strains developed strong antibody responses to O. anthropi and Brucella antigen extracts at 4 and 6 weeks postimmunization, as detected by Western blot analysis and indirect ELISA (data not shown). O. anthropi 49237SOD also stimulated a strong antibody response to purified Brucella SOD, while other strains did not (Fig. 2). However, O. anthropi 49237SOD stimulated a mix of immunoglobulin G1 (IgG1) and IgG2a responses to SOD, while RB51SOD stimulated an IgG2a response exclusively (Fig. 2), suggesting the development of both Th1 and Th2 types of response. Since the dose might play an important role in directing the development of a predominantly Th1 or Th2 immune response (6, 12, 20), experiment 3 was carried out to study the effects of different doses of O. anthropi strain 49237SOD on protection. None of the doses used induced protection (data not shown). The overall data suggested that a mix of Th1 and Th2 responses was induced after immunization with the O. anthropi strains and that the Th2 responses might be interfering with the protective immune mechanisms. We then hypothesized that switching the observed mixed Th1 and Th2 responses to a predominantly Th1 response could facilitate induction by O. anthropi 49237SOD of a protective immune response against virulent Brucella infection.
TABLE 1.
Inability of O. anthropi strains 49237 and 49237SOD to induce protective immunity against B. abortus strain 2308 challenge
| Group | Vaccinea | Log10 CFU in spleen (x ± SD) | P |
|---|---|---|---|
| 1 | Saline | 5.37 ± 0.19 | Control |
| 2 | RB51 | 4.24 ± 0.29 | <0.05∗b |
| 3 | RB51SOD | 3.01 ± 0.34 | <0.01∗∗ |
| 4 | O. anthropi 49237 | 5.27 ± 0.33 | >0.05 |
| 5 | O. anthropi 49237pBB | 5.40 ± 0.21 | >0.05 |
| 6 | O. anthropi 49237SOD | 5.29 ± 0.19 | >0.05 |
For groups 2 through 6, the dose of vaccine was 5 × 108 CFU.
∗ and ∗∗, significantly different from the saline group and from each other.
FIG. 2.
Comparison of Brucella Cu,Zn SOD-specific IgG, IgG1, and IgG2a responses in sera of mice vaccinated with O. anthropi strain 49237SOD (⧫) and Brucella RB51SOD (▪). Groups of five mice either were immunized i.p. with either O. anthropi strain 49237, O. anthropi strain 49237SOD, B. abortus strain RB51, or B. abortus strain RB51SOD or were inoculated with saline alone. Mice were bled individually at 6 weeks after immunization, and their serum samples were used for detection of IgG, IgG1, and IgG2a antibodies specific to purified Brucella Cu,Zn SOD by indirect ELISA. Each serum sample was tested in duplicate. Neither saline, O. anthropi strain 49237, nor Brucella RB51 induced detectable antibody responses to purified Brucella Cu,Zn SOD (data not shown)
O. anthropi strains plus CpG-ODN induced significant protection against virulent Brucella infection.
Vaccination of mice with a combination of O. anthropi 49237 or 49237SOD and the immunostimulatory CpG-ODN resulted in significant protection compared to infection of saline-inoculated controls. (P < 0.01) (Table 2, experiment A). However, O. anthropi strain 49237SOD with CpG-ODN stimulated significantly better protection than strain 49237 with CpG-ODN (P < 0.05) (Table 2, experiment A), further confirming that SOD is a protective Brucella antigen when the right type of immune response is induced. Stimulation of protective immunity by O. anthropi strain 49237SOD with CpG-ODN was demonstrated in two other repeat experiments (Table 2, experiments B and C). In all experiments, mice inoculated with CpG-ODN alone, E. coli plus CpG-ODN, O. anthropi 49237 alone, or O. anthropi 49237SOD alone showed no protection.
TABLE 2.
Induction of protective immunity against B. abortus 2308 challenge by immunization with O. anthropi 49237 or 49237SOD in combination with CpG-ODN
| Group | Vaccine (dose) | Log10 CFU in spleens (x ± SD) | P |
|---|---|---|---|
| Experiment A | |||
| 1 | Saline | 5.31 ± 0.16 | Control |
| 2 | CpG-ODN alone | 5.33 ± 0.21 | >0.05 |
| 3 | E. coli (1 × 106) + CpG-ODN | 5.28 ± 0.18 | >0.05 |
| 4 | O. anthropi 49237 (5 × 108) alone | 5.25 ± 0.18 | >0.05 |
| 5 | O. anthropi 49237 (5 × 108) + CpG-ODN | 4.04 ± 0.95 | <0.05*a |
| 6 | O. anthropi 49237SOD (5 × 108) | 5.25 ± 0.21 | >0.05 |
| 7 | O. anthropi 49237SOD (5 × 108) + CpG-ODN | 2.30 ± 1.01 | <0.01** |
| Experiment B | |||
| 1 | Saline | 5.69 ± 0.14 | Control |
| 2 | CpG-ODN alone | 5.49 ± 0.33 | >0.05 |
| 3 | O. anthropi 49237SOD (5 × 108) | 5.24 ± 0.49 | >0.05 |
| 4 | O. anthropi 49237SOD (5 × 108) + CpG-ODN | 3.73 ± 1.22 | <0.01** |
| Experiment C | |||
| 1 | Saline | 5.30 ± 0.12 | Control |
| 2 | CpG-ODN alone | 5.28 ± 0.15 | >0.05 |
| 3 | O. anthropi 49237 (5 × 108) alone | 5.25 ± 0.15 | >0.05 |
| 4 | O. anthropi 49237SOD (5 × 108) | 5.25 ± 0.15 | >0.05 |
| 5 | O. anthropi 49237SOD (5 × 108) + CpG-ODN | 2.88 ± 1.23 | <0.01** |
* and **, significantly different from the saline group and each other.
Addition of CpG-ODN facilitated the development of a predominantly IgG2a antibody response against Brucella Cu,Zn SOD.
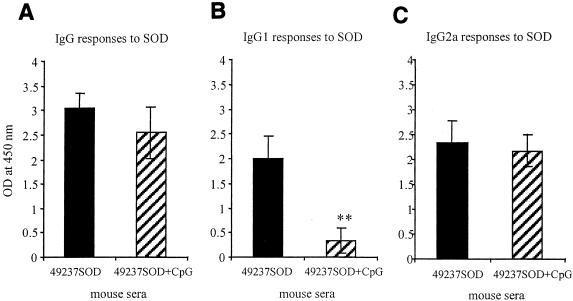
Mice vaccinated with O. anthropi strain 49237SOD developed specific antibodies to the B. abortus Cu,Zn SOD antigen which were detectable as early as 2 weeks postvaccination, and the antibody levels increased at 4 and 6 weeks (data not shown). Mice vaccinated with O. anthropi strain 49237 did not develop any antibody response to the SOD antigen, as expected (data not shown). It was found that addition of CpG-ODN to the vaccination protocol with strain 49237SOD significantly suppressed the SOD-specific IgG1 response (P < 0.01) (Fig. 3 B); the SOD-specific IgG and IgG2a levels remained primarily unchanged (Fig. 3 A and C).
FIG. 3.
Brucella Cu,Zn SOD-specific antibody isotype responses stimulated by O. anthropi strain 49237SOD with (hatched bars)or without (solid bars) the CpG DNA adjuvant. Groups of five mice were immunized i.p. with O. anthropi strain 49237SOD with or without the CpG DNA adjuvant. Mice were bled individually at 5 weeks after immunization, and their serum samples were used at a 1:100 dilution for detection of IgG (A), IgG1 (B), and IgG2a (C) responses to purified Brucella Cu,Zn SOD by indirect ELISA. Each serum sample was tested in duplicate. A significant difference was shown only in the production level of IgG1 between vaccinations with O. anthropi strain 49237SOD alone and O. anthropi strain 49237SOD plus CpG-ODN (B) (P < 0.01).
Addition of CpG-ODN to the vaccination protocol resulted in increased IFN-γ secretion and suppressed IL-4 secretion.
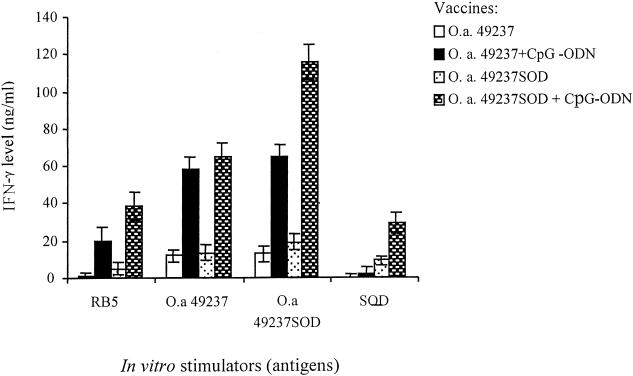
Splenocytes from mice immunized with O. anthropi strain 49237 or 49237SOD produced IFN-γ upon stimulation with heat-inactivated O. anthropi strain 49237 or 49237SOD; however, splenocytes from mice immunized with O. anthropi strain 49237 or 49237SOD in combination with CpG-ODN produced significantly increased IFN-γ levels upon stimulation with the antigens (P < 0.05) (Fig. 4). When stimulated in vitro with heat-inactivated strain 49237SOD, splenocytes from mice immunized with strain 49237SOD plus CpG-ODN produced significantly higher IFN-γ levels than splenocytes from mice immunized with strain 49237 plus CpG-ODN (P < 0.05) (Fig. 4). RB51 induced a minimum amount of IFN-γ secretion from the splenocytes of mice immunized with bacteria only but induced a large amount of IFN-γ secretion from the splenocytes of mice immunized with bacteria in combination with CpG (Fig. 4). More specifically, in vitro stimulation with purified SOD stimulated significantly higher IFN-γ secretion from the splenocytes of mice immunized with O. anthropi strain 49237SOD plus CpG-ODN than from the splenocytes of mice immunized with strain 49237SOD only (Fig. 4).
FIG. 4.
IFN-γ production by splenocytes from immunized mice upon in vitro stimulation with different antigens. BALB/c mice were inoculated i.p. with O. anthropi strain 49237 (5 × 108 CFU/mouse) or recombinant strain 49237SOD (5 × 108 CFU/mouse) with or without CpG adjuvant as described in the text. Mice were killed after 6 weeks. A total of 5 × 105 splenocytes were isolated and cultured in 96-well plates in triplicate and stimulated with either heat-inactivated B. abortus strain RB51 (106 CFU/well), O. anthropi strain 49237 (106 CFU/well), O. anthropi strain 49237SOD (106 CFU/well), or purified Cu,Zn SOD. After 5 days, supernatants were collected and tested for IFN-γ production by a sandwich ELISA. Upon stimulation with different antigens, splenocytes from mice immunized with O. anthropi strain 49237 or 49237SOD in combination with CpG-ODN produced significantly more IFN-γ than splenocytes from mice immunized with the respective strains without CpG-ODN (P < 0.05). The heat-inactivated O. anthropi strain 49237SOD stimulated significantly higher IFN-γ production by splenocytes from mice immunized with strain 49237SOD plus CpG-ODN than by splenocytes from mice immunized with strain 49237 plus CpG-ODN (P < 0.05). Stimulations with heat-inactivated RB51 or purified Cu,Zn SOD also resulted in significant differences in IFN-γ production between mice immunized with O. anthropi strain 49237SOD with and without CpG-ODN coinjection (P < 0.05).
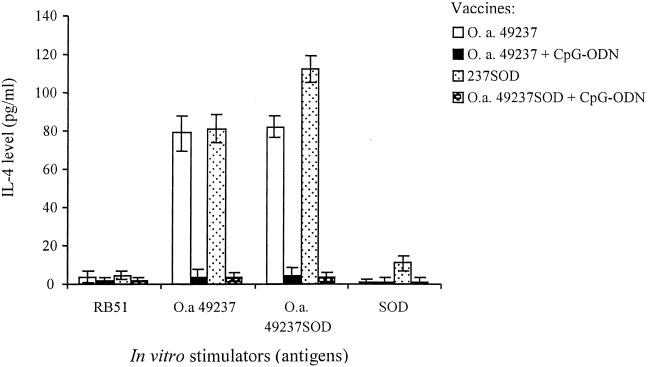
Since IL-4 cytokine levels in supernatants were too low to detect, PMA and ionophore were used to trigger IL-4 production in the last 16 h of culturing as described previously (35). As shown in Fig. 5, O. anthropi 49237 or 49237SOD could stimulate high levels of IL-4 production by splenocytes from mice vaccinated with O. anthropi 49237 or 49237SOD. Addition of CpG-ODN to the vaccination protocol significantly decreased the production of IL-4 (P < 0.01). Interestingly, in vitro stimulation with RB51 did not lead to production of IL-4 from the splenocytes of mice vaccinated with O. anthropi 49237 or 49237SOD with or without CpG adjuvant (Fig. 5). Stimulation with Brucella Cu,Zn SOD resulted in high levels of IL-4 production from the splenocytes of mice immunized with strain 49237SOD, but none from the splenocytes of mice immunized with strain 49237SOD plus CpG-ODN (Fig. 5).
FIG. 5.
IL-4 production by splenocytes from mice upon in vitro stimulation with different antigens. Culturing and stimulation of splenocytes were performed as described in the legend to Fig. 4. After 4 days, the cultured cells were restimulated with PMA-ionophore for 16 h, and supernatants were collected and tested for IL-4 production by a sandwich ELISA. Addition of CpG-ODN to the vaccination protocols significantly decreased IL-4 production compared to that with the protocols without CpG-ODN (P < 0.01). Stimulation with purified Brucella Cu,Zn SOD produced significantly more IL-4 in the splenocytes of mice immunized with strain 49237SOD alone than in the splenocytes of mice immunized with strain 49237SOD plus CpG-ODN (P < 0.01).
Bacterial persistence.
To investigate whether there were any differences in the in vivo survival abilities of O. anthropi 49237 and 49237SOD with or without the CpG adjuvant, clearance of the bacteria from spleens of immunized mice was assessed. As shown in Table 3, the CpG adjuvant did not influence the survival status of O. anthropi strain 49237SOD (P > 0.05). The spleens of mice in all the groups were free of any bacteria by day 14 postinoculation. However, O. anthropi strain 49237SOD survived in greater numbers than strain 49237 at days 5 and 8 postinoculation (P < 0.05), suggesting that SOD facilitated the survival of O. anthropi strain 49237. Analysis of strain 49237SOD colonies recovered from spleens of mice at days 5 and 8 postinoculation indicated that all the bacteria contained the plasmid and expressed SOD (data not shown).
TABLE 3.
Clearance of O. anthropi strains 49237, 49237pBB, and 49237SOD with or without CpG adjuvant from mouse spleens
| No. of days postinoculation | Mean log10 CFU/spleen ± SDa in mice inoculatedb with:
|
||||
|---|---|---|---|---|---|
| 49237pBB | 49237 | 49237 + CpG | 49237SOD | 49237SOD + CpG | |
| 5 | 4.08 ± 0.36 | 3.27 ± 0.36 | 3.59 ± 0.26 | 4.91 ± 0.33 | 4.74 ± 0.51 |
| 8 | 2.06 ± 0.49 | 2.01 ± 0.24 | 2.11 ± 0.22 | 2.89 ± 0.26 | 2.70 ± 0.35 |
| 14 | —c | — | — | — | — |
No significant difference was found between O. anthropi strain 49237 alone and 49237 plus CpG-ODN (P > 0.05) or between O. anthropi strain 49237SOD alone and strain 49237SOD plus CpG-ODN (P > 0.05) at 5 or 8 days postinoculation. However, strain 49237 (or 49237 plus CpG-ODN) had significantly lower CFU than strain 49237SOD (or 49237SOD plus CpG-ODN) at 5 or 8 days postinoculation (P < 0.05). Clearance of strain 49237pBB was significantly different from that for all the other groups at 5 days postinoculation (P < 0.05) but different only from strain 49237SOD alone and strain 49237SOD plus CpG-ODN (P < 0.05) at 8 days postinoculation.
Inoculation doses, 3.6 × 108 (log10 8.56) for O. anthropi 49237 and 49237pBB; 2.2 × 108 (log10 8.34) for O. anthropi 49237SOD. Each of five mice per group were inoculated i.p.
—, undetectable level (detection limit, 20 CFU/spleen, or log10 1.3 CFU/spleen).
DISCUSSION
Many antigens of O. anthropi share immunological cross-reactivity with those of Brucella. However, neither strain 49237 nor recombinant 49237SOD expressing the protective Brucella antigen SOD stimulated protection against virulent B. abortus infection. Analysis of immune responses of mice indicated that strains 49237 and 49237SOD stimulated a mix of Th1 and Th2 types of responses to the O. anthropi and SOD antigens, as characterized by IgG1 and IgG2a serum antibody production and secretion of both IFN-γ and IL-4 by in vitro-stimulated lymphocytes. A predominantly or exclusively Th1 type of immune response is usually needed for protection against intracellular pathogens (49). In infection with Leishmania major, it is well demonstrated that a Th2 type immune response interferes with development of a Th1 response and blocks protection against virulent infection (49). It is known that a Th1 response, and CMI in general, is critical for protection against Brucella infections (3, 4, 34, 41, 44). Therefore, we hypothesized that the recombinant O. anthropi strain 49237SOD would stimulate protection against virulent Brucella infection if the immune response could be switched to an exclusive Th1 type. This was achieved by use of the DNA adjuvant CpG-ODN, which is known to favor the development of Th1 responses (9, 10, 49). After switching of immune responses to a predominantly Th1 type, both O. anthropi strains 49237 and 492237SOD provided protection against Brucella challenge. The recombinant strain 49237SOD gave better protection than strain 49237. These results suggested that the cross-reactive antigens in O. anthropi can stimulate protection against Brucella infection if the right type of immune response is induced but that expression of Brucella SOD enhances this protection.
The CpG motifs have been widely studied as vaccine adjuvants. Synthetic DNA motifs containing an unmethylated CpG dinucleotide flanked by two 5′ purines (optimally GpA) and two 3′ pyrimidines (optimally TpC or TpT) mimic the immunostimulatory effects of bacterial DNA. Due to CpG methylation and CpG suppression in eukaryotic genomes, these sequence motifs are 20 times more common in prokaryotic than eukaryotic DNA (15, 25). Bacterial DNA or synthetic CpG-ODN stimulate an innate immune response characterized by activation of B cells, T cells, and macrophages. Together with protein-based antigens, in vivo injection of CpG-ODN induces a Th1-dominant antigen-specific immune response. CpG-ODN can even switch an antigen-specific Th2-dominant immune response to a Th1-dominant immune response through increased secretion of IL-12 and IFN-γ (10). It has been reported that CpG-ODN triggered protective Th1 responses in a lethal Th2-driven L. major infection (49). CpG-ODN were curative even when injected as late as 20 days after lethal L. major infection, indicating that CpG-ODN reversed an established Th2 response (49). It is therefore not surprising that in our studies CpG-ODN changed the immune responses to O. anthropi to a Th1 type and that consequently immunization led to protection, since Th1 responses are required for anti-Brucella immunity. Our study also suggests that an exclusive Th1 type of specific immune response is required for protection against brucellosis.
O. anthropi strain 49237SOD induced an average of 2.1 log10 units of protection against Brucella infection when coadministered with CpG-ODN. In three separate experiments, B. abortus strain RB51 induced only an average of 1.07 log10 units of protection against Brucella infection. This suggests that vaccination with O. anthropi strain 49237 SOD plus CpG is more effective than immunization with strain RB51. The experiments gave additional evidence that Brucella Cu,Zn SOD is a protective antigen, since good protection levels were reached when the antigen-specific immune response was of the correct Th1 type. Although SOD is a protective antigen, vaccination with strain RB51 does not stimulate an antibody response or a CMI response to SOD (44), probably due to its low expression level in strain RB51. However, overexpression of SOD by strain RB51 results in significantly higher protection and induction of both antibody and CMI responses to Cu,Zn SOD (44).
Cu,Zn SOD is an enzyme involved in protecting cells from exogenous sources of superoxide, such as the oxidative burst of phagocytes (21). It is therefore often regarded as a virulence factor in many bacterial pathogens. Overexpression of Cu,Zn SOD in the attenuated B. abortus strain RB51 does not alter its in vivo survival ability (44). However, in the present study, we observed that expression of Cu,Zn SOD in O. anthropi aided in the in vivo survival of the organism, although both O. anthropi strain 49237 and its recombinant strain expressing Brucella Cu,Zn SOD were cleared within 2 weeks postinoculation (data not shown). This suggests that Brucella Cu,Zn SOD can be considered a mild virulence factor under the right conditions. Even so, the fast clearance of O. anthropi strain 49237SOD makes this organism a potentially good, highly attenuated Brucella vaccine.
Production of IFN-γ during a Th1 response is very important for protection against virulent Brucella infection (18, 45, 46, 48). IFN-γ activates macrophages and enhances their bactericidal effects through production of reactive oxygen intermediates and nitrogen intermediates (24). It has been shown that addition of IFN-γ in vitro inhibits Brucella replication in macrophages (24), and injection of recombinant murine IFN-γ in vivo increased the resistance of mice to infection with B. abortus (36). When endogenous IFN-γ was depleted with an anti-IFN-γ monoclonal antibody, increased numbers of B. abortus were found in the spleens and livers of infected mice (47). IL-4 is typically produced by Th2 lymphocytes and can down-regulate the production of a Th1 response (5). It has been shown that vaccination with live Brucella induced IFN-γ-producing CD4+ Th1 cells, while vaccination with soluble Brucella proteins induced IL-4-producing CD4+ Th2 cells (48). Furthermore, IFN-γ-producing CD4+ Th1 cells from immunized donor mice were able to mediate resistance against virulent Brucella challenge, but IL-4-producing CD4+ Th2 cells from immunized mice failed to provide resistance (48). O. anthropi strain 49237 or 49237SOD immunization was unable to induce protection and stimulated both antigen-specific IFN-γ and IL-4 production. This finding suggests that the IL-4-producing Th2 response to Brucella antigens can interfere with the IFN-γ-producing Th1 response and prevent protection against virulent Brucella challenge. On the other hand, as demonstrated in our study, protection is induced once the immune response is directed to an exclusive Th1 type, as characterized by a lack of IL-4 secretion and enhanced IFN-γ production by the antigen-specific lymphocytes.
Studies with other intracellular pathogens suggest that an appropriate dose of vaccine is needed for eliciting an exclusive Th1 type of protective immune response (6, 20, 26, 27, 32). The failure of strains 49237 and 49237SOD to induce a predominant Th1 type immune response leading to protection against a Brucella challenge might be due to incorrect vaccination doses. However, four different doses of strain 49237SOD were used in this study and no protection was observed with any of them. In addition, two immunizations with strain 49237SOD failed to result in any protection against Brucella infection. In every case, both Th1 and Th2 types of Cu,Zn SOD-specific immune responses were detected (data not shown). Based on these findings, it appears that O. anthropi at any given dose can only stimulate a mixture of Th1 and Th2 responses. This is in contrast to Brucella, which stimulates a predominantly Th1 type of immune response (2). It would be interesting to identify the molecular and immunological bases for this difference between these two genetically closely related bacteria.
The cross-protection strategy has been used in the development of experimental vaccines against bacterial pathogens. Many attenuated bacteria, e.g., the Brucella vaccine strain RB51 (22), the mycobacterial vaccine strain bacillus Calmette-Guérin (BCG) (28), Salmonella enterica serovar Typhimurium (13), and E. coli (19), have been successfully manipulated as vaccine vectors to express foreign antigens specified by cloned genes from other pathogens. In addition, some bacteria can be used directly as vaccines against different bacterial pathogens for the cross-protection strategy. For instance, active immunization with either the Smith diffuse strain of Staphylococcus aureus or a type Ia strain of group G streptococci protected against challenge by either the homologous or heterologous bacterial strains (23). However, our study is distinct in that it used a nonpathogenic, cross-reactive bacterium as a vaccine vector to express a protective antigen of a pathogenic bacterium and induced protection against the virulent pathogen only after directing the immune responses to a Th1 type. Ongoing studies in our laboratory are directed at examining the ability of O. anthropi strain 49237SOD to induce protective immunity in domestic animals that are natural target species for brucellosis.
Editor: R. N. Moore
REFERENCES
- 1.Acha, P., and B. Szyfres. 1980. Zoonoses and communicable diseases common to man and animals, p. 28-45. Pan American Health Organization, Washington, D.C.
- 2.Agranovich, I., D. E. Scott, D. Terle, K. Lee, and B. Golding. 1999. Down-regulation of Th2 responses by Brucella abortus, a strong Th1 stimulus, correlates with alterations in the B7.2-CD28 pathway. Infect. Immun. 67:4418-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araya, L. N., P. H. Elzer, G. E. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 4.Araya, L. N., and A. J. Winter. 1990. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect. Immun. 58:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae, J. 1999. Generation of baculovirus-Brucella abortus heat shock protein recombinants; mouse immune responses against the recombinants, and B. abortus superoxide dismutase and L7/L12 recombinant proteins. Ph.D thesis. Virginia Polytechnic Institute and State University, Blacksburg, Va.
- 6.Bretscher, P. A., O. Ogunremi, and J. N. Menon. 1997. Distinct immunological states in murine cutaneous leishmaniasis by immunising with different amounts of antigen: the generation of beneficial, potentially harmful, harmful and potentially extremely harmful states. Behring Inst. Mitt. 98:153-159. [PubMed] [Google Scholar]
- 7.Bricker, B. J., L. B. Tabatabai, B. A. Judge, B. L. Deyoe, and J. E. Mayfield. 1990. Cloning, expression, and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect. Immun. 58:2935-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheville, N. F., S. C. Olsen, A. E. Jensen, M. G. Stevens, M. V. Palmer, A. M. Glorance, H. S. Houng, E. S. Drazek, R. L. Warren, T. L. Hadfield, and D. L. Hoover. 1996. Bacterial persistence and immunity in goats vaccinated with a purE deletion mutant or the parental 16M strain of Brucella melitensis. Infect. Immun. 64:2431-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, R. S., D. Askew, E. H. Noss, A. Tobian, A. M. Krieg, and C. V. Harding. 1999. CpG oligodeoxynucleotides down-regulate macrophage class II MHC antigen processing. J. Immunol. 163:1188-1194. [PubMed] [Google Scholar]
- 10.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloeckaert, A., A. Tibor, and M. S. Zygmunt. 1999. Brucella outer membrane lipoproteins share antigenic determinants with bacteria of the family Rhizobiaceae. Clin. Diagn. Lab. Immunol. 6:627-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 13.Curtiss, R., III, and J. O. Hassan. 1996. Nonrecombinant and recombinant avirulent Salmonella vaccines for poultry. Vet. Immunol. Immunopathol. 54:365-372. [DOI] [PubMed] [Google Scholar]
- 14.De Ley, J., P. Mannheim, P. Segers, A. Lievens, M. Denijn, M. Vanhoucke, and M. Gillis. 1987. Ribosomal ribonucleic acid cistron similarities and taxonomic neighborhood of Brucella and CDC group Vd. Int. J. Syst. Bacteriol. 37:35. [Google Scholar]
- 15.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 16.Elzer, P. H., F. M. Enright, J. R. McQuiston, S. M. Boyle, and G. G. Schurig. 1998. Evaluation of a rough mutant of Brucella melitensis in pregnant goats. Res. Vet. Sci. 64:259-260. [DOI] [PubMed] [Google Scholar]
- 17.Elzer, P. H., S. D. Hagius, G. T. Robertson, R. W. Phillips, J. V. Walker, M. B. Fatemi, F. M. Enright, and, R. M. Roop II. 1996. Behaviour of a high-temperature-requirement A (HtrA) deletion mutant of Brucella abortus in goats. Res. Vet. Sci. 60:48-50. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes, D. M., X. Jiang, J. H. Jung, and C. L. Baldwin. 1996. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunol. Med. Microbiol. 16:193-203. [DOI] [PubMed] [Google Scholar]
- 19.Finke, M., M. Duchene, A. Eckhardt, H. Domdey, and B. U. von Specht. 1990. Protection against experimental Pseudomonas aeruginosa infection by recombinant P. aeruginosa lipoprotein I expressed in Escherichia coli. Infect. Immun. 58:2241-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golding, B., and D. E. Scott. 1995. Vaccine strategies: targeting helper T cell responses. Ann. N. Y. Acad. Sci. 754:126-137. [DOI] [PubMed] [Google Scholar]
- 21.Gort, A. S., D. M. Ferber, and J. A. Imlay. 1999. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol. Microbiol. 32:179-191. [DOI] [PubMed] [Google Scholar]
- 22.Holmes, B., M. Popoff, M. Kiredjian, and K. Kersters. 1988. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int. J. Syst. Bacteriol. 38:406. [Google Scholar]
- 23.Ichiman, Y., and K. Yoshida. 1982. Cross protection in mice with the Smith diffuse strain of Staphylococcus aureus versus a type Ia strain of group B streptococci. Can. J. Microbiol. 28:726-732. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, X., and C. L. Baldwin. 1993. Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61:124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinman, D. M., K. M. Barnhart, and J. Conover. 1999. CpG motifs as immune adjuvants. Vaccine 17:19-25. [DOI] [PubMed] [Google Scholar]
- 26.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 27.Lagrange, P. H., G. B. Mackaness, and T. E. Miller. 1974. Influence of dose and route of antigen injections on the immunological induction of T cells. J. Exp. Med. 139:528-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langermann, S., S. R. Palaszynski, J. E. Burlein, S. Koenig, M. S. Hanson, D. E. Briles, and C. K. Stover. 1994. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guerin vaccines expressing pneumococcal surface protein A. J. Exp. Med. 180:2277-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McQuiston, J. R., G. G. Schurig, N. Sriranganathan, and S. M. Boyle. 1995. Transformation of Brucella species with suicide and broad host-range plasmids. Methods Mol. Biol. 47:143-148. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 31.Onate, A. A., R. Vemulapalli, E. Andrews, G. G. Schurig, S. Boyle, and H. Folch. 1999. Vaccination with live Escherichia coli expressing Brucella abortus Cu,Zn superoxide dismutase protects mice against virulent B. abortus. Infect. Immun. 67:986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero, C., C. Gamazo, M. Pardo, and I. Lopez-Goni. 1995. Specific detection of Brucella DNA by PCR. J. Clin. Microbiol. 33:615-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schurig, G. G., R. M. Roop II, T. Bagchi, S. Boyle, D. Buhrman, and N. Sriranganathan. 1991. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28:171-188. [DOI] [PubMed] [Google Scholar]
- 35.Soo, S. S., B. Villarreal-Ramos, C. M. Anjam Khan, C. E. Hormaeche, and J. M. Blackwell. 1998. Genetic control of immune response to recombinant antigens carried by an attenuated Salmonella typhimurium vaccine strain: Nramp1 influences T-helper subset responses and protection against leishmanial challenge. Infect. Immun. 66:1910-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens, M. G., G. W. Pugh, Jr., and L. B. Tabatabai. 1992. Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect. Immun. 60:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabatabai, L. B., and G. W. Pugh, Jr. 1994. Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 38.Velasco, J., J. A. Bengoechea, K. Brandenburg, B. Lindner, U. Seydel, D. Gonzalez, U. Zahringer, E. Moreno, and I. Moriyon. 2000. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect. Immun. 68:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velasco, J., R. Diaz, M. J. Grillo, M. Barberan, C. Marin, J. M. Blasco, and I. Moriyon. 1997. Antibody and delayed-type hypersensitivity responses to Ochrobactrum anthropi cytosolic and outer membrane antigens in infections by smooth and rough Brucella spp. Clin. Diagn. Lab. Immunol. 4:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velasco, J., C. Romero, I. Lopez-Goni, J. Leiva, R. Diaz, and I. Moriyon. 1998. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int. J. Syst. Bacteriol. 48:759-768. [DOI] [PubMed] [Google Scholar]
- 41.Vemulapalli, R., S. Cravero, C. L. Calvert, T. E. Toth, N. Sriranganathan, S. M. Boyle, O. L. Rossetti, and G. G. Schurig. 2000. Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin. Diagn. Lab. Immunol. 7:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vemulapalli, R., A. J. Duncan, S. M. Boyle, N. Sriranganathan, T. E. Toth, and G. G. Schurig. 1998. Cloning and sequencing of yajC and secD homologs of Brucella abortus and demonstration of immune responses to YajC in mice vaccinated with B. abortus RB51. Infect. Immun. 66:5684-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vemulapalli, R., Y. He, S. M. Boyle, N. Sriranganathan, and G. G. Schurig. 2000. Brucella abortus strain RB51 as a vector for heterologous protein expression and induction of specific Th1 type immune responses. Infect. Immun. 68:3290-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vemulapalli, R., Y. He, S. Cravero, N. Sriranganathan, S. M. Boyle, and G. G. Schurig. 2000. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect. Immun. 68:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaitseva, M. B., H. Golding, M. Betts, A. Yamauchi, E. T. Bloom, L. E. Butler, L. Stevan, and B. Golding. 1995. Human peripheral blood CD4+ and CD8+ T cells express Th1-like cytokine mRNA and proteins following in vitro stimulation with heat-inactivated Brucella abortus. Infect. Immun. 63:2720-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan, Y., and C. Cheers. 1998. Control of IL-12 and IFN-gamma production in response to live or dead bacteria by TNF and other factors. J. Immunol. 161:1447-1453. [PubMed] [Google Scholar]
- 47.Zhan, Y., and C. Cheers. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhan, Y., A. Kelso, and C. Cheers. 1995. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect. Immun. 63:969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]