Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) strains produce a 20-kDa zinc metalloprotease toxin (BFT) associated with diarrheal disease of animals, young children, and adults. BFT stimulates secretion in intestinal loops in vivo and modifies epithelial cell morphology in vitro. The B. fragilis toxin (bft) gene from ETBF strain 86-5443-2-2 (piglet; bft-2) revealed significant nucleotide and predicted amino acid differences when compared to the bft gene from ETBF strain VPI 13784 (lamb; bft-1). This study compares BFT-1 and BFT-2, respectively, produced by ETBF strains VPI 13784 and 86-5443-2-2 purified using the Van Tassell method (38) and a modified purification scheme described herein. Multiple differences in the protein toxins produced by these ETBF strains were identified. First, purified BFT-1 eluted from a high-resolution anion-exchange column (Mono Q) at 0.22 ± 0.005 M NaC1 versus 0.18 ± 0.001 M NaC1 for BFT-2 (P < 0.001). Second, BFT-1 and BFT-2 exhibited different electrophoretic mobilities on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and reverse-phase fast protein liquid chromatography. Third, each BFT reacted with greater specificity to homologous rather than heterologous antisera. Fourth, BFT-2 had modest, but consistently, greater biological activity than BFT-1 when tested on HT29/C1 cells (P ≤ 0.01). Together, these data indicate that these ETBF strains produce two distinct isotypes of BFT, termed BFT-1 (VPI 13784 BFT) and BFT-2 (86-5443-2-2 BFT) to recognize the order in which the proteins were purified and genetic sequences identified. The modified purification scheme described in this report yields about two to three times more purified BFT protein than previous protocols and is less time consuming.
Strains of Bacteroides fragilis (enterotoxigenic B. fragilis [ETBF]) which produce a ca. 20-kDa protein toxin termed B. fragilis toxin (BFT) have been associated with diarrheal disease of animals, young children, and adults (11, 29, 30, 36, 45) (M. G. Menozzi, M. Malpeli, S. Covan, S. Rossi, G. Benaglia, G. Montanarini, P. Vitali, A. Pantosit, G. Gherardini, and C. Chezzi, Abstr. 2nd World Congr. Anaerob. Bacteria Infect., abstr. 5.007, 1998). The ligated lamb intestinal loop (LLIL) assay (21) and a tissue culture assay using immortalized human intestinal epithelial cells (HT29/C1) (20, 41) have been described to detect the toxigenicity of ETBF. BFT stimulates secretion in the LLIL assay and causes swelling and morphological changes associated with the rearrangement of F actin in HT29/C1 cells (5, 15). BFT also reduces the barrier function and/or stimulates chloride secretion when polarized monolayers of epithelial cells as well as human colon are treated (2, 24, 28, 43). Most recently, BFT has been reported to cleave the extracellular domain of the zonula adherens protein, E cadherin (44). This proteolytic event is hypothesized to trigger the reported F-actin rearrangement and pathophysiologic sequelae stimulated by BFT.
Since the recognition of this enteric pathogen, comparison of the biologic activities of crude culture supernatants of ETBF strains in LLIL and the HT29/C1 cell assay have revealed variable responses with some strains classified as high BFT producers and others as moderate or low BFT producers (20, 22, 23, 40, 41). Three distinct nucleotide sequences for bft (B. fragilis toxin) were first reported for ETBF strains VPI 13784 (lamb) (14), 86-5443-2-2 (piglet) (6, 14) and Korea 419 (human) (3, 12). Alignment of these sequences revealed 87 to 96% identity in the predicted protein sequences of the mature BFT proteins, termed BFT-1 (VPI 12784), BFT-2 (86-5443-2-2), and BFT-3 (Korea 419) (3). These data revealed a surprising degree of interstrain diversity of the BFT protein and suggested that one possible explanation for the various biologic responses to culture supernatants of ETBF strains was differences in the protein toxin secreted by these strains. Alternatively, differences in the efficiency of synthesis and secretion of BFT by different strains and/or accessory virulence genes present in some, but not all, ETBF strains may account for the strain-dependent biologic activities detected to date.
In this paper, we compare BFT-1 and BFT-2 by using the method described by Van Tassell et al. (38) and a newly developed modified purification method. Our data reveal that BFT's from these ETBF strains are two distinct proteins by biochemical criteria, electrophoretic mobility, immunologic reactivity with antisera produced to each BFT, and protein sequence analysis. In addition, when the purification method described by Van Tassell et al. (38) was used, BFT produced by both strains copurified with another nonprotein molecule that most likely represents a portion of the capsular polysaccharide of these B. fragilis strains. Our new purification method achieves separation of BFT from this nonprotein molecule, significantly increases the yield of purified BFT, and is time-efficient. Lastly, ETBF strains which are low BFT-producers secrete protein toxins similar biochemically and by biologic activity to the respective isotypes of BFT isolated from high BFT-producing ETBF strains (i.e., BFT-1 or BFT-2 secreted by strains VPI 13784 or 86-5443-2-2, respectively). These results indicate that differences in the secreted BFT protein most likely do not account for the variable toxigenicity of ETBF strains.
MATERIALS AND METHODS
Chemicals and reagents.
Ammonium sulfate, glycine, and sodium dodecyl sulfate (SDS) were purchased from J. T. Baker Inc. (Phillipsburg, N.J.); acrylamide and bisacrylamide were from Gibco BRL Technologies (Grand Island, N.Y.) and International Biotechnologies Inc. (New Haven, Conn.), respectively; and tosyl phenylalanyl-chloro-methyl ketone (TPCK) and urea were from Boehringer GmbH (Mannheim, Germany). Q Sepharose fast flow, SP Sepharose fast flow, Mono Q columns, and a Superdex 75 HR 10/30 gel filtration column were obtained from Pharmacia Biotech Inc. (Piscataway, N.J.). Phenyl agarose and other chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless stated otherwise. Purified capsular polysaccharide capsule from B. fragilis strain NCTC 9343 was purified as previously described (37) and was provided by A. Tzianabos (Harvard Medical School).
Strains and culture medium.
The ETBF strains classified as high BFT producers and used in this study were 86-5443-2-2 (piglet isolate; from Lyle Myers, Montana State University) and VPI 13784 (lamb isolate; from Tracy Wilkins, Virginia Polytechnic Institute and State University); the ETBF strains classified as low BFT producers were 20793-3 and J38-1 (human isolates; from R. Bradley Sack, Johns Hopkins University School of Medicine). B. fragilis strains were grown in BHC medium (37 g of brain heart infusion base [Difco Laboratories, Detroit, Mich.]/liter, 5 g of yeast extract [Difco]/liter, and 1 μg of vitamin K/ml, 5 μg of hemin/ml, and 0.5 g of l-cysteine/ml [all from Sigma]) as previously described (20).
Purification of BFT. (i) Van Tassell purification method.
Purification of BFT was initially performed as described by Van Tassell et al. (38). Briefly, B. fragilis cultures were incubated anaerobically in 2 liters of BHC broth at 37°C for 16 to 18 h (A600 1.5 to ∼1.8). Following removal of cells by centrifugation at 4,800 × g for 40 min at 4°C, the culture supernatant was precipitated with 70% saturated ammonium sulfate for 2 h at room temperature, followed by incubated overnight at 4°C. The precipitate was collected by centrifugation (6,928 × g for 30 min), then dissolved in 100 ml of 0.05 M Tris-HCl buffer (pH 7.5) containing 1 mg of TPCK/ml, and dialyzed against 10 liters of buffer A (0.05 M Tris-HCl buffer [pH 7.5]) overnight at 4°C. This dialysate was applied to a Q Sepharose anion-exchange column (Pharmacia-LKB Biotechnology) equilibrated with buffer A. The column was washed with buffer A, and bound material was eluted by applying a 0 to 0.5 M NaCl gradient. Ten-milliliter fractions were collected and assayed for BFT by the HT29/C1 cell assay (see below). Toxin-containing fractions were pooled, and the NaCl concentration was adjusted to 1.5 M. The sample was then applied to a phenyl-agarose hydrophobic interactive chromatography column (Pharmacia-LKB Biotechnology) equilibrated in buffer B (0.05 M Tris-HCl [pH 7.5]-1.5 M NaCl). The column was sequentially washed with 1.5, 1.0, and 0.5 M NaCl in buffer A and finally eluted with 25% ethanol. The column eluate, monitored by UV absorption (280 nm), was fractionated, and toxin-containing fractions were pooled. After overnight dialysis at 4°C against 1 liter of buffer A, the BFT fraction was applied to a Mono Q HR 5/5 high-resolution anion-exchange column equilibrated in buffer A. After washing with the same buffer, bound material was eluted with a 0 to 0.5 M NaCl gradient. The column eluate was monitored for BFT activity by the HT29/C1 cell assay.
(ii) Urea-based purification method.
Five hundred milliliters of BHC broth inoculated with B. fragilis was cultured as described above, and the bacterial cells were removed by centrifugation at 4°C as described above. After sterilization of the culture filtrate (0.22-μm-pore size filter), the supernatant was concentrated five- to sixfold at 4°C by ultrafiltration using a membrane with a molecular mass exclusion of 10 kDa (Millipore Corporation, Bedford, Mass.). The concentrated ultrafiltrate was chromatographed on a phenyl-agarose hydrophobic interactive column as described above. The subsequent Mono Q chromatography step was modified by adding 6 M urea to the sample as well as the elution buffers. The column eluate was monitored by UV absorption (280 nm). After dialysis to remove the urea from protein-containing peaks, BFT activity of the fractions was tested by the HT29/C1 cell assay.
(iii) Accessory purification and analysis steps. (a) Analytical reverse-phase FPLC.
Reverse-phase fast protein liquid chromatography (FPLC) was performed on selected BFT samples to assess purity. Following Mono Q chromatography, samples (30 to 80 μg) were injected onto a PepRPC 10/10 column (Pharmacia) equilibrated in water-0.05% trifluoroacetic acid (TFA). Bound material was eluted with a gradient of 0 to 100% acetonitrile-0.05% TFA. The column eluate was monitored by UV absorption at 226 nM.
(b) Protein sequence analysis.
Purified BFT was subjected to automated Edman degradation on an Applied Biosystems Model 470 automated sequencer to confirm the toxin identity and to assess purity of the sample. Phenylthiohydantoin-derivatized amino acids were separated and quantified by the Model 470 onboard high-performance liquid chromatographer and peak integrator.
Protein concentration estimation, electrophoresis, and immunoblotting.
Protein concentration was estimated using the Protein Assay kit (Bio-Rad, Richmond, Calif.) or BCA Protein Assay Reagent (Pierce, Rockford, Ill.). Native polyacrylamide gel electrophoresis (PAGE) and denaturing PAGE in the presence of sodium dodecyl sulfate (SDS-PAGE) were performed as described by Sambrook et al. (34) at room temperature or 4°C. Rainbow protein molecular weight markers (Amersham Life Science Inc., Arlington Heights, Ill.) were used to estimate the molecular weights of proteins on SDS-PAGE gels.
To recover a specific protein from a native polyacrylamide gel, one lane of the gel was excised following PAGE and stained with CuCl2 as described by Lee et al. (16), and then the corresponding region of another lane was sliced from the gel. Gel slices were minced and soaked in 0.05 M Tris (pH 7.5) overnight at 4°C; following removal of gel fragments by centrifugation, the recovered protein was concentrated using a Centricon concentrator (cutoff of 10 kDa) and analyzed further as described in the text.
Immunoblotting (Western blotting) was performed as described by Sambrook et al. (34). Proteins separated by native or SDS-PAGE were electrophoretically transferred to nitrocellulose membrane sheets (Bio-Rad). After blocking with 10% nonfat dry milk in Tris-buffered saline, the membrane was probed with one of three primary antibodies: (i) polyclonal rabbit anti-BFT-2 serum (produced as previously described [20] using purified BFT-2 as the immunogen), (ii) monospecific goat anti-BFT-1 IgG (using purified BFT-1 as the immunogen [39, 40]; gift of Tracy Wilkins), or (iii) polyclonal goat anti-BFT-1 serum produced by using crude BFT-1 as the immunogen (39; gift of Tracy Wilkins)). Membranes were washed with Tris-buffered saline-0.1% Tween 20 and incubated with appropriate dilutions of secondary antibodies (peroxidase-conjugated donkey anti-rabbit immunoglobulin G [IgG] or peroxidase-conjugated rabbit anti-goat IgG [Sigma]). After washing to remove unbound antibody, the membranes were developed using the ECL system (Western blot Chemiluminescence Reagent [Amersham Pharmacia Biotech] or Supersignal Chemiluminescent Substrate [Pierce]).
For 2-dimensional gel electrophoresis, isoelectric focusing (IEF) was first performed overnight using a 7-cm Ready IPG strip (pH 3 to 6; Bio-Rad) and the Protein IEF System (Bio-Rad) at the voltage recommended by the manufacturer. Two-dimensional SDS-PAGE separation on a 15% acrylamide gel was done using a Mini-Protein II Cell (Bio-Rad). Gels were silver stained with the Silver Quest Silver Staining Kit (Invitrogen Corp., Carlsbad, Calif.).
Measurement of carbohydrate in BFT preparations. (i) Phenol-sulfuric acid assay.
The glucose content of purified BFT samples was determined by the phenol-sulfuric acid method (31).
(ii) Periodate oxidation.
BFT was fractionated by SDS-PAGE and transferred to nitrocellulose followed by treatment with 10 mM sodium metaperiodate in 0.02 M sodium acetate buffer (pH 3.8) at 4°C in the dark for 2 h (8).
(iii) Carbohydrate stain.
ECL Glycoprotein Detection System (Amersham Life Scientific Inc.) was used to detect carbohydrate. This assay is based on the oxidation of carbohydrate by sodium metaperiodate with a biotin-streptavidin-horseradish peroxidase detection method.
Proteolysis of BFT.
BFT-1 and BFT-2 were digested with trypsin (1:2, 1:100, 1:1,000 [wt/wt]) for 1, 2, 3, 6, and 18 h at room temperature in a 50 mM ammonium bicarbonate-1 mM CaC12 buffer (pH 8.0). Proteolytic fragments of BFT-1 and BFT-2 separated by SDS-15% PAGE were visualized by silver staining (Silver Quest Silver Staining Kit).
HT29/C1 cell assay.
HT29/C1 cells were cultured and used as previously described (20, 41). Ninety six-well microtiter plates (Costar, Cambridge, Mass.) or eight-well slides (Lab-Tek; Nunc Inc., Naperville, Ill.) were used for the titration of BFT in culture supernatants or fractions of each purification step. Fourfold or 10-fold serial dilutions were used to assay samples on 96-well plates, and fourfold dilutions were used for eight-well slides. Results of the 96-well plate assays were assessed by phase-contrast light microscopy at a 100-fold magnification, and the results of the eight-well slide assays were assessed by transmitted light microscopy at a 100-fold magnification of Giemsa-stained preparations (20). Total units of BFT activity were defined by the inverse of the endpoint titer at which HT29/C1 cells exhibited altered morphology 3 h after treatment with either purified or crude BFT preparations.
Statistical analysis.
Data are presented as means ± standard error of the mean (SEM) unless otherwise stated. Comparison of means was done by either the paired or unpaired Student's test. A P value of ≤0.05 was considered to designate a significant difference.
RESULTS
Characterization of BFT production and purification from ETBF strains VPI 13784 and 86-5443-2-2.
Crude culture supernatants of ETBF strains VPI 13784 and 86-5443-2-2 (containing BFT-1 and BFT-2, respectively) were assessed for biological activity using the HT29/C1 cell culture assay. Following anaerobic growth in BHC broth for 16 h at 37°C and adjustment to the same bacterial density (A600), supernatants were prepared by centrifugation and the cell-active titer was determined on HT29/C1 cells. The HT29/C1 cell-specific activity for culture supernatants prepared from ETBF strain 86-5443-2-2 (BFT-2) was 4.75 ± 0.4 U of cell activity/μg of protein and, for ETBF strain VPI 13784 (BFT-1), was 2.39 ± 0.8 U/μg of protein (n = 5, P ≤ 0.03; Table 1). These results are consistent with our previous observations on the HT29/C1 biological activity of crude culture supernatants of these ETBF strains (11). HT29/C1 cells treated with either crude culture supernatants or purified BFT from these two strains exhibited similar morphological changes (e.g., cell rounding and separation of cells in clusters) by light microscopy as control cells (data not shown).
TABLE 1.
BFTs purified by Van Tassell and urea-based methods
| Method and protein | Total protein (μg) (mean ± SEM) | Total activity (U) (mean ± SEM) | Sp act (U/μg) (mean ± SEM) | % Activity recovered (mean ± SEM) | Final titer (ng/ml) (mean ± SEM) |
|---|---|---|---|---|---|
| Van Tassell | |||||
| BFT-1 (n = 5) | |||||
| Culture supernatant | 9.93 (±0.66) × 106 | 23.10 (±7.34) × 106 | 2.39 (±0.8) | ||
| Mono Q fraction | 22.44 (±5.29) | 3.23 (±0.67) × 106 | 1.49 (±0.20) × 105 | 17 (±2.57) | 0.37 ± 0.06 |
| BFT-2 (n = 5) | |||||
| Culture supernatant | 10.55 (±1.23) × 106 | 48.19 (±1.81) × 106 | 4.75 (±0.4) | ||
| Mono Q fraction | 26.02 (±3.32) | 10.83 (±1.72) × 106 | 4.34 (±0.65) × 105 | 23 (±4.11) | 0.13 ± 0.02 |
| Urea based | |||||
| BFT-2 (n = 3) | |||||
| Culture supernatant | 8.46 (±1.45) × 106 | 53.33 (±10.67) × 106 | 6.23 (±0.24) | ||
| Urea Mono Q fraction | 77.31 (±29.14) | 23.39 (±1.38) × 106 | 3.24 (±0.45) × 105 | 44.0 (±10.44) | 0.07 ± 0.02 |
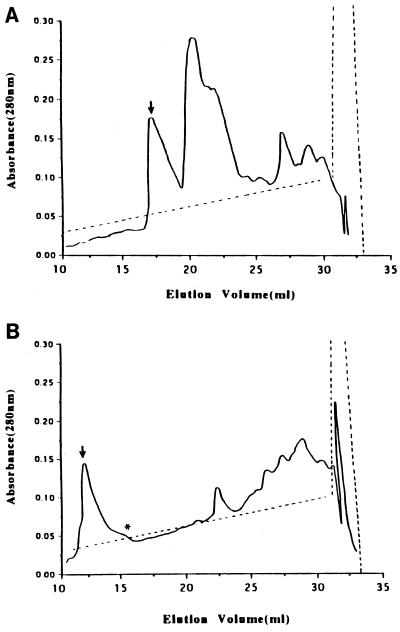
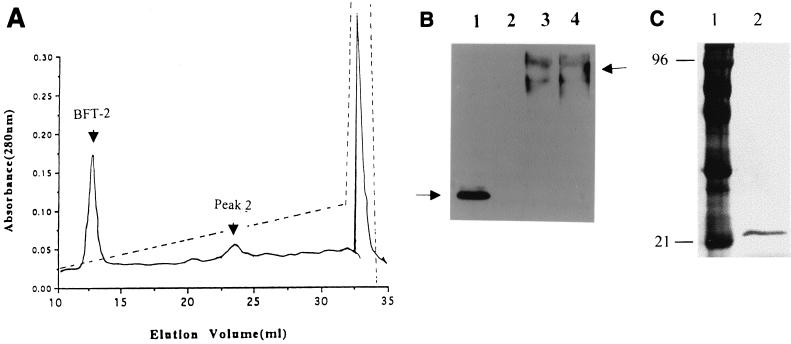
Using the Van Tassell purification protocol, no significant differences in the purification profiles of the culture supernatants of ETBF strains 86-5443-2-2 and VPI 13784 were observed during the Q-Sepharose and phenyl-agarose chromatography steps. In contrast, BFT purified from these two strains exhibited a marked difference at the Mono Q chromatography step. BFT-1 eluted at 0.21 to 0.24 M NaCl (mean ± SEM, 0.22 ± 0.005 M) as a single peak (Fig. 1A), whereas BFT-2 eluted at 0.17 to 0.18 M NaCl (mean ± SEM, 0.18 ± 0.001 M, P value of <0.001 compared to BFT-1) (Fig. 1B). The yield of BFT was 14.2 to 32.3 μg/liter (26.02 ± 3.3 μg/liter of the original culture; n = 5) from ETBF strain 86-5443-2-2 and 14.5 to 43.3 μg/liter (22.44 ± 5.3 μg/liter; n = 5) from ETBF strain VPI 13784 (the P value was not significant [NS]). BFT-1 and BFT-2 were active when tested in the HT29/C1 cell assay at 0.25 to 0.58 ng/ml (mean ± SEM, 0.37 ± 0.06 ng/ml) and at 0.08 to 0.16 ng/ml (mean ± SEM, 0.13 ± 0.02 ng/ml), respectively (n = 5, P ≤ 0.05; Table 1).
FIG. 1.
Profiles of elution of BFT-1 and BFT-2 from Mono Q anion-exchange chromatography. Shown are representative traces of the Mono Q elution profiles of proteins from culture supernatants of ETBF strains VPI 13784 (A) and 86-5443-2-2 (B) after previous purification by Q Sepharose (anion-exchange chromatography) and phenyl-agarose (hydrophobic interactive chromatography) columns. The biologically active BFT fractions as determined by the HT29/C1 cell assay are marked by arrows. (B) The fraction marked with an arrow contains 1 μg of carbohydrate/μg of BFT; the fraction marked with an asterisk contains 2 μg of carbohydrate/μg of BFT. Solid lines, absorbance; dotted lines, NaC1 gradient.
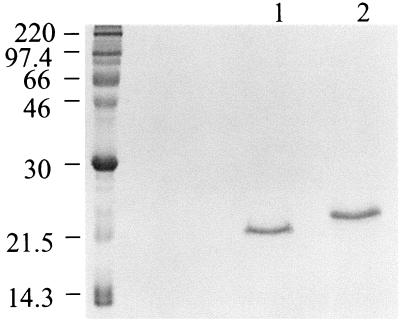
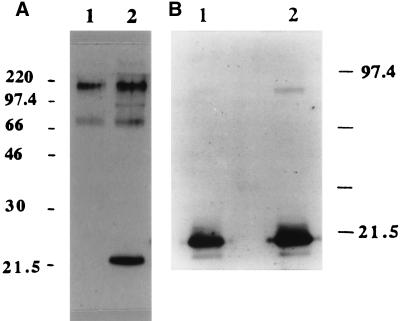
Based on previously reported data (19, 38), the purification of BFT from ETBF culture supernatants was expected to yield a single protein with a molecular mass of ca. 20 kDa on SDS-PAGE. However, analysis of purified BFT-2 by SDS-PAGE stained with Coomassie blue revealed a major band at 20 kDa with faint bands in some preparations at ca. 70 and 100 kDa (data not shown). The purified BFT-1 preparations revealed a major band at ca. 22 to 23 kDa with fainter bands in some preparations at ca. 70 and 100 kDa (data not shown). These results were confirmed when the gels were silver stained. As shown in Fig. 2, BFT-1 and BFT-2 exhibit different electrophoretic mobilities.
FIG. 2.
Appearance of BFT-1 and BFT-2 on SDS-PAGE. BFT purified by the Van Tassell method from ETBF strains VPI 13784 and 86-5443-2-2 (BFT-1 and BFT-2, respectively) was fractionated by SDS-PAGE and stained with Coomassie blue. BFT-1 was detected at ∼23 kDa (lane 2) and BFT-2 was detected at ∼20 kDa (lane 1). Each lane was loaded with 6 μg of protein.
Further analysis of BFT-2 by native PAGE revealed bands at ca. 36, 90, and 200 kDa (stained with Coomassie blue) or a smear in a higher molecular mass range (when stained with CuC12) (data not shown). After elution from gel slices following native PAGE, both the 36-kDa and higher-molecular-mass bands were biologically active when tested in the HT29/C1 cell assay. The material eluted from native PAGE gel slices was also reanalyzed by SDS-PAGE. The 36-kDa molecular mass band on native PAGE migrated to ca. 20 kDa, whereas the higher-molecular-mass bands on native PAGE migrated to ca. 70 to 100 kDa on SDS-PAGE (data not shown). N-terminal amino acid sequence analysis of the higher-molecular-mass bands eluted from SDS-PAGE was not successful.
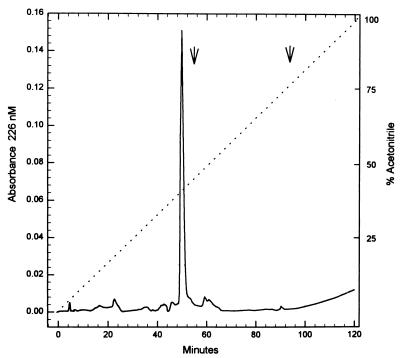
Because of the unexpected complexity on SDS-PAGE and native PAGE of the Mono-Q-purified BFT preparations, the molecular compositions of Mono-Q-purified BFT-2 and BFT-1 were further analyzed by reverse-phase FPLC. Using the BFT-2 preparation, a single sharp peak eluted from this column (Fig. 3). Thus, despite the apparent complexity of the BFT-2 preparation by SDS-PAGE, the Van Tassell purification procedure yielded a single protein from ETBF strain 86-5443-2-2 culture supernatants which was estimated to be at least 95% pure. This result also suggested that the higher-molecular-mass molecules detected by SDS-PAGE were either not proteins or were aggregates of BFT that were disrupted by chromatography in acetonitrile. N-terminal amino acid sequence analysis of the protein recovered from the Pep RPL column revealed Ala-Val-Pro-Ser-Glu-Pro-Lys-Thr-Val-Tyr-Val-Ile-Cys-Leu-Arg. This sequence matches our previous analysis of the amino terminus of BFT-2 (6) and is also identical to the published N-terminal amino acid sequence of BFT-1 (38). The reverse-phase FPLC profile of BFT-1 purified by the Van Tassell method also revealed one peak. This peak eluted at a different retention time than BFT-2 (51.8 versus 49.5 min; 43.1% versus 41.3% acetonitrile; BFT-1 versus BFT-2, respectively).
FIG. 3.
Profile of BFT-2 using analytical reverse-phase FPLC. A single sharp peak was eluted at 41.3% acetonitrile when BFT-2 purified by the Van Tassell method was analyzed by this technique. Solid line, absorbance; dotted line, % acetonitrile.
Immunoblot analysis of BFT-1 and BFT-2.
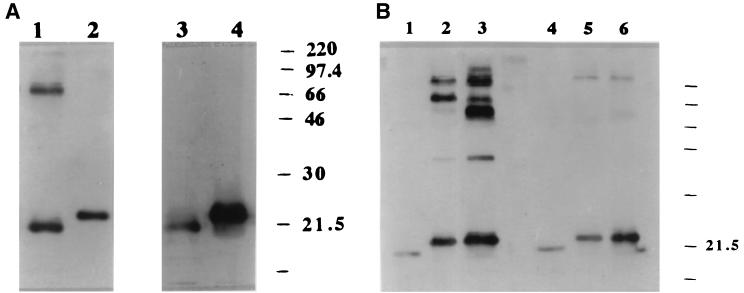
BFT-1 and BFT-2 purified by the Van Tassell method were further analyzed by immunoblotting. When BFT-2 was separated by SDS-PAGE and probed using polyclonal anti-BFT-2 serum, strong signals were observed at the 20-kDa position as well as at ca. 70 and 200 kDa (Fig. 4A, lane 1). Using the same antiserum, only a 22- to 23-kDa band was detected at lower intensity in the BFT-1 preparation (Fig. 4A, lane 2). Immunoblot analysis of these same toxin preparations using the monospecific anti-BFT-1 IgG revealed only a ∼20-kDa band in the BFT-2 and a more intense ∼23-kDa band in the BFT-1 preparations (Fig. 4A, lanes 3 and 4).
FIG. 4.
Comparison of the immunoreactivity of BFT purified from ETBF strains 86-5443-2-2 and VPI 13784 by using the Van Tassell method. (A) Western blot probed with polyclonal anti-BFT-2 serum (1/1,000 dilution). Each lane was loaded with 70 ng of protein. Polyclonal anti-BFT-2 serum detected three bands (ca. 20, 70, and 200 kDa) when using BFT-2 as the antigen (lane 1) but detected only a ∼23-kDa band at lower intensity in the BFT-1 preparation (lane 2). In contrast, probing the same Western blot with the monospecific anti-BFT-1 serum reveals only lower-molecular-mass bands with different intensities in these BFT preparations (lane 3, BFT-2; lane 4, BFT-1). (B) Western blot probed with polyclonal anti-crude BFT-1 (1/1,000 dilution) and monospecific anti-BFT-1 sera (1/500 dilution). Each lane was loaded with 70 ng of protein. Polyclonal anti-crude BFT-1 serum weakly detected only a 20-kDa band in the BFT-2 preparation (lane 1). In contrast, this polyclonal antibody detected both a ∼23-kDa band with greater intensity and additional higher-molecular-mass bands in BFT-1 purified in our laboratory (lane 2, VPI-m5) or in the laboratory of Tracy Wilkins (lane 3, VPI). In contrast, monospecific anti-BFT-1 serum detected predominantly the ca. 20-kDa protein band with various intensities dependent on the source of the BFT (lane 4, BFT-2; lane 5, VPI-m5 BFT; lane 6, VPI-BFT). In addition, fainter ca. 200-kDa bands were detected in the VPI-m5 and VPI BFTs by using the monospecific anti-BFT-1 serum.
In contrast, when using the polyclonal antiserum to crude BFT-1 as the primary antibody to probe both the BFT-1 and BFT-2 preparations, the 22- to 23-kDa band of BFT-1 was detected with greater intensity than the 20-kDa band detected in the BFT-2 preparation (Fig. 4B, lanes 1 to 3). In addition, two or more bands with higher apparent molecular masses were detected in the BFT-1 preparations but not in the BFT-2 preparations. When monospecific anti-BFT-1 IgG was used as the primary antibody to probe the same membranes, the ∼20 (BFT-2) or ∼23 (BFT-1) kDa bands were detected with different intensities and faint higher-molecular-mass bands were detected in some, but not all, BFT-1 preparations (Fig. 4B, lanes 4 to 6). In contrast, higher-molecular-mass bands were not detected with these antisera in any BFT-2 preparations. These results suggest that the polyclonal antibodies produced using BFT-1 as the immunizing antigen had a lower specificity for BFT-2 than BFT-1.
Determination that BFT copurifies with carbohydrate.
The position and appearance of the higher-molecular-mass bands detected in both the BFT-1 and BFT-2 preparations purified by the Van Tassell method suggested that these bands may represent a portion of the B. fragilis capsule (26). Consistent with this hypothesis, BFT-containing samples from each purification step were positive for carbohydrate by the phenol-sulfuric assay (data not shown). After the Mono Q column chromatography, approximately 1 μg carbohydrate/μg of BFT was measurable in purified BFT-2 by the phenol-sulfuric acid assay (Fig. 1) (31). To further assess if BFT copurifies with a nonprotein molecule, BFT-2 was treated with proteinase K (50 μg/ml for 60 min at 37°C) with subsequent analysis of this sample by SDS-PAGE and Western blot analysis. After proteinase K treatment, no bands were detectable by Coomassie blue staining of BFT-2 fractionated by SDS-PAGE. In contrast, by Western blot analysis using the polyclonal antisera to BFT-2, the 20-kDa band was absent but the higher-molecular-mass bands were still detected, albeit at reduced intensity (Fig. 5A). After inhibition of the proteinase K with phenylmethylsulfonyl fluoride (5 mM), the biological activity of the proteinase K-treated BFT-2 was either eliminated (n = 1) or greatly reduced (ca. 60-fold; n = 1). Similarly, oxidation of BFT-2 with 10 mM sodium metaperiodate resulted in a reduced intensity of the higher-molecular-mass band without alteration of the 20-kDa band (Fig. 5B). To further determine if the proteinase K-resistant, metaperiodate-sensitive higher-molecular-mass bands detectable by Western blot analysis were carbohydrate, the bands were analyzed using a glycoprotein detection kit (see Materials and Methods). In some preparations, a positive signal at the high-molecular-mass position was obtained (data not shown). These results suggest that the copurified molecules may have been carbohydrate but that the quantity of this carbohydrate in some preparations was at the limit of detection.
FIG. 5.
BFT copurifies with carbohydrate molecules. (A) Western blot of the proteinase K digestion of BFT-2. Using polyclonal anti-BFT-2 sera (1/1,000 dilution), only higher-molecular-mass bands were detected in BFT-2 purified by the Van Tassell method after digestion with proteinase K (50 μg/ml, 60 min, 37°C) (lane 1). After SDS-PAGE fractionation, Coomassie blue staining of BFT-2 treated with proteinase K revealed no bands (data not shown). Lane 2 shows the appearance of the BFT-2 preparation prior to proteinase K digestion. Each lane was loaded with 70 ng of protein. (B) Western blot of BFT-2 treated with sodium metaperiodate. Lane 1, BFT-2 treated with 10 mM sodium metaperiodate as described in Materials and Methods; lane 2, untreated BFT-2. Immunostaining was performed using polyclonal anti-BFT-2 sera (1/1,000 dilution).
To assess whether the copurified carbohydrate moiety altered BFT activity, capsular polysaccharide purified from B. fragilis strain NCTC 9343 was examined for biological activity on HT29/C1 cells. No cellular activity was detected using 20 μg of this capsular polysaccharide/ml alone nor did addition of capsular polysaccharide to 50 ng of BFT/ml alter the endpoint HT29/C1 cell titer when compared to BFT alone. In addition, the biological activity of two Mono Q fractions with different amounts of carbohydrate (0.95 μg of carbohydrate/μg of BFT versus 2 μg of carbohydrate/μg of BFT; Fig. 1B) were directly compared in the HT29/C1 cell assay at equivalent protein concentrations. The endpoint titers of both fractions were identical, suggesting that the copurified carbohydrate did not modulate BFT activity as detected by the HT29/C1 cell assay.
BFT purification by urea-based anion-exchange chromatography.
Because the Van Tassel purification method and additional experiments using gel filtration and cation-exchange column chromatography (data not shown) to separate the high-molecular-mass bands from the 20-kDa BFT band were not successful, the BFT purification protocol was modified as described in Materials and Methods. The critical change in approach was the addition of 6 M urea to the buffers and samples utilized in the Mono Q chromatography step. Figure 6A shows the sharp peak of BFT-2 eluted by Mono Q chromatography at 0.18 M NaCl in the presence of 6 M urea and contrasts with the broader elution peak observed using the Van Tassell purification method (Fig. 1B). A similar elution profile was obtained for BFT-1 except that the toxin peak eluted at 0.25 M NaCl (data not shown). Figure 6B shows the Western blot of the identified fractions probed with the polyclonal BFT-2 serum (compare to Fig. 4A). Only the fraction identified as “BFT-2” (Fig. 6A) contained biologically active toxin as assessed in the HT29/C1 cell assay whereas peak 2 revealed bands ca. 70 and 95 kDa by immunoblotting. However, no biological activity could be detected in this and all other fractions. In addition, after treatment with proteinase K, the BFT-2 band was eliminated by Western blot analysis (Fig. 6B, lane 2) or Coomassie blue staining (data not shown), whereas the band of peak 2 was unaltered by this treatment (Fig. 6B, compare lanes 3 and 4). Figure 6C shows that BFT-2 purified by urea-based Mono Q anion-exhange chromatography yields a single band on a silver-stained SDS-PAGE gel. The experimental isoelectric points (pIs) of BFT-1 and BFT-2 were 4.9 and 5.6, respectively. Table 1 compares the purification of BFT-2 using the Van Tassell procedure (32) and the modified urea-containing procedure described herein.
FIG. 6.
BFT purified by a urea-based modified method. (A) Profile of eluted BFT-2 from urea-based Mono Q anion-exchange chromatography. Far left arrow indicates the sharp peak of BFT-2 eluted at 0.18 M NaCl in the presence of 6 M urea. This compares with the broader elution peak observed using the Van Tassell purification method and shown in Fig. 1B. (B) Western blot of urea-based Mono Q peaks. Peaks indicated by arrows were analyzed by Western blot analysis using polyclonal BFT-2 antiserum (1/1,000 dilution). Both peaks were treated with proteinase K as described in the text. Lane 1, BFT-2 fraction; lane 2, BFT-2 fraction after proteinase K treatment; lane 3, peak 2; lane 4, peak 2 after proteinase K treatment. Proteinase K treatment eliminated the BFT-2 peak but did not alter the higher-molecular-mass peaks shown in lanes 3 and 4. (C) Silver stain of BFT-2 after purification by urea-based Mono Q anion-exchange chromatography and SDS-PAGE electrophoresis. Lane 1, molecular weight markers; lane 2, BFT-2 (200 ng of protein).
Protease digestion of BFT-1 and BFT-2.
Initial experiments revealed that BFT-1 and BFT-2 were resistant to digestion by trypsin when used at concentrations of 1:1,000 and 1:100 (wt/wt), respectively. At a higher concentration of trypsin (1:2 [wt/wt]), BFT-1 and BFT-2 were partially cleaved after 24 h. The patterns of tryptic peptides from both toxins were identical (data not shown).
Analysis of BFT secreted by low-producing ETBF strains.
The mechanisms by which some ETBF strains are high BFT producers rather than low BFT producers are unknown. Our prior work indicated that the particular bft isotype (i.e., bft-1 versus bft-2) present in the strain did not correlate with the extent of biological activity (6). To further assess the biologic diversity of BFT, BFT was purified by the urea-based modified purification protocol from low BFT-producing ETBF strains J38-1 (bft-1 isotype) and 20793-3 (bft-2 isotype) and compared to BFT-1 and BFT-2 purified from the high BFT-producing strains VPI 13784 and 86-4332-2-2, respectively. These experiments revealed that the BFT purified from each low-BFT-producing strain was comparable in biochemical profile and in biologic activity to the BFT purified from the high-BFT-producing strain containing the same bft allele (data not shown).
DISCUSSION
The data in this report indicate that the ETBF strains VPI 13784 and 86-5443-2-2 that contain distinct bft alleles secrete two biochemically different isotypes of BFT. Several lines of evidence support this conclusion. First, elution of each toxin from a high-resolution anion-exchange column (Mono Q) revealed different profiles, suggesting that the two proteins have different net charges. Differences in elution profile were also obtained with FPLC. Second, the two proteins had distinct electrophoretic mobilities on SDS-PAGE consistent with their predicted variable amino acid composition. Reported data suggest that even one or two amino acid changes in aliphatic, aromatic, and/or acidic residues may alter the SDS-PAGE migration of protein homologues (18). Third, examination of the two toxins by using antisera developed to each toxin reveals that homologous rather than heterologous antisera detected each toxin with greater specificity. Lastly, although the qualitative effect of each BFT on HT29/C1 cell morphology appeared similar, the two toxins had modest, but consistent, quantitative differences in HT29/C1 cellular activity. These observations are consistent with the fact that the mature toxin proteins, BFT-1 (from strain VPI 13784) and BFT-2 (from strain 86-5433-2-2), are predicted to share only 88.7 and 86.6% identity in nucleotide and predicted protein sequences, respectively (6, 14, 19). Further scrutiny of the amino acid sequences of these proteins reveals that they differ in 12 residues, including 4 residues in the zinc binding motif and 8 of the 31 residues of the carboxy terminus of the proteins, regions defined as the catalytic domain in zinc-dependent proteases (17). These changes yield two proteins with different theoretical pIs (4.94 versus 5.02, BFT-1 versus BFT-2 [http://caexpasy.org/cgi-bin/pi_tool]) and hydrophobicity (−2.31 versus −2.23, BFT-1 versus BFT-2 [http://ca.expasy.org/tools/pscale/Hphob.Doolittle.html]). Consistent with this theoretical analysis and the charge differences predicted by anion-exchange chromatography, the experimental pIs of BFT-1 and BFT-2 differed. However, tryptic digestion of BFT-1 and BFT-2 yielded similar peptic fragments, suggesting similar overall protein tertiary structures. Together, these data indicate that ETBF strains VPI 13784 and 86-5443-2-2 secrete two distinct isotypes of BFT. BFT produced by ETBF strain VPI 13784 has been termed BFT-1, and BFT produced by ETBF strain 86-5443-2-2 is termed BFT-2. This terminology recognizes the order in which both proteins were purified and genetic sequences were identified. More recently, ETBF strains isolated in Korea (3) and Japan (12) have been identified to contain a third distinct bft allele. The predicted protein sequence from BFT-3 has 95.7% identity with BFT-2 but only 89.2% identity with BFT-1. Consistent with this genetic analysis (3), the protein profile of BFT-3 by Mono Q chromatography and the migration speed of purified BFT-3 on SDS-PAGE are identical to those of BFT-2 (data not shown).
Another major observation of our current experiments is that although the purification procedure described by Van Tassell et al. (38) results in purification of a single protein which is BFT, this protein copurifies with carbohydrate most likely representing a portion of the ETBF capsular polysaccharide. This conclusion is supported by our observations that (i) the high molecular mass bands detected by polyclonal antisera to the BFT-2 preparation were largely insensitive to proteinase K digestion but sensitive to sodium metaperiodate treatment, (ii) the detection of a single protein with the N-terminal amino acid sequence of BFT was achieved by reverse-phase FPLC, (iii) direct measurement of carbohydrate was achieved by the phenol-sulfuric assay in purified BFT preparations, (iv) direct detection of the higher-molecular-mass band was indicated by a specific carbohydrate stain, and (v) the strain-specific nature of the high-molecular-mass bands was revealed by immunoblot analysis. Consistent with our observations, published data reveal that the B. fragilis capsular polysaccharide appears as a broad band on a silver-stained SDS-PAGE gel between ca. 80 and 200 kDa (26, 37) and that B. fragilis strains express multiple immunologically distinct capsular polysaccharides (27). The other major possibility for a nonprotein carbohydrate-containing molecule copurifying with BFT is lipopolysaccharide (LPS). However, B. fragilis expresses rough LPS with a molecular mass of less than 20 kDa (27, 42) and no proteinase K-insensitive molecules of this size were detected by our experiments, making copurification of LPS and BFT very unlikely.
Examination of the biologic activity of the higher-molecular-mass bands recovered under nondenaturing conditions (i.e., native PAGE), the presence of bands in the higher-molecular-mass range staining with Coomassie blue on SDS-PAGE, and the diminished intensity of these higher-molecular-mass bands after proteinase treatment (Fig. 5A) suggest that BFT purified by the Van Tassell method is most likely partially adherent to the copurified carbohydrate moieties. However, our data suggest that the copurified carbohydrate moieties do not alter the biological activity of BFT. The isoelectric point (pI) of BFT-2 is 5.6, and the pIs of the B. fragilis capsular polysaccharides A and B are approximately 7.3 and 2.5, respectively (37). Previous data indicate that both B. fragilis capsular polysaccharides elute from anion-exchange columns at NaCl concentrations of 0 to 0.5 M (37). These conditions are similar to the Mono Q elution profile of BFT (Fig. 1 and 6). Thus, the similarities in the charges of the polysaccharide molecules and BFT under the purification conditions employed probably contributed to the inability to separate them by this technique. However, inclusion of 6 M urea in the elution buffers for the Mono Q chromatography step enabled the complete separation of BFT and the associated carbohydrate. After removal of the urea by dialysis, BFT retained its biologic activity consistent with prior results, indicating urea does not fully denature all proteins (7). Of note, the modified purification protocol described herein is also more time-efficient due to the elimination of the ammonium sulfate precipitation and Q Sepharose anion-exchange columns steps in the Van Tassell procedure and yields more BFT (2 days to purify ∼35 μg of BFT from 500 ml of crude culture supernatant versus 1 week to purify ∼80 μg of BFT from 3 liters with the Van Tassell method).
Since the initial description of the secretory response to ETBF culture supernatants in the LLIL assay (22, 23), the biological activity of the culture supernatants of ETBF strains has been reported to be variable. Examination of the effect of culture supernatants of ETBF strains on the morphology of HT29/C1 cells and the amount of toxin detectable from ETBF strains by ELISA also revealed strain-to-strain variability in biological activity and the amount of secreted BFT (4, 20, 40, 41). Several possibilities exist for the variable toxigenicity of ETBF culture supernatants including differences in the expression and/or secretion of BFT from the bacterial cells, differences in the BFT protein secreted by various strains and/or the presence of additional virulence factors that modulate the activity of BFT. In this report, we determined that BFT-1 or -2 purified from culture supernatants of low-BFT-producing strains is similar in HT29/C1 biologic activity to its respective BFT subtype secreted by high-BFT-producing strains (i.e., 86-5443-2-2 or VPI 13784). These data suggest that low-BFT-producing strains differ from high-BFT-producing strains either in the transcriptional regulation of bft or in the efficiency of secretion of BFT but that mutations in bft-1 or bft-2 are not likely to account for the biological diversity of ETBF strains. Our initial data suggest that transcriptional regulation is the key mechanism involved (A. A. Franco, R. K. Cheng, A. Goodman, C. Polyak, C. L. Sears, unpublished data).
Although ETBF have been associated with diarrheal disease in children between the ages of 1 and 5 (1, 11, 29, 30, 36) (Menozzi et al., Abstr. 2nd World Congr. Anaerob. Bacteria Infect.), and, more recently, adults of all ages (45), much remains to be learned about the clinical illnesses associated with this enteric pathogen. Recent data identifying that extraintestinal strains (particularly bloodstream isolates) of B. fragilis produce BFT suggest a wider spectrum of disease for ETBF (9, 10, 20) (M. V. Whitcher, R. B. Mann, L. Powers, and R. J. Obiso, Abstr. 100th Gen. Meet. Am Soc. Microbiol., abstr. B-156, 2000). Additional studies are required to correlate disease expression with either the amount and/or isotype of BFT secreted by ETBF strains. Regardless of the range of clinical illnesses associated with ETBF infections, the BFT proteins secreted by these strains exhibit striking biological activities, including changes in the morphology of human intestinal epithelial cells with rearrangement of F actin (5, 15, 32, 33); stimulation of secretion in the ligated intestinal segments of rats, rabbits, lambs and calves (21, 23, 25); alteration of the physiology of intestinal epithelial cells and human colonic tissue (2, 15, 24, 28, 43); stimulation of interleukin-8 secretion (13, 35; S. Wu, J. Powell, and C. L. Sears, unpublished results); and cleavage of the zonula adherens protein, E cadherin (44). Further research to characterize the mechanisms of cellular intoxication by the BFT proteins should yield new insights into the regulation of intestinal secretion and the cytoskeletal network of intestinal epithelial cells.
Acknowledgments
We gratefully acknowledge the gifts of B. fragilis strains from Lyle Myers (Montana State University, Bozeman), R. Bradley Sack (Johns Hopkins University School of Medicine, Baltimore, Md.), and Tracy Wilkins (Virginia Polytechnic Institute and State University, Blacksburg) and of purified BFT from ETBF strain VPI 13784 and monospecific and polyclonal anti-VPI 13784 BFT sera from Tracy Wilkins. We thank David Maneval (Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, Md.) for use of the Superdex 75 HR 10/30 column.
This work was supported by NIH-DK 45496 (to C.L.S.).
Editor: J. T. Barbieri
REFERENCES
- 1.Caceres, M., G. Zhang, A. Weintraub, and C.-E. Nord. 2000. Prevalence and antimicrobial susceptibility of enterotoxigenic Bacteroides fragilis in children with diarrhoea in Nicaragua. Anaerobe 6:143-148. [Google Scholar]
- 2.Chambers, F. G., S. S. Koshy, R. F. Saidi, D. P. Clark, R. D. Moore, and C. L. Sears. 1997. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84 cells) in vitro. Infect. Immun. 65:3561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung, G. T., A. A. Franco, S. Wu, G. E. Rhie, R. Cheng, H. B. Oh, and C. L. Sears. 1999. Identification of a third metalloprotease toxin gene in extraintestinal isolates of Bacteroides fragilis. Infect. Immun. 67:4945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.d'Abusco, A. S., M. Del Grosso, S. Censini, A. Covacci, and A. Pantosti. 2000. The alleles of the bft gene are distributed differently among enterotoxigenic Bacteroides fragilis strains from human sources and can be present in double copies. J. Clin. Microbiol. 38:607-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donelli, G., A. Fabbri, and C. Fiorentini. 1996. Bacteroides fragilis enterotoxin induces cytoskeletal changes and surface blebbing in HT-29 cells. Infect. Immun. 64:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco, A. A., L. M. Mundy, M. Trucksis, S. Wu, J. B. Kaper, and C. L. Sears. 1997. Cloning and characterization of the Bacteroides fragilis metalloprotease toxin gene. Infect. Immun. 65:1007-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewlett, E. L., V. M. Gordon, J. D. McCaffery, W. M. Sutherland, M. C. Gray. 1989. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecular. J. Biol. Chem. 264:19379-19384. [PubMed] [Google Scholar]
- 8.Jett, M., G. A. Jamieson, and S. L. DeBernardo. 1971. The carbohydrate sequence of the glycopeptide chains of human transferrin. J. Biol. Chem. 246:3686-3693. [PubMed] [Google Scholar]
- 9.Kato, N., A. Karuniawati, R. Jotwani, H. Kato, K. Watanabe, and K. Ueno. 1995. Isolation of enterotoxigenic Bacteroides fragilis from extraintestinal sites by cell culture assay. Clin. Infect. Dis. 20(Suppl. 2):S141.. [DOI] [PubMed] [Google Scholar]
- 10.Kato, N., H. Kato, K. Watanabe, and K. Ueno. 1996. Association of enterotoxigenic Bacteroides fragilis with bacteremia. Clin. Infect. Dis. 23(Suppl. 1):S83-S86. [DOI] [PubMed] [Google Scholar]
- 11.Kato, N., C. Liu, H. Kato, K. Watanabe, H. Nakamura, N. Iwai, and K. Ueno. 1999. Prevalence of enterotoxigenic Bacteroides fragilis in children with diarrhea in Japan. J. Clin. Microbiol. 37:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato, N., C. X. Liu, H. Kato, K. Watanabe, Y. Tanaka, T. Yamamoto, K. Suzuki, and K. Ueno. 2000. A new subtype of the metalloprotease toxin gene and the incidence of the three bft subtypes among Bacteroides fragilis isolates in Japan. FEMS Microbiol. Lett. 182:171-176. [DOI] [PubMed] [Google Scholar]
- 13.Kim, J. M., Y. K. Oh, Y. J. Kim, H. B. Oh, and Y. J. Cho. 2001. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-kappa B plays a major role in the regulation of IL-8 expression. Clin. Exp. Immunol. 123:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kling, J. J., R. L. Wright, J. S. Moncrief, and T. D. Wilkens. 1997. Cloning and characterization of the gene for the metalloprotease enterotoxing of Bacteroides fragilis. FEMS Microbiol. Lett. 146:279-284. [DOI] [PubMed] [Google Scholar]
- 15.Koshy, S. S., M. H. Montrose, and C. L. Sears. 1996. Human intestinal epithelial cells swell and demonstrate actin rearrangement in response to the metalloprotease toxin of Bacteroides fragilis. Infect. Immun. 64:5022-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, C., A. Levin, and D. Branton. 1987. Copper staining: a five-minute protein stain for sodium dodecyl sulfate- polyacrylamide gels. Anal. Biochem. 166:308-312. [DOI] [PubMed] [Google Scholar]
- 17.Massova, I., L. P. Kotra, R. Fridman, and S. Mobashery. 1998. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 12:1075-1095. [PubMed] [Google Scholar]
- 18.Miyake, J., S. Ochiai-Yanagi, T. Kasumi, T. Takagi. 1978. Isolation of a membrane protein from R rubrum chromatophores and its abnormal behavior in SDS-polyacrylamide gel electrophoresis due to a high binding capacity for SDS. J. Biochem. 83:1679-1686. [DOI] [PubMed] [Google Scholar]
- 19.Moncrief, J. S., R. Obiso, L. A. Barroso, J. J. Kling, R. L. Wright, R. L. Van Tassell, D. M. Lyerly, and T. D. Wilkins. 1995. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect. Immun. 63:175-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mundy, L. M., and C. L. Sears. 1996. Detection of toxin production by Bacteroides fragilis: assay development and screening of extraintestinal clinical isolates. Clin. Infect. Dis. 23:269-276. [DOI] [PubMed] [Google Scholar]
- 21.Myers, L. L., B. D. Firehammer, D. S. Shoop, and M. M. Border. 1984. Bacteroides fragilis: a possible cause of acute diarrheal disease in newborn lambs. Infect. Immun. 44:241-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers, L. L., D. S. Shoop, and T. D. Byars. 1987. Diarrhea associated with enterotoxigenic Bacteroides fragilis in foals. Am. J. Vet. Res. 48:1565-1567. [PubMed] [Google Scholar]
- 23.Myers, L. L., D. S. Shoop, B. D. Firehammer, and M. M. Border. 1985. Association of enterotoxigenic Bacteroides fragilis with diarrheal disease in calves. J. Infect. Dis. 152:1344-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obiso, R. J., Jr., A. O. Azghani, and T. D. Wilkins. 1997. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect. Immun. 65:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obiso, R. J., Jr., D. M. Lyerly, R. L. Van Tassell, and T. D. Wilkins. 1995. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect. Immun. 63:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantosti, A., A. O. Tzianabos, A. B. Onderdonk, and D. L. Kasper. 1991. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect. Immun. 59:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantosti, A., A. O. Tzianabos, B. G. Reinap, A. Onderdonk, and D. L. Kasper. 1993. Bacteroides fragilis strains express multiple capsular polysaccharides. J. Clin. Microbiol. 31:1850-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riegler, M., M. Lotz, C. Sears, C. Pothoulakis, I. Castagliuolo, C. C. Wang, R. Sedivy, T. Sogukoglu, E. Cosentini, G. Bischof, W. Feil, B. Teleky, G. Hamilton, J. T. LaMont, and E. Wenzl. 1999. Bacteroides fragilis toxin 2 damages human colonic mucosa in vitro. Gut 44:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sack, R. B., M. J. Albert, K. Alam, P. K. B. Neogi, and M. S. Akbar. 1994. Isolation of enterotoxigenic Bacteroides fragilis from Bangladeshi children with diarrhea: a case-control study. J. Clin. Microbiol. 32:960-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sack, R. B., L. L. Myers, J. Almeido-Hill, D. S. Shoop, W. C. Bradbury, R. Reid, and M. Santosham. 1992. Enterotoxigenic Bacteroides fragilis: epidemiologic studies of its role as a human diarrhoeal pathogen. J. Diarrhoeal Dis. Res. 10:4-9. [PubMed] [Google Scholar]
- 31.Saha, S. K., and C. F. Brewer. 1994. Determination of the concentrations of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohydr. Res. 254:157-167. [DOI] [PubMed] [Google Scholar]
- 32.Saidi, R. F., K. Jaeger, M. H. Montrose, S. Wu, and C. L. Sears. 1997. Bacteroides fragilis toxin alters the actin cytoskeleton of HT29/C1 cells in vivo qualitatively but not quantitatively. Cell Motility Cytoskeleton 37:159-165. [DOI] [PubMed] [Google Scholar]
- 33.Saidi, R. F., and C. L. Sears. 1996. Bacteroides fragilis toxin rapidly intoxicates human intestinal epithelial cells (HT29/C1) in vitro. Infect. Immun. 64:5029-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sanfilippo, L., C. K. Seth, R. Li, T. J. Balwin, M. G. Menozzi, and Y. R. Mahida. 2000. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-beta) by human colonic epithelial cells. Clin. Exp. Immunol. 119(3):456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.San Joaquin, V. H., J. C. Griffis, C. Lee, and C. L. Sears. 1995. Association of Bacteroides fragilis with childhood diarrhea. Scand. J. Infect. Dis. 27:211-215. [DOI] [PubMed] [Google Scholar]
- 37.Tzianabos, A. O., A. Pantosti, H. Baumann, J. R. Brisson, H. J. Jennings, and D. L. Kasper. 1992. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J. Biol. Chem. 267:18230-18235. [PubMed] [Google Scholar]
- 38.Van Tassell, R. L., D. M. Lyerly, and T. D. Wilkins. 1992. Purification and characterization of an enterotoxin from Bacteroides fragilis. Infect. Immun. 60:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Tassell, R. L., D. M. Lyerly, and T. D. Wilkins. 1994. Production of antisera against the enterotoxin of Bacteroides fragilis and their use in a cytotoxicity neutralization assay of HT-29 cells. Clin. Diagn. Lab. Immunol. 1:473-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Tassell, R. L., D. M. Lyerly, and T. D. Wilkins. 1994. Characterization of enterotoxigenic Bacteroides fragilis by a toxin-specific enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 1:578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weikel, C. S., F. D. Grieco, J. Reuben, L. L. Myers, and R. B. Sack. 1992. Human colonic epithelial cells, HT29/C1, treated with crude Bacteroides fragilis enterotoxin dramatically alter their morphology. Infect. Immun. 60:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weintraub, A., U. Zahringer, and A. A. Lindberg. 1985. Structural studies of the polysaccharide part of the cell wall lipopolysaccharide from Bacteroides fragilis NCTC 9343. Eur. J. Biochem. 151:657-661. [DOI] [PubMed] [Google Scholar]
- 43.Wells, C. L., E. M. A. Van De Westerlo, R. P. Jechorek, B. A. Feltis, T. D. Wilkins, and S. L. Erlandsen. 1996. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology 110:1429-1437. [DOI] [PubMed] [Google Scholar]
- 44.Wu, S., K.-C. Lim, J. Huang, R. F. Saidi, and C. L. Sears. 1998. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 95:14979-14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, G., B. Svenungsson, A. Karnell, and A. Weintraub. 1999. Prevalence of enterotoxigenic Bacteroides fragilis in adult patients with diarrhea and healthy controls. Clin. Infect. Dis. 29:590-594. [DOI] [PubMed] [Google Scholar]