Abstract
Oophorectomized, estrogen-treated rats were immunized by the intravaginal or intranasal route with a mannoprotein extract (MP) or secreted aspartyl proteinases (Sap) of Candida albicans, with or without cholera toxin as a mucosal adjuvant. Both routes of immunization were equally effective in (i) inducing anti-MP and anti-Sap vaginal antibodies and (ii) conferring a high degree of protection against the vaginal infection by the fungus. These data suggest that appropriate fungal antigens and adjuvant can be used to protect against candidal vaginitis, by either route.
Clinical studies on recurrent vulvovaginal candidiasis as well as several investigations with estrogen-dependent murine models of this disease led to the suggestion that local rather than systemic humoral and/or cellular immunity plays a defensive role (5, 6, 11-13, 18). Members of our group have specifically addressed the issue of the protective role of antibodies against specific virulence factors of Candida albicans in a model of estrogen-dependent rat vaginitis (7, 8). Several lines of evidence support this role. In particular, a substantial therapeutic effect by local administration of monoclonal antibodies against mannoprotein (MP) (16) or secretory aspartyl proteinase (Sap) of the fungus was obtained (7). Moreover, active intravaginal immunization with MP or Sap conferred an elevated degree of antibody-mediated protection from vaginal infection by C. albicans (4-7). These results and those found by others (14, 15) laid the foundation for studies on the preventative or therapeutic use of vaccines against vaginal candidiasis.
Particular attention has recently been focused on mucosal immunization and its capacity to induce a high level of antibody and cell-mediated immune response at distant mucosal sites or even systemically (1). It is well known that the modalities of antigen administration and the type of adjuvant exert a critical role in the induction of protection, a notation of particular importance for a multifaceted, multiorgan disease such as candidiasis in normal or immunocompromised patients (2, 17). Several microbial toxins, among which are the cholera toxin (CT) produced by Vibrio cholerae, and nontoxic derivatives from this and other microorganisms have shown a great potential as mucosal adjuvants for local and systemic antibody responses (9, 10, 22). Considering that all previous evidence of antibody protective responses at the vaginal level were obtained by local immunization (4-7), while there would be several advantages in immunizing at a nonvaginal mucosal site, we have now compared intranasal to intravaginal immunization with soluble Candida antigens and CT as a mucosal adjuvant for induction of a specific antibody response at the vaginal level and outcome of vaginal infection by C. albicans.
The yeast used throughout this study was C. albicans SA-40, first isolated from the vaginal secretion of a subject with acute vaginitis. The modalities of fungal growth, induction, and assessment of experimental vaginal infection in oophorectomized rats were as previously described (4, 6-8).
For active immunization, groups of five rats were immunized by the intravaginal (i.v.g.) or intranasal (i.n.) route, three times at weekly intervals, with 100 μg of MP or a Sap preparation, mostly consisting of the Sap 2 component (7), added with 10 μg of CT (kindly provided by Swiss Serum and Vaccine Institute, Bern, Switzerland). For i.n. immunization the rats were lightly anesthetized by intraperitoneal administration of 45 mg of 10% ketamine/kg of body weight (Ketavet 100; Farmaceutici Gellini Spa Aprilia, Latina, Italy) and 5 mg of 2% Xilazine/kg (Rompum Bayer AG, Leverkusen, Germany). The inoculum was delivered as two applications of 5 μl to each nostril for a total volume of 10 μl per dose.
Control animals for both i.n. and i.v.g. immunizations received only CT or only sterile saline. One week after the last immunization, all animals were challenged i.v.g. with 107 cells of C. albicans, and the infection was monitored by enumeration of CFU, as reported elsewhere (4, 6, 7). Three independent experiments for each immunization (MP or Sap plus or minus CT) and each immunization route (i.n. or i.v.g.) were carried out. The presence of antibodies was assayed in the vaginal wash by a previously described enzyme-linked immunosorbent assay (ELISA) (4, 7). Briefly, 200 μl of an in-house mannoprotein extract solution (MP) (17) or Sap (kindly provided by P. A. Sullivan, Massey University, Palmerston North, New Zealand) (5 μg/ml in 0.2 M sodium carbonate) was used as the coating antigen for the detection of any specific antibody and was dispensed into the wells of a polystyrene microtitration plate which was kept overnight at 4°C. After three washes with Tween 20-phosphate-buffered saline buffer, 1:2 dilutions of vaginal fluids were distributed in triplicate wells and the plates were incubated for 1 h at room temperature. Each well was washed again with Tween 20-phosphate-buffered saline buffer, and predetermined optimal dilutions of alkaline phosphatase conjugate, sheep anti-rat immunoglobulin G (IgG), IgM, or IgA (obtained from Serotec Ltd; Kidlington, Oxford, United Kingdom) were added. Bound alkaline phosphatase was detected by the addition of a solution of para-nitrophenyl phosphate in diethanolamine buffer, and the plates were read at A405 with an automated microreader (Labsystem Multiscan, Helsinki, Finland) blanked against air. A vaginal fluid was considered positive for a determined antibody when the optical density was greater than 2 times the value of the well coated with the same antigen and tested with the vaginal fluid of an unimmunized, uninfected rat (these 1:2-diluted fluids never exceeded the readings of 0.14 (for IgA) and 0.10 (for both IgM and IgG). ELISA-positive vaginal fluids were confirmed by Western blotting, as described elsewhere (4).
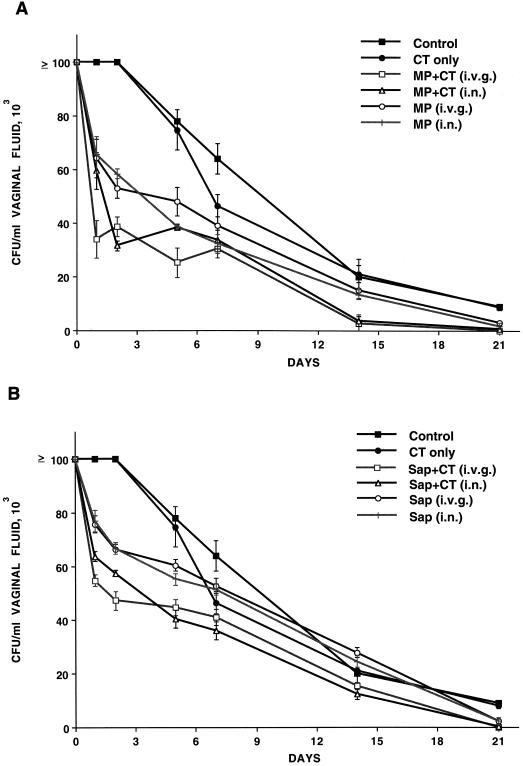
Since it was previously shown that active i.v.g. immunization with MP or Sap in Freund's adjuvant conferred protection against vaginal C. albicans challenge (4, 7), we preliminarily addressed the confirmation of previous data by using CT instead of Freund's adjuvant. In addition, we examined whether protection could be achieved by i.n. immunization with the same antigens, with or without CT. Rats given only CT or saline i.v.g. or i.n. served as controls. As shown in Fig. 1A (which refers to one typical experiment out of the three performed with similar results), the rats immunized with MP by either the i.n. or the i.v.g. route were characterized by early clearance of the fungal cells from the vagina compared to rats given the adjuvant or saline only, as demonstrated by a nearly 50% reduction of vaginal Candida counts by the first 48 h after challenge. This early, 2-day clearance rate was significantly more pronounced in the animals immunized, by either route, with the antigen plus CT, an effect which persisted for at least 2 weeks after challenge (Fig. 1). There was no significant difference at any but the occasional time point in the candidal CFU between the animals immunized by the i.n. and those immunized by the i.v.g. route, and in both cases the infection was cleared by 21 days after challenge, when the rats given saline or CT only were still infected with an average of around 10 × 103 CFU/ml of vaginal fluid (Fig. 1A).
FIG. 1.
(A) Outcome of vaginal infection by C. albicans in rats immunized with MP (A) or Sap (B), plus or minus CT, and controls, as indicated. Each curve represents the mean (± standard error) of the number of fungal CFU for five rats. Statistical significance was assessed by Student's t test (two-tailed), set at P < 0.05. In Fig. 1A, at all indicated time points, there was a statistically significant difference between the vaginal number of CFU for controls and CT-only animals and those for animals given MP and MP plus CT, as well as between the number of vaginal CFU for animals given MP only and those given MP plus CT, by either route, with the exception of the number of CFU on day 5 (difference nonsignificant between the number of CFU for animals given intranasal MP or MP plus CT). (B) At days 1, 2 and 5, there was a statistically significant difference between the numbers of vaginal CFU for controls and CT-only animals and those for animals given Sap or Sap plus CT, by either route, as well as between the number of vaginal CFU for animals given Sap only and those given Sap plus CT, by either route. On days 7 and 14, the animals given CT only had significantly lower counts than the control rats, yet the animals immunized with Sap plus CT had significantly fewer vaginal CFU than those given CT only.
As shown in Fig. 1B (which also refers to one out of three experiments with similar results), immunization with Sap also resulted in statistically significant acceleration of fungal clearance from the vagina in the first week of infection, both when the animals were immunized with the antigen only and, more significantly, when CT was coadministered with Sap (particularly in the first 5 days after challenge). The effect on clearance somewhat faded away in the second and third weeks of infection, although, on day 21, all immunized animals had no fungal cells in their vaginas, in contrast to the controls, which were all still infected (from 8 × 103 to 14 × 103 cells/ml of vaginal fluid; Fig. 1B). Comparison of data in Fig. 1A and B, together with the two other experiments performed and not shown, suggests that MP immunization was slightly more effective than Sap immunization in accelerating clearance of Candida organisms from the vagina, both after i.n. and i.v.g. immunization, somewhat confirming previous results of ours (4, 7).
Overall, the data in Fig. 1A and B cumulatively indicated that administration of MP and Sap was protective against C. albicans vaginal challenge, that the degree of protection was generally greater when the antigen was administered with the mucosal adjuvant, and finally, that the two routes of antigen and adjuvant administration conferred a substantially equivalent protection in terms of the rate of fungus clearance from rat vaginas.
We also examined whether and to what extent antigen-specific antibodies were induced in the rat vagina following protective immunization with MP or Sap, with or without CT. To this aim, the vaginal fluids of i.n.- or i.v.g.-immunized rats and controls were harvested before (time zero) and after (28 days) immunization with the respective antigen and/or CT and tested in ELISA for specific antibody. We also sought for anti-MP and anti-Sap antibodies after challenge with C. albicans, which would theoretically constitute a booster immunization, considering that both MP and Sap are secreted during C. albicans infection (5, 19, 20). Tables 1 and 2 show the results of one of two independent determinations of vaginal anti-MP and anti-Sap antibodies expressed as the optical density readings of the ELISA with MP or Sap as the coating antigen. Animals immunized i.v.g. or i.n. with MP antigen in conjunction with CT developed a measurable level of anti-MP antibody (IgG and IgA) compared to the animals given MP only (which showed barely detectable antibody levels) and those given saline or CT only, which showed no antibodies at all (Table 1). The anti-MP antibody levels were significantly boosted by C. albicans challenge in animals immunized with MP plus CT but not in those previously immunized with MP only, indicating that the barely detectable ELISA reading in the vaginal fluid of these animals was probably an aspecific background. The booster effect was particularly noticeable for IgG and with the i.n. route. No antibodies against MP were detected in animals immunized with Sap, with or without CT, and none were detected in controls animals either, i.e., rats not immunized or given adjuvant only, even after C. albicans challenge (Table 1), thus indicating the absence of immunogenic mannoprotein constituents in our Sap preparation.
TABLE 1.
Anti-MP antibody levelsa in the vaginal fluids of rats immunized i.v.g. and i.n. with MP or Sap and challenged or not with C. albicansb
| Immunizationc | Level of anti-MP antibodyd for group
|
|||||
|---|---|---|---|---|---|---|
| Immunized only
|
Immunized and challenged
|
|||||
| IgG | IgM | IgA | IgG | IgM | IgA | |
| None | 0.09 | 0.07 | 0.14 | 0.20 | 0.09 | 0.22 |
| CT, i.n. | 0.09 | 0.10 | 0.09 | 0.18 | 0.15 | 0.22 |
| CT, i.v.g. | 0.08 | 0.10 | 0.08 | NDe | ND | ND |
| MP, i.n. | 0.22 | 0.19 | 0.21 | 0.21 | 0.18 | 0.18 |
| MP, i.v.g. | 0.25 | 0.09 | 0.24 | 0.20 | 0.14 | 0.21 |
| MP + CT, i.n. | 0.34 | 0.11 | 0.34 | 0.89 | 0.48 | 0.64 |
| MP + CT, i.v.g. | 0.38 | 0.09 | 0.33 | 0.55 | 0.34 | 0.51 |
| Sap, i.n. | 0.14 | 0.10 | 0.16 | 0.23 | 0.22 | 0.24 |
| Sap, i.v.g. | 0.15 | 0.10 | 0.16 | 0.22 | 0.11 | 0.24 |
| Sap + CT, i.n. | 0.19 | 0.18 | 0.17 | 0.18 | 0.22 | 0.26 |
| Sap + CT, i.v.g. | 0.16 | 0.15 | 0.18 | 0.22 | 0.11 | 0.24 |
Measured by ELISA on pools of vaginal washes from each rat (five per group) (diluted 1:2) taken at the end of the immunization protocol (day 28 since the first administration of the immunogen) and on day 7 after challenge.
Challenge: 107 C. albicans cells in 0.1 ml.
See the text for the protocol of immunization.
The values are optical density readings at 405 nm. They are those of the experiment referred to in Fig. 1A.
ND, not done.
TABLE 2.
Anti-Sap antibody levelsa in the vaginal fluids of rats immunized i.v.g. and i.n. with MP or Sap and challenged or not with C. albicansb
| Immunizationc | Level of anti-Sap antibodyd for group
|
|||||
|---|---|---|---|---|---|---|
| Immunized only
|
Immunized and challenged
|
|||||
| IgG | IgM | IgA | IgG | IgM | IgA | |
| None | 0.10 | 0.09 | 0.11 | 0.18 | 0.09 | 0.20 |
| CT, i.n. | 0.07 | 0.09 | 0.09 | 0.18 | 0.10 | 0.17 |
| CT, i.v.g. | 0.09 | 0.08 | 0.09 | NDe | ND | ND |
| MP, i.n. | 0.17 | 0.18 | 0.21 | 0.14 | 0.11 | 0.17 |
| MP, i.v.g. | 0.21 | 0.17 | 0.18 | 0.19 | 0.18 | 0.21 |
| MP + CT, i.n. | 0.29 | 0.22 | 0.28 | 0.30 | 0.22 | 0.33 |
| MP + CT, i.v.g. | 0.28 | 0.18 | 0.26 | 0.36 | 0.28 | 0.31 |
| Sap, i.n. | 0.23 | 0.20 | 0.26 | 0.24 | 0.18 | 0.26 |
| Sap, i.v.g. | 0.25 | 0.22 | 0.24 | 0.28 | 0.21 | 0.31 |
| Sap + CT, i.n. | 0.34 | 0.22 | 0.36 | 0.39 | 0.24 | 0.48 |
| Sap + CT, i.v.g. | 0.38 | 0.36 | 0.34 | 0.45 | 0.29 | 0.52 |
Measured by ELISA on pools of vaginal washes from each rat (five per group) (diluted 1:2) taken at the end of the immunization protocol (day 28 since the first administration of the immunogen) and on day 7 after challenge.
Challenge: 107 C. albicans cells in 0.1 ml.
See the text for the protocol of immunization.
The values are optical density readings at 405 nm. They are those of the experiment referred to in Fig. 1B.
ND, not done.
Anti-Sap antibody levels are shown in Table 2. These antibodies were consistently detected only in animals immunized with the specific antigen and CT, and the booster effect of C. albicans challenge appeared to be little and probably relevant only for the IgA levels (Table 2). Some anti-Sap antibodies were detected also in the animals immunized with MP and CT, but with no booster effect by C. albicans challenge (Table 2), indicating that our in-house mannoproteic extract could contain Sap traces.
Overall, these results demonstrate the elicitation of both anti-MP and anti-Sap antibodies in animals immunized with the respective antigen and with CT as an adjuvant to substantially similar extents via the i.n. and the i.v.g. routes. They also demonstrate that (i) the most relevant protective isotypes (IgG and IgA) were produced, and (ii ) the infection by C. albicans constituted a potent booster of vaginal antibody formation, mainly against MP, a highly represented antigenic cell-surface and secretory constituent of the fungus (19, 20, 21).
Experimental models of vaginitis in rodents have recently been addressed to study the anti-Candida response and mechanisms of antifungal protection at the vaginal level (3-8, 11-14). In our rat model of vaginal candidiasis, the presence of protective antibodies and T-helper type 1 cytokines in the vaginal fluids, the in vitro proliferation of vaginal lymphocytes in response to Candida antigenic stimulation, and the increased number of activated CD4+ cells after C. albicans challenge constitute good evidence for induction of locally expressed Candida-specific antibody and helper-T-cell responses, potentially synergizing anticandidal protection at the vaginal level (6).
In this paper, we have demonstrated that i.n. immunization with MP or Sap, mixed with the potent mucosal adjuvant CT, induced a high degree of protection against vaginal challenge with C. albicans. Importantly, the degree of protection after i.n. immunization was totally comparable to that achievable with i.v.g. immunization, as shown in this and previous papers (4, 6, 7), demonstrating the substantial equivalence of the two routes of mucosal immunization and the well-known communication among mucosal compartments (1, 5). This acquires particular significance for anti-Candida immunization owing to the recognized advantages of the i.n. route in terms of ease of administration and subject compliance to immunization itself.
Our data again emphasize the role of specific humoral immunity at the vaginal level by both the i.n. and the i.v.g. routes of immunization. Although our MP extract contained some protein in addition to the polysaccharide, previous investigations with the use of monoclonal antibodies against mannan or the protein constituent of MP suggested that the protection was conferred by the antimannan antibodies (7). In this context, the potent booster effect exerted by Candida challenge on antibody response to MP might play a role in the protection achieved by immunization or by the clearance of a primary infection (4, 6), considering that MP contains protective epitopes (mostly β 1-2 oligosaccharides) of the fungus cell surface (16, 19, 20, 21). Although no ad hoc transfer experiments were here performed and no specific T-cell responses were investigated, we previously demonstrated the protective role of both anti-Sap and anti-MP antibodies by transfer experiments with vaginal fluids and use of anti-MP and anti-Sap monoclonal antibodies (4, 7). Antibody-mediated anti-Candida protection was recently confirmed by Han and colleagues in a mouse vaginitis model (14).
We have shown here that CT can induce protective immunity when coadministered with suitable, protective fungal antigens. CT has long been shown to promote the development of both humoral and cell-mediated immune responses against several pathogens in mucosal compartments (1, 9, 10). The most recent evidence for this activity in the field of candidiasis has been provided by Cardenas-Freytag et al. (2), who showed the effectiveness of an i.n.-delivered vaccine consisting of whole inactivated Candida cells and a CT-like mucosal adjuvant in conferring protection from a systemic fungus challenge.
Overall, our data demonstrate that protection from candidiasis in our experimental model can be induced by immunization with a specific subunit vaccine preparation and with CT as an adjuvant by the i.n. route rather than by local immunization. It is logical to expect from the wealth of information already published that CT could easily be replaced by similarly active, nontoxic derivatives of this and other microbial toxins. This opens the way to an easier modality of immunization in harmony with the functional absence of compartmentation for anti-Candida protection at the mucosal level. The association between antibody elicitation and protection, together with previous results (4, 6, 7), also confirms and extends the potential of passive immunotherapy with specific antibodies against virulence factors of the fungus for anticandidal protection at vaginal and possibly other mucosal and nonmucosal sites.
Acknowledgments
This work was supported in part by the National AIDS Research Program under ISS contract 50D.2.
Thanks are due to Giusi Mandarino for help in the preparation of the manuscript.
Editor: R. N. Moore
REFERENCES
- 1.Boyaka, P. N., M. Marinaro, J. L. Vancott, I. Takahashi, K. Fujihashi, M. Yamamoto, F. W. van Ginkel, R. J. Jackson, H. Kiyono, and J. R. McGhee. 1999. Strategies for mucosal vaccine development. Am. J. Trop. Med. Hyg. 60:35-45. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas-Freytag, L., E. Cheng, P. Mayeux, J. E. Domer, and J. D. Clements. 1999. Effectiveness of a vaccine composed of heat-killed Candida albicans and a novel mucosal adjuvant LT(R192G) against systemic candidiasis. Infect. Immun. 67:826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A. 1995. Antibody immunity and invasive fungal infection. Infect. Immun. 63:4211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone, A., M. Boccanera, D. Ariani, G. Santoni, and F. De Bernardis. 1995. Rat clearing a vaginal infection by Candida albicans acquired specific antibody-mediated resistance to vaginal reinfection. Infect. Immun. 63:2619-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bernardis, F., and M. Boccanera. 2002. Mucosal infection and immunity in candidiasis, p. 461-482 In R. Calderone and R. Chilar (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker, Inc., New York, N.Y.
- 6.De Bernardis, F., G. Santoni, M. Boccanera, E. Spreghini, D. Adriani, L. Morelli, and A. Cassone. 2000. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect. Immun. 68:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3339-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bernardis, F., R. Lorenzini, and A. Cassone. 1999. Rat model of Candida vaginal infection, p. 735-740. In O. Zak and M. A. Sande (ed.), Handbook of animal models in infection. Academic Press, New York, N.Y.
- 9.Elson, C. O. 1989. Cholera toxin and its subunits as potential oral adjuvants. Immunol. Today 146:29-33. [DOI] [PubMed] [Google Scholar]
- 10.Elson, C. O. 1996. Cholera toxin as mucosal adjuvant, p. 59-72. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines. Academic Press, Inc., San Diego, Calif.
- 11.Fidel, P. L., Jr., and J. D. Sobel. 1996. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 9:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1993. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect. Immun. 61:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidel, P. L., Jr., J. Wluo, N. Chabain, A. Wolf, and E. Van Buren. 1997. Use of cellular depletion analysis to examine circulation of immune effector function between the vagina and the periphery. Infect. Immun. 65:3939-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur, S., G. Virella, J. Koistinen, E. O. Horger, A. Mahviand, and H. H. Fudenberg. 1992. Humoral immunity in vaginal candidiasis. Infect. Immun. 15:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mencacci, A., A. Torosantucci, R. Spaccapelo, L. Romani, F. Bistoni, and A. Cassone. 1994. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production and anticandidal protection in mice. Infect. Immun. 62:5353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers, T. J., and E. Balish. 1980. Immunity to Candida albicans. Microbiol. Rev. 44:660-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobel, J. D. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14:148-153. [DOI] [PubMed] [Google Scholar]
- 19.Torosantucci, A., M. J. Gomez, C. Bromuro, I. Casalinuovo, and A. Cassone. 1991. Biochemical and antigenic characterization of mannoprotein constituents released from yeast and mycelial form of Candida albicans. J. Med. Vet. Mycol. 29:361-372. [DOI] [PubMed] [Google Scholar]
- 20.Torosantucci, A., C. Bromuro, M. J. Gomez, C. M. Ausiello, F. Urbani, and A. Cassone. 1993. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J. Infect. Dis. 168:427-435. [DOI] [PubMed] [Google Scholar]
- 21.Trinel, P. A., C. Faille, P. M. Jacquinot, J. C. Caillez, and D. Poulain. 1992. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect. Immun. 60:3845-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu-Amano, J., R. J. Jackson, K. Fujihashi, H. Kiyono, H. F. Staats, and J. R. McGhee. 1994. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine 12:903-911. [DOI] [PubMed] [Google Scholar]