Abstract
Attachment and ingestion of Mycobacterium avium subsp. paratuberculosis by two epithelial cell lines were enhanced by soluble fibronectin (FN). Peptide blocking of the FN attachment protein (FAP-P) inhibited the internalization of M. avium subsp. paratuberculosis. Disruption of FAP-P expression significantly reduced attachment and ingestion of M. avium subsp. paratuberculosis by T-24 and Caco-2 cells. The results indicate that the interaction between FN and FAP-P facilitates attachment and internalization of M. avium subsp. paratuberculosis by epithelial cells.
Johne's disease poses a significant economic threat to the dairy cattle industry (13, 21). Johne's disease is a granulomatous enteritis of ruminants that is caused by Mycobacterium avium subsp. paratuberculosis. M. avium subsp. paratuberculosis invades macrophages that reside in ileal Peyer's patches, subsequently resulting in granuloma formation. Infiltration of intestinal lamina propria by inflammatory cells and an increase in the size and number of granulomas disrupt intestinal absorption, resulting in wasting and death. Animals are usually infected before 6 months of age; however, the cachexia and profuse diarrhea that are the hallmarks of Johne's disease are not observed until several years postinfection.
The ability of a microorganism to bind fibronectin (FN) may potentially facilitate its colonization of the host through attachment to the extracellular matrix in areas of epithelial damage. Furthermore, because several host cell integrins have binding sites for FN, the ability of a microorganism to bind soluble FN establishes a bridge between the organism and the host cell cytoskeleton, a condition necessary for the internalization of the microbe by the cell (3, 6). Fibronectin attachment proteins (FAPs) comprise a family of FN-binding glycoproteins that are expressed by several species of mycobacteria (16-19, 24). Expression of FAP is critical for the attachment of Mycobacterium bovis BCG and M. avium subsp. avium to tissue in vivo and ex vivo (11, 24). Moreover, the internalization of M. bovis BCG and Mycobacterium leprae by cultured epithelial cells was shown to be a FAP-dependent process (10, 18).
M. avium subsp. paratuberculosis also expresses a FAP (designated FAP-P) which mediates soluble FN binding (19). However, unlike the FAPs of other mycobacteria, FAP-P is not present on the surface of the organism (19). Thus, the manner in which FAP-P engages FN may sequester the cell binding domain from host cell receptors. As a result, binding and ingestion of M. avium subsp. paratuberculosis by host cells would be FN independent.
To examine the effect of the interaction of FAP-P and FN on the ability of M. avium subsp. paratuberculosis to attach to and invade epithelial cells, FN-opsonized and nonopsonized organisms were used to infect T-24 human bladder carcinoma cells (a gift from J. S. Schorey, University of Notre Dame, South Bend, Ind.) and Caco-2 human intestinal adenocarcinoma cells (supplied by M. Popielarczyk, Purdue University, West Lafayette, Ind.). T-24 cells were propagated as described previously (18). Caco-2 cells were propagated in minimum essential medium with Earle's salts, l-glutamine, and nonessential amino acids (Life Technologies) supplemented with 20% fetal bovine serum (FBS) and antibiotic-antimycotic solution. M. avium subsp. paratuberculosis strain 5781 (19) was propagated in Middlebrook 7H9 broth (Becton Dickinson, Cockeysville, Md.) supplemented with 10% oleic acid-albumin-dextrose-catalase (Becton Dickinson), 0.05% Tween 80 (Sigma, St. Louis, Mo.), and 2 μg of mycobactin J (Allied Monitor, Fayetteville, Mo.) per ml in tissue culture flasks.
M. avium subsp. paratuberculosis cultures were centrifuged for 15 min at 1,600 × g. The bacterial pellet was mixed thoroughly by vigorous pipetting and vortexing in phosphate-buffered saline (PBS) containing 0.05% Tween 20 (Sigma) to generate a single-cell suspension and resuspended in a volume of PBS containing 0.05% Tween 20 sufficient to yield 5 × 106 CFU/ml. Five milliliters of bacterial suspension was centrifuged as described above, and the pellet was resuspended in 5 ml of ANT buffer (10 mM ammonium acetate, 0.85% sodium chloride, 0.05% Tween 20; pH 3) After incubation for 5 min at room temperature, the suspension was centrifuged as before. The pellet was resuspended in 5 ml of ANT buffer (pH 6) and split into four 1-ml units. Twenty micrograms of bovine FN (Biomedical Technologies, Stoughton, Mass.) was added to each of two tubes. After incubation for 1 h at 37°C, the bacterial suspensions were diluted 1:1 with either Iscove’s modified Dulbecco’s medium or minimum essential medium basal medium (no FBS or antibiotics) and used to infect T-24 or Caco-2 cells, respectively.
T-24 and Caco-2 cells (5 × 104) in complete media were seeded into each well in the top and bottom rows, respectively, of an eight-well chamber slide (Lab-Tek II; Nunc, Napierville, Ill.). The wells were precoated with 100 μg of murine laminin (Sigma)/ml. After being seeded, cells were allowed to attach for 9 h at 37°C in 5% CO2. The wells were washed twice with prewarmed PBS, and 0.2 ml of FN-opsonized or nonopsonized bacteria, prepared as outlined above, was added to the appropriate wells to yield a multiplicity of infection of 10. After incubation for 3 h at 37°C in 5% CO2, the wells were washed twice with PBS and bacterial attachment and invasion were assessed by the double immunofluorescence assay described previously (10). Briefly, the wells were blocked with 5% FBS in PBS followed by incubation with a 1:100 dilution of rabbit anti-M. bovis BCG immunoglobulin G (IgG; Dako, Carpinteria, Calif.). After being washed in PBS, the slides were incubated with a 1:200 dilution of tetramethyl rhodamine isothiocyanate-conjugated goat anti-rabbit IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) to label extracellular bacteria. Host cell membranes were rendered permeable by exposure to methanol for 5 min, and the staining procedure was repeated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Antibodies, Inc., Davis, Calif.). Slides were examined with an Optiphot-2 epifluorescence microscope (Nikon, Mellville, N.Y.). Attached bacteria fluoresced both red and green, whereas internalized bacteria stained green only. For most experiments, 200 cells were counted per treatment, and the number of cells having attached or internalized mycobacteria was recorded. Three hundred cells were counted for each antisense strain and for vector controls. Cells with both attached and internalized mycobacteria were scored as ingesting cells.
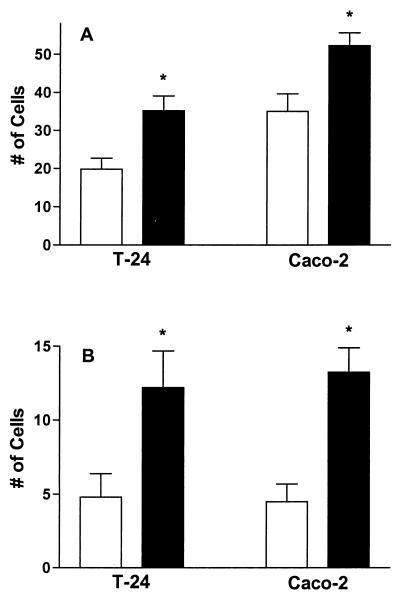
Opsonization of bacteria with FN resulted in a nearly 2-fold increase in the number of T-24 cells binding mycobacteria and a 1.5-fold enhancement in bacterial attachment to Caco-2 cells (Fig. 1A). FN-opsonized bacteria were also more readily internalized, with 2.5- and 3-fold increases in ingestion of organisms by T-24 and Caco-2 cells, respectively (Fig. 1B). These observations indicate that opsonization of M. avium subsp. paratuberculosis with FN enhances the ability of the organism to adhere to and invade epithelial cells. In this and all other experiments, no more than 10% of the cells binding mycobacteria that were untreated or treated only with FN had three or more organisms attached. Similarly, no more than 12% of the ingesting cells counted in any experiment contained more than two mycobacteria.
FIG. 1.
FN opsonization enhances attachment and internalization of M. avium subsp. paratuberculosis by T-24 and Caco-2 cells. M. avium subsp. paratuberculosis was incubated with (filled bars) or without (open bars) FN prior to infection of the cells. The data shown represent the means and standard errors of the results for at least four independent experiments. The attachment and ingestion of FN-opsonized mycobacteria by each cell line were compared to those of nonopsonized organisms by Student's t test. An asterisk indicates a P value of <0.05. (A) Total number of cells binding mycobacteria; (B) total number of cells with ingested mycobacteria.
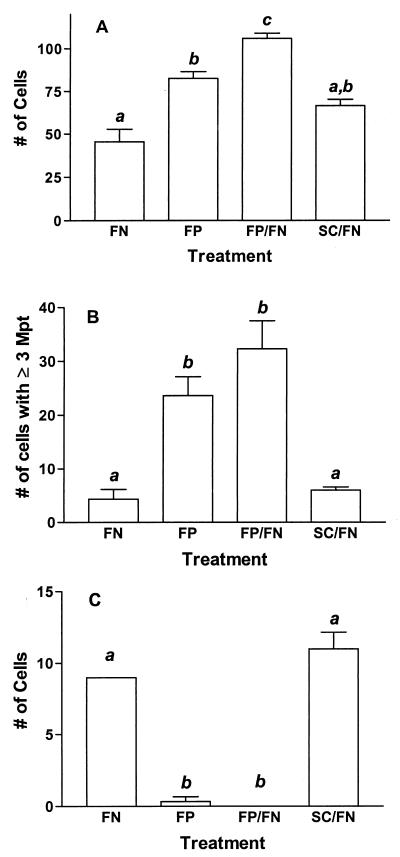
While initial experiments indicated that attachment and internalization of M. avium subsp. paratuberculosis by both cell lines were significantly enhanced in the presence of exogenous FN, it was not clear whether cellular binding and ingestion of untreated mycobacteria were mediated by residual FN from the culture medium or if these observations suggested the presence of FN-independent attachment and invasion mechanisms. To resolve this question and to determine the involvement of FAP-P in these processes, 20 μg of bovine FN in 0.5 ml of ANT buffer (pH 6) was pretreated with 200 μg of either FAP-A peptide (GNRQRWFVVWLGTSNDPVDKVAAK) (17) or a control peptide (WNQVTFAGPNWDVLKKVGRRVADS) or was left untreated for 1 h at room temperature. Acid-pretreated M. avium subsp. paratuberculosis (5 × 106 CFU in 0.5 ml of ANT buffer, pH 6) was added to each of the FN solutions and to a solution containing 200 μg of FAP-A peptide only, and the solutions were incubated at 37°C for 1 h. Bacterial suspensions were then used to infect both epithelial cell lines as described above. Treatment with FAP-A peptide significantly enhanced mycobacterial attachment to T-24 cells, and this effect was magnified in the presence of FN (Fig. 2A). No significant effect was seen for the control peptide. Furthermore, whereas fewer than 10% of T-24 cells binding FN-opsonized M. avium subsp. paratuberculosis had more than two organisms attached, FAP-A peptide treatment dramatically increased the number of cells binding three or more mycobacteria (28 to 30% of T-24 cells binding organisms) (Fig. 2B). However, the internalization of M. avium subsp. paratuberculosis was almost eliminated by FAP-A peptide treatment (Fig. 2C). The levels of attachment of organisms to Caco-2 cells were similar across all treatment groups, but FAP-A peptide blocked ingestion of mycobacteria in a manner nearly identical to that seen for T-24 cells (data not shown). These results demonstrate that the internalization of M. avium subsp. paratuberculosis by epithelial cells is primarily an FN-dependent process that is mediated by FAP-P.
FIG. 2.
FN-binding peptide from FAP-A enhances attachment of M. avium subsp. paratuberculosis to T-24 cells but inhibits internalization. M. avium subsp. paratuberculosis was treated with FN, FAP-A peptide (FP), a combination of FN and FP (FP/FN), or a combination of FN and a control peptide (SC/FN) for 1 h prior to infection of T-24 cells. The data from three independent experiments were combined and analyzed by one-way analysis of variance and Tukey's multiple-comparison test. Groups labeled with the same lowercase letter (a, b, or c) are not significantly different at P values of <0.05. (A) Total number of cells binding mycobacteria; (B) number of cells binding three or more mycobacteria; (C) total number of cells ingesting mycobacteria.
We sought to attenuate FAP-P expression by creating antisense FAP-P mutants in order to investigate the significance of the FN-FAP-P interaction on the binding and ingestion of M. avium subsp. paratuberculosis by epithelial cells in the absence of exogenous inhibitors. Portions of the FAP-P gene were amplified by PCR with either 1-314A (CATGTCGACGTAAACACGGTAGGTTCTTCGCCATGGATCAG) or 31-316A(TATGTCGACAGGTGGAAGCGACCTCGACACGCCGCAAAG) as the forward primer and 31-316B (CATACCGGTGGTGGGGCCGCGTTGGGATCATTC) as the reverse primer. A promoter cassette was amplified from the M. bovis hsp60 promoter present in the mycobacterial shuttle plasmid pMV261 (22) (a gift from Mark Hickey, Pathogenesis Corp., Seattle, Wash.) with PHSP60-U2 (TATCTAGATCGGGGACGTCTGCGGCCGACCATTT) as the forward primer and PHSP60D (ACTCACCGGTCGCGAGTGCCAACGTTATTC) as the reverse primer. Both FAP-P cassettes were digested with AgeI (Promega, Madison, Wis.) and ligated separately to the AgeI-digested promoter cassette. The product of each ligation reaction was amplified with PSHP60-U2 as the forward primer and either 1-314A or 31-316A as the reverse primer. The PCR products were digested with XbaI and SalI (Promega) and ligated separately to a similarly digested pMV261. The resulting shuttle plasmids, designated pTS026 (containing nucleotides −22 to +295 of the FAP-P coding sequence) and pTS028 (containing nucleotides +7 to +295 of the coding sequence), were separately introduced into M. avium subsp. paratuberculosis strain 5781 by electroporation in accordance with a previously described protocol (14). A vector control strain was created by using pWES4 (provided by Amy Parker, Kuzell Institute, San Francisco, Calif.), which was derived from pMV261 and expresses green fluorescent protein from the hsp60 promoter (15). Fluorescence microscopy performed with pWES4-transformed M. avium subsp. paratuberculosis revealed that the hsp60 promoter was constitutively active in this organism (data not shown).
One M. avium subsp. paratuberculosis clone transformed with pTS026 (designated strain 5781/26A) and two clones transformed with pTS028 (designated strains 5781/28F and 5781/28H) were chosen for further study following screening of kanamycin-resistant colonies for their FN binding capacities (19). The presence of antisense FAP-P vectors in each strain was confirmed by Southern hybridization, and the vectors were estimated by densitometry to be present at roughly 60 copies per organism in strain 5781/28H and approximately 40 copies per organism in the other strains (data not shown). Soluble FN binding by strains 5781/26A and 5781/28F was reduced by approximately 10% relative to that of the vector control strain (designated 5781/W4C), and that of strain 5781/28H was reduced by approximately 30% (data not shown).
Equal amounts of total protein from antisense mutant lysates were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, electroblotted to nitrocellulose, probed with anti-FAP IgG (purified by protein A chromatography from rabbit anti-FAP serum supplied by J. S. Schorey), and subjected to densitometric analysis (Scion Image; Scion Corp., Frederick, Md.). Different patterns of FAP-P expression were revealed in each of the three mycobacterial strains harboring antisense FAP-P vectors (Fig. 3). Cell-associated FAP-P from strain 5781/W4C migrated in sodium dodecyl sulfate-12% polyacrylamide gels as two bands: a minor band at 54 kDa and a major band at 49 kDa. This banding pattern is consistent with that previously reported for wild-type M. avium subsp. paratuberculosis (19), and it has been speculated that the low-Mr form of FAP is generated by C-terminal cleavage of the high-Mr form (5). Both bands were present in sonicates of strain 5781/26A and had essentially the same proportionate intensities as those of the vector control strain, but total FAP-P expression was reduced by 10 to 15%. Strain 5781/28H also expressed both forms of FAP-P in the same proportion as that seen for strain 5781/W4C. Two additional anti-FAP-reactive species migrating as 52- and 48.5-kDa bands were also observed. These probably represent truncated variants of the 54- and 49-kDa forms of FAP-P, respectively. Given this, FAP-P expression in strain 5781/28H was approximately 72% of that observed for strain 5781/W4C. The reduction in FAP-P expression by strains 5781/26A and 5781/28H appeared to correlate with the reduction in soluble FN binding observed for these strains (r2 = 0.93). The FAP-P banding pattern was reversed in strain 5781/28F, with the intensity of the 54-kDa form being twice that of the vector control and the 49-kDa form appearing as a faint band. Total FAP-P production by this strain was 31% lower than that by strain 5781/W4C.
FIG. 3.
Attenuated FAP-P expression in M. avium subsp. paratuberculosis transformed with vectors containing antisense FAP-P fragments. Total protein from sonicated M. avium subsp. paratuberculosis transformed with pWES4 (W4C), pTS026 (26A), or pTS028 (28F and 28H) was separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with anti-FAP IgG. The bar at the left of the figure corresponds to the 52.5-kDa band of the molecular mass marker. Asterisks indicate apparent truncated FAP-P forms.
Because strains 5781/28F and 5781/28H were transformed with the same plasmid and because the patterns of FAP-P expression in strains 5781/26A and 5781/28H were similar to that in strain 5781/W4C, we suspected that the banding pattern observed for strain 5781/28F was due to a genomic mutation. To confirm this, we sequenced the C-terminal half of FAP-P DNA amplified from the genomic DNA of strain 5781/28 (GenBank accession no. AF395912). A silent mutation (A for G) was noted at position 678 (relative to the A residue of the start codon). Deletion of the G residue at position 1092 was also observed in this strain, a change which would alter the last four codons of the fapP gene and eliminate the stop codon. The frameshift mutation in strain 5781/28F may have altered a protease recognition site, which may explain the atypical pattern of FAP-P expression observed for this strain. This frameshift would be expected to cause a fusion of FAP-P with elements immediately downstream. The fact that no apparent increase was observed in the size of either of the FAP-P bands extracted from strain 5781/28F may indicate that the fusion length was limited by transcription termination.
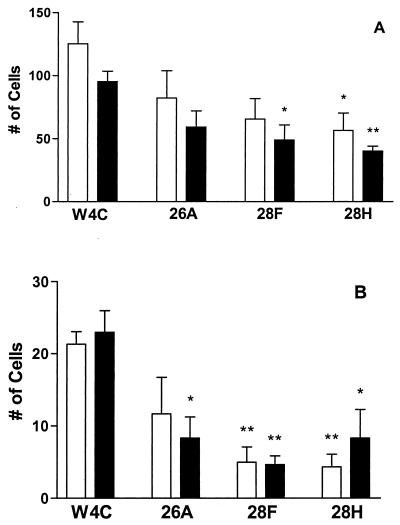
To determine the effect of attenuated FAP-P expression on the attachment and internalization of M. avium subsp. paratuberculosis by epithelial cells, infections of T-24 cells and Caco-2 cells with FN-treated FAP-P mutant mycobacteria and with FN-opsonized strain 5781/W4C were compared. In preliminary experiments, strain 5781/W4C bound to T-24 and Caco-2 cells at levels similar to those of the parent strain (20 to 25% and 25 to 30%, respectively; data not shown). However, in the experiments shown in Fig. 4, FN-opsonized strain 5781/W4C attached to 40% of the T-24 cells counted. The reason for this observation was not immediately clear. The attachment of the vector control strain to Caco-2 cells and the invasion of both cell lines by this strain (5 to 7% of cells counted) were similar to those previously noted for strain 5781. The binding of strain 5781/26A by both cell lines was approximately 35% lower than that observed for the vector control strain; however, this difference was not significant (Fig. 4A). The attachment of strain 5781/28F to T-24 and Caco-2 cells was diminished by almost 50%, and this reduction was significant for Caco-2 cells. Strain 5781/28H bound to 55% fewer T-24 cells and 57% fewer Caco-2 cells than did the vector control strain.
FIG. 4.
M. avium subsp. paratuberculosis antisense FAP-P mutants are inefficiently attached and internalized by epithelial cells. M. avium subsp. paratuberculosis strains containing pTS026 (26A), pTS028 (28F and 28H), or the vector control pWES4 (W4C) were incubated with FN and used to infect T-24 (open bars) and Caco-2 (filled bars) cells. The data from three independent experiments were combined and compared by Student's t test. A single asterisk indicates a P value of <0.05; a pair of asterisks indicates a P value of <0.01. (A) Total number of cells binding mycobacteria; (B) total number of cells ingesting mycobacteria.
Cellular entry was similarly affected in FAP-P mutants (Fig. 4B). The levels of internalization of strain 5781/26A by T-24 cells and Caco-2 cells were 55 and 36%, respectively, of that observed for the vector control strain. Invasion by strain 5781/28F was diminished by 77% in T-24 cells and 80% in Caco-2 cells, and ingestion of strain 5781/28H by these cell lines was also significantly reduced relative to that of strain 5781/W4C. These results show that efficient attachment of M. avium subsp. paratuberculosis to epithelial cells in the presence of FN requires FAP-P and confirm that internalization of these cells by M. avium subsp. paratuberculosis principally involves the FN-FAP-P interaction.
In a previous study, M. avium subsp. paratuberculosis bound FN most efficiently at pH 3 (19), suggesting that FN binding is activated by passage through the ruminant digestive tract, allowing the organism to efficiently bind FN that is secreted into the duodenum (23). The present observation that bacterial attachment to and ingestion by both T-24 and Caco-2 cell lines are markedly enhanced by FN imparts additional significance to FN binding as a potential virulence trait of M. avium subsp. paratuberculosis.
Because treatment with FAP-A peptide did not interfere with M. avium subsp. paratuberculosis attachment to Caco-2 cells and actually enhanced its attachment to T-24 cells regardless of the presence of FN, these results suggest that this organism may also attach to these cells in an FN-independent manner. If that is the case, these unidentified cellular receptors are not likely to be associated with the cytoskeleton, since internalization was abolished in FAP-A peptide-treated replicates. It is also possible that FAP-A peptide may have served to nonspecifically bridge mycobacteria to the cells.
The reduction in FN binding observed for antisense FAP-P mutant strains was disappointingly low. This was not surprising, given that the antisense fragments used were very G+C rich (75 and 77% G+C for the fapP inserts present in pTS026 and pTS028, respectively) and were therefore likely to be able to form stable secondary structures. Nevertheless, FAP-P expression was clearly attenuated in all strains carrying antisense expression cassettes, and the number of antisense plasmid copies present in these strains may have driven this effect.
The molecular basis for the origin of the additional anti-FAP-reactive bands in strain 5781/28H is unclear. Antisense RNA may have mediated the truncation of FAP-P in strain 5781/28H, possibly through latent nuclease activity. The large difference in the number of antisense plasmid copies between strains 5781/28F and 5781/28H may explain why these additional bands were not observed in the former strain. Alternatively, the presence of these additional anti-FAP-reactive bands may indicate the presence of a persistent subpopulation of organisms that contain a fapP mutation other than that observed in strain 5781/28F.
Studies with neonatal bovine calves and goat kids have shown that like many other invasive enteropathogens, M. avium subsp. paratuberculosis enters Peyer's patches through M cells, which are specialized antigen-sampling cells present in the dome epithelium (12, 20). The mechanism by which nonprofessional phagocytes such as M cells ingest these organisms is presently unknown. T-24 cells have been shown to bind and ingest mycobacteria via α5β1 integrin in a manner that requires the interaction of FN and FAP (10, 18). However, this cell line may not be representative of intestinal epithelial cells. The means by which Caco-2 cells, which also express α5β1 integrin (4), bind and ingest organisms is likely to be more relevant to Johne's disease, particularly because these cells can be induced to develop an M-cell-like phenotype in vitro (9). The fact that disruption of the interaction of FAP-P and FN inhibits bacterial ingestion by Caco-2 cells implies that α5β1 integrin serves as the receptor for FN-opsonized M. avium subsp. paratuberculosis in these cells, as well. The results of the present study indicate that FN-bound FAP-P in the cell envelope is a significant adhesin and the dominant invasin of M. avium subsp. paratuberculosis for Caco-2 cells as well as T-24 cells. M cells, unlike other intestinal epithelial cells, express β1 integrins on their apical surfaces (1). Therefore, the mechanism described for the attachment and invasion of the cell lines used herein may be relevant in vivo.
The incubation time used to assess attachment and ingestion of M. avium subsp. paratuberculosis in this study was identical to that used for M. leprae in a previous investigation (18) but considerably longer than that typically used to study these events with other invasive pathogens. Despite this extended time period, the frequencies of internalization of M. avium subsp. paratuberculosis strains 5781 and 5781/W4C by T-24 cells were approximately half those reported for M. bovis BCG and M. leprae (10, 18). This may reflect differences in the subcellular location of FAP in these species: FAP-B and FAP-L are surface exposed in M. bovis BCG and M. leprae, respectively, whereas FAP-P is not (10, 16, 18, 19). This, in turn, may affect the way in which FAP-P-bound FN is able to engage host cell receptors. Indeed, the proportionate reductions in attachment and internalization of the antisense FAP-P mutants to epithelial cells were greater than those that could be predicted from the soluble-FN binding capacity of these strains. The effect of antisense FAP-P mutations on ingestion by epithelial cells was particularly acute. The effect of the manner in which FAP-P binds FN in the mycobacterial cell envelope may have combined with that of diminished FAP-P expression in antisense mutants to interfere with receptor clustering, which is necessary to initiate integrin-mediated internalization of microorganisms (2, 6).
The relatively slow penetration of cells observed for M. avium subsp. paratuberculosis in vitro is consistent with earlier in vivo observations. Although M. avium subsp. paratuberculosis was detected in the dome epithelium covering Peyer's patches within 30 min of inoculation into caprine gut loops (20), very few acid-fast bacilli were observed in cross sections of ileal domes 5 h after injection into bovine calf gut loops (12).
M. avium subsp. paratuberculosis may express additional colonization and penetration factors that are not apparent in the present in vitro system. Reducing the influence of FAP-P through mutation may aid in the identification of factors other than FAP-P produced by this organism that may participate in attachment and internalization processes. Defining the nature of the initial host-pathogen interface will allow the design of adhesin or receptor analogues that can be added to milk replacer or feed to competitively block the attachment of M. avium subsp. paratuberculosis to the host. These approaches have been successfully employed against human mucosal pathogens in animal model systems (7, 8). The development of the FAP-P mutants described herein will facilitate the investigation of the role of FAP-P in the attachment and internalization of M. avium subsp. paratuberculosis by M cells in vivo and provide insight into the pathobiology of this organism and the pathogenesis of Johne's disease.
Acknowledgments
We thank Jeffrey Schorey for providing T-24 cells and anti-FAP antiserum, Mark Hickey for supplying pMV261, Amy Parker for contributing pWES4, and Melissa Popielarczyk for furnishing Caco-2 cells.
This work was supported by a Purdue University School of Veterinary Medicine Internal Grant.
Editor: V. J. DiRita
REFERENCES
- 1.Clark, M. A., B. H. Hirst, and M. A. Jepson. 1998. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect. Immun. 66:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dersch, P., and R. R. Isberg. 1999. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J. 18:1199-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 4.Honore, S., V. Pichard, C. Penel, V. Rigot, C. Prevot, J. Marvaldi, C. Briand, and J. B. Rognoni. 2000. Outside-in regulation of integrin clustering processes by ECM components per se and their involvement in actin cytoskeleton organization in a colon adenocarcinoma cell line. Histochem. Cell Biol. 114:323-335. [DOI] [PubMed] [Google Scholar]
- 5.Horn, C., A. Namane, P. Pescher, M. Riviere, F. Romain, G. Puzo, O. Barzu, and G. Marchal. 1999. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. J. Biol. Chem. 274:32023-32030. [DOI] [PubMed] [Google Scholar]
- 6.Jones, S. L., F. P. Lindberg, and E. J. Brown. 1999. Phagocytosis, p. 997-1020. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 7.Kelly, C. G., and J. S. Younson. 2000. Anti-adhesive strategies in the prevention of infectious disease at mucosal surfaces. Expert Opin. Investig. Drugs 9:1711-1721. [DOI] [PubMed] [Google Scholar]
- 8.Kelly, C. G., J. S. Younson, B. Y. Hikmat, S. M. Todryk, M. Czisch, P. I. Haris, I. R. Flindall, C. Newby, A. I. Mallet, J. K. Ma, and T. Lehner. 1999. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat. Biotechnol. 17:42-47. [DOI] [PubMed] [Google Scholar]
- 9.Kerneis, S., A. Bogdanova, J. P. Kraehenbuhl, and E. Pringault. 1997. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277:949-952. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda, K., E. J. Brown, W. B. Telle, D. G. Russell, and T. L. Ratliff. 1993. Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells. J. Clin. Investig. 91:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton, A. M., M. V. Chadwick, A. G. Nicholson, A. Dewar, R. K. Groger, E. J. Brown, and R. Wilson. 2000. The role of Mycobacterium avium complex fibronectin attachment protein in adherence to the human respiratory mucosa. Mol. Microbiol. 38:381-391. [DOI] [PubMed] [Google Scholar]
- 12.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 13.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179-192. [DOI] [PubMed] [Google Scholar]
- 14.Parish, T., and N. G. Stoker. 1998. Electroporation of mycobacteria. Methods Mol. Biol. 101:129-144. [DOI] [PubMed] [Google Scholar]
- 15.Parker, A. E., and L. E. Bermudez. 1997. Expression of the green fluorescent protein (GFP) in Mycobacterium avium as a tool to study the interaction between mycobacteria and host cells. Microb. Pathog. 22:193-198. [DOI] [PubMed] [Google Scholar]
- 16.Ratliff, T. L., R. McCarthy, W. B. Telle, and E. J. Brown. 1993. Purification of a mycobacterial adhesin for fibronectin. Infect. Immun. 61:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schorey, J. S., M. A. Holsti, T. L. Ratliff, P. M. Allen, and E. J. Brown. 1996. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol. Microbiol. 21:321-329. [DOI] [PubMed] [Google Scholar]
- 18.Schorey, J. S., Q. Li, D. W. McCourt, M. Bong-Mastek, J. E. Clark-Curtiss, T. L. Ratliff, and E. J. Brown. 1995. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect. Immun. 63:2652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secott, T. E., T. L. Lin, and C. C. Wu. 2001. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 69:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigurdardottir, O. G., C. M. Press, and O. Evensen. 2001. Uptake of Mycobacterium avium subsp. paratuberculosis through the distal small intestinal mucosa in goats: an ultrastructural study. Vet. Pathol. 38:184-189. [DOI] [PubMed] [Google Scholar]
- 21.Stabel, J. R. 1998. Johne's disease: a hidden threat. J. Dairy Sci. 81:283-288. [DOI] [PubMed] [Google Scholar]
- 22.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 23.Yu, J. L., R. Andersson, and A. Ljungh. 1996. Protein adsorption and bacterial adhesion to biliary stent materials. J. Surg. Res. 62: 69-73. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, W., J. S. Schorey, M. Bong-Mastek, J. Ritchey, E. J. Brown, and T. L. Ratliff. 2000. Role of a bacillus Calmette-Guerin fibronectin attachment protein in BCG-induced antitumor activity. Int. J. Cancer 86:83-88. [DOI] [PubMed] [Google Scholar]