Abstract
Eimeria spp. are a group of highly successful intracellular protozoan parasites that develop within enterocytes. Eimeria maxima from the chicken is characterized by high immunogenicity (a small priming infection gives complete immunity to subsequent homologous challenge) and naturally occurring antigenically variant populations that do not completely cross-protect. In this study we examined the expression of antigenic diversity in E. maxima, as manifested by cross-strain protection in a series of inbred chicken lines. The IAH line of Light Sussex chickens and all lines of inbred White Leghorns were susceptible to primary infections with either of two strains (H and W) of E. maxima and were protected completely against challenge with the homologous strain of parasite. The extent of cross-protection against the heterologous parasite strain varied from 0 to almost 100% depending on host genetics. Interestingly, in one inbred line of chickens (line 15I) the cross-protective phenotype was directional and intensely influenced by the infection history of the host. The basis for the observed variation in cross-protection is not known, but our results suggest that the major histocompatibility complex is not a major genetic component of the phenotype. These results are discussed in relation to the number of protective antigens presented by complex pathogens and the development of immunoprotective responses in hosts of different genetic backgrounds.
Antigenic diversity is a feature of many classes of pathogens, including viruses (1, 9, 37, 72, 81), bacteria (3, 24, 32, 53, 56, 85), protozoa (2, 25, 30, 36, 57, 60, 61), and helminths (86). There are two types of antigenic diversity. The first arises by switching between variant proteins encoded by large polymorphic gene families (12, 13, 20, 52, 57, 59, 62, 69) and facilitates pathogen persistence within a single vertebrate host in the face of immune system attack (20, 26, 62, 80). The second involves genetic diversity within the pathogen so that an infection with one subpopulation may fail to induce cross-protection against challenge with another (1, 2, 3, 9, 10, 23, 43). Immunologically relevant genetic diversity within a pathogen population allows different strains of a pathogen to infect hosts that are immune to other antigenic variants and is most likely driven by the adaptive immune response of the host population (8, 10). The appearance in the field of pathogens that are antigenically variant and immunologically non-cross-protective poses a significant complication to the design and successful application of vaccines.
Experimentally, studies of antigenic polymorphism by parasites have generally focused on the specificity of the immune response induced by infection rather than the specificity of immune protection. Studies that have examined protection have focused on a single host genotype rather than a broader investigation of any variation in protection associated with host genetics. In this paper we demonstrate that host genetics and infection history are important parts of the host-pathogen relationship and act in concert to determine the level of cross-protective immunity generated by infection.
Eimeria spp. are obligate intracellular apicomplexan protozoan parasites and are the cause of intestinal coccidiosis. Infections of the vertebrate host with these parasites result in the induction of potent protective immune responses (63), and protection against challenge infections is confined absolutely to homologous species (63), even though both T- and B-cell responses exhibit cross-species reactivity (15).
Eimeria maxima is the most immunogenic of the seven species of Eimeria that infect the domestic chicken, and ingestion of only a few oocysts induces almost complete (>99.99%) protection against challenge with homologous parasites (44, 65). However, different populations, including field isolates, exhibit substantial immunological diversity (23, 43, 47, 48, 54, 75). The antigenicity of a strain of E. maxima appears to be stable with time (74), and this stability is distinct from the dynamic process of antigenic variation that occurs with some other protozoa.
In this report we examined the contribution of host genetics and infection history to the expression of cross-strain immune protection. We used two reference laboratory strains of E. maxima that cross protect incompletely in Light Sussex (LS) chickens (48) to assess their capacity to induce cross-protection in six inbred lines of White Leghorn chickens. While all hosts were susceptible to the two strains of E. maxima and were protected completely against homologous challenge, we show for the first time that some hosts developed no immunity to challenge with a heterologous strain. Our results demonstrate the complexity of the immunological relationship, in terms of antigenic diversity, in eucaryotic pathogens and the genetics of their hosts. These issues are highly relevant to the development of vaccine strategies against coccidiosis and parasitic organisms where polymorphisms in protective antigens have been identified.
MATERIALS AND METHODS
Parasites.
The Houghton (H) reference strain of E. maxima was isolated in 1954 at the Houghton Laboratory (formerly the Houghton Poultry Research Station) of the Institute for Animal Health. The Weybridge (W) reference strain was kindly provided by Janet Catchpole of the Veterinary Laboratories Agency (Weybridge, Surrey, United Kingdom). Both strains are the progeny of a single oocyst. Methods for maintaining the parasites, preparing infective doses, and quantifying infections by detecting or counting the numbers of oocysts in feces between 5 and 10 days postinfection have been described elsewhere (45). The two strains of E. maxima were passaged at frequent intervals and used within 21 days of sporulation for each experiment. All passages of the two strains of parasites in chickens were undertaken in separate isolated rooms, each fitted with two antelobbies and fumigated before and after use with methyl bromide. Furthermore, oocysts of the two strains of E. maxima were recovered from fecal material in purpose-built rooms which were disinfected afterwards by a minimum of 7 h of exposure to gaseous ammonia. All experiments were conducted in methyl bromide-treated rooms to avoid the ingress of coccidial parasites.
Animals.
The IAH strain of outbred LS chickens and six inbred lines of White Leghorn chickens, lines 61, 72, 15I, N, C.B4, and C.B12, were used because of their different susceptibilities to infections with E. maxima (18). Lines C.B4 and C.B12 have the same genetic background but are different at the B locus (major histocompatibility complex [MHC]) (18). Lines 61 and 72 have the same B locus haplotype (B2) but differ at many other non-B genetic loci.
A hemagglutination assay using blood collected by venipuncture from the brachial vein in heparinized tubes (Vacutainer Systems Europe, Becton Dickinson) (17) was used to determine the MHC (B locus) of the progeny of a backcross between line 15I and C.B12 chickens; individuals were either homozygous for B15 MHC or heterozygous for B15 and B12 MHC, with 75% of the non-MHC genes derived from the line 15I background.
Chickens were raised free from coccidia and used at 3 to 4 weeks old. For experiments they were housed either individually (experiment 7) or in groups of three or four in metal cages with wire floors that were covered with plastic mesh inserts for birds up to 6 weeks old. Throughout they had unlimited access to unmedicated food and water.
Design of experiments.
For all experiments chickens were given a primary infection of 100 oocysts of E. maxima at 3 or 4 weeks of age and, together with a group of previously uninfected control birds housed in a separate room, were challenged 3 weeks later with 250 oocysts of the same or a heterologous strain. In all experiments the mean values given in the figures were calculated from the results of experiments with four subgroups of at least three (usually four) chickens. Percent cross-protection was calculated as 100 × [1 − (number of oocysts per bird produced by immunized birds after challenge/number of oocysts per bird produced by unimmunized birds after challenge)].
RESULTS
Two reference strains of E. maxima do not completely cross protect in LS chickens.
In experiment 1 (Fig. 1) groups of 3-week-old LS chickens were given 100 oocysts of either the H or W strain of E. maxima and then challenged 3 weeks later with 250 oocysts of the homologous or heterologous strain.
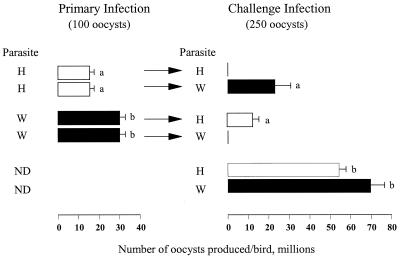
FIG. 1.
Outputs of oocysts of H and W strains of E. maxima in LS chickens arising from primary infections and challenge with either homologous or heterologous strains. The values are means calculated from the results of experiments with four subgroups of at least three chickens. Standard errors of the group means are indicated. Groups within columns annotated with different letters differ significantly (P < 0.05) from each other. ND, not done.
Primary infection with either strain induced complete protection (>99.99%) against homologous challenge (Fig. 1; Table 1), whereas substantial numbers of oocysts were produced after challenge with the heterologous parasite to give cross-protection values of 63 (H, primary, W, challenge) and 79% (W, primary, H, challenge). Significantly more oocysts were produced by the W strain in the 3-week-old birds used at the start of the experiment, but no differences were found when the birds were 6 weeks of age (Fig. 1).
TABLE 1.
Influence of host genetics and infection history on cross-protective immunity between two strains of E. maxima
| Chicken | Strain of E. maxima for:
|
% Protection against challenge infectiona | |
|---|---|---|---|
| Primary infection | Challenge infection | ||
| LS | H | W | 69 (1) |
| W | H | 79 (1) | |
| Line N | H | W | 71 (2) |
| H | W | 96 (6) | |
| W | H | 68 (3) | |
| W | H | 89 (6) | |
| Line 15I | H | W | 100 (2) |
| H | W | 100 (6) | |
| W | H | 0 (3) | |
| W | H | 0 (NS) | |
| W | H | 61 (6) | |
| Line C.B4 | H | W | 0 (4) |
| W | H | 0 (4) | |
| Line C.B12 | H | W | 0 (2) |
| H | W | 0 (4) | |
| W | H | 0 (4) | |
| W | H | 0 (2) | |
| Line 61 | H | W | 40 (2) |
| H | W | 65 (5) | |
| W | H | 0 (3) | |
| W | H | 0 (5) | |
| Line 72 | H | W | 58 (5) |
| W | H | 81 (5) | |
Numbers in parentheses are the numbers of the figures that show the original data. NS, data not shown.
Host genetics and the order of immunization affect the level of cross-protection between the H and W strains of E. maxima.
Six inbred lines of White Leghorn chickens were immunized with either the H or W strain of E. maxima and then challenged with either the homologous or heterologous strain 3 weeks later. Line 15I birds were consistently more susceptible to primary infection with either strain of E. maxima than lines N, 61, 72, C.B12, and C.B4 (Fig. 23 to 4). Complete protection against the homologous strain of parasite was found in all six inbred lines (Fig. 2 and 3).
FIG. 2.
Outputs of oocysts of H and W strains of E. maxima in four lines of White Leghorn chickens arising from primary infections and challenge with the homologous or heterologous W strain. The values are means calculated from the results of experiments with four subgroups of at least three chickens. Standard errors of the group means are indicated. Groups within columns annotated with different letters differ significantly (P < 0.05) from each other. ND, not done.
FIG. 3.
Outputs of oocysts of H and W strains of E. maxima in four lines of White Leghorn chickens arising from primary infections and challenge with the homologous or heterologous H strain. The values are means calculated from the results of experiments with four subgroups of at least three chickens. Standard errors of the group means are indicated. Groups within columns annotated with different letters differ significantly (P < 0.05) from each other. ND, not done.
FIG. 4.
Outputs of oocysts of H and W strains of E. maxima in lines C.B4 and C.B12 of White Leghorn chickens arising from primary infections and challenge with either homologous or heterologous strains. The values are means calculated from the results of experiments with four subgroups of at least three chickens. Standard errors of the group means are indicated. Groups within columns annotated with different letters differ significantly (P < 0.05) from each other. ND, not done.
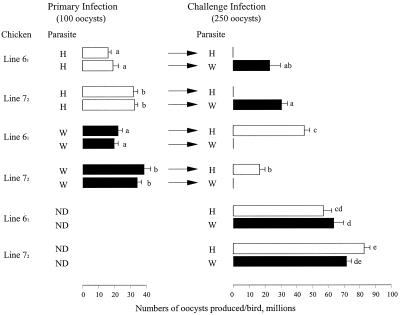
In contrast to complete protection against reinfection with the homologous strain of E. maxima, the level of protection against heterologous challenge infection varied dramatically, depending on the line of chicken (Fig. 12345 to 6 and Table 1). Line C.B4 and C.B12 chickens failed to develop any measurable degree of cross-immunity (Fig. 2 and 4; Table 1), whereas line N chickens always developed approximately 70 to 80% cross-strain immunity (Fig. 2, 3, and 6; Table 1). Line 61 and 72 chickens usually expressed no, or low levels of, cross-protective immunity (Fig. 2, 3, and 5; Table 1).
FIG. 5.
Outputs of oocysts of H and W strains of E. maxima in lines 61 and 72 of White Leghorn chickens arising from primary infections and challenge with either homologous or heterologous strains. The values are means calculated from the results of experiments with four subgroups of at least three chickens. Standard errors of the group means are indicated. Groups within columns annotated with different letters differ significantly (P < 0.05) from each other. ND, not done.
FIG. 6.
Outputs of oocysts of H and W strains of E. maxima in lines 15I and N of White Leghorn chickens arising from primary infections and challenge with either homologous or heterologous strains. The values are means calculated from the results of experiments with four subgroups of at least three chickens. Standard errors of the group means are indicated. Groups within columns annotated with different letters differ significantly (P < 0.05) from each other. ND, not done.
In most cases, the choice of immunizing and challenge strains of parasite had little, if any, effect on the level of cross-protective immunity generated by a particular chicken genotype. For example line N chickens were always 70% cross-protected and line C.B4 or C.B12 chickens were never cross-protected. However, with line 72 and line 15I the order of infection affected the level of cross-protection against challenge with the heterologous strain of parasite. This feature was most dramatic with line 15I chickens, whereby strong cross-protection was obtained after immunization with the H strain of E. maxima (95 [Fig. 6] and 98% [Fig. 2]) and substantially less protection was obtained after immunization with the W strain (0 [Fig. 3] and 60% [Fig. 6]). The experiment with line 15I chickens was repeated a third time with different immunizing doses of the W strain, and no cross-protection was observed after challenge with the H strain (data not shown).
Exposure of line 15I chickens to the W strain of E. maxima does not prevent the development of immunity to the H strain.
To test whether the complete lack of cross-protection between the W and H strains in line15I chickens (after immunization with the W strain) was due to specific immune tolerance, we challenged chickens a third time with both strains of E. maxima. Line N chickens, which exhibit reasonably strong cross-protection, were also examined in this experiment. Outputs of oocysts during the primary infections and the degrees of cross-protection against secondary challenges were consistent with results of earlier experiments, viz., line N chickens were well protected against heterologous challenge (approximately 90 to 95%), whereas in line 15I birds the outcomes of protection to secondary heterologous challenge were determined by the order in which the parasite strains were given (Fig. 6; Table 1). Thus, line 15I birds given a primary infection with the H strain were completely protected against challenge with the W strain but only 60% protected when the W strain preceded a challenge with the H strain.
In comparison to the outputs of oocysts from an age-matched control group, all line N and line 15I birds given both the H and W strains serially were immune to a tertiary infection (Fig. 6). However, line 15I chickens given two prior infections with only the W strain remained partially susceptible to challenge with the H strain.
MHC haplotype does not influence the degree of cross-protection.
Since the cross-protection phenotype is a consequence of adaptive immunity and since T cells have been demonstrated to be the major mediator of immunity to E. maxima, it was reasonable to test for an association with the MHC haplotype. The influence of the MHC (B locus) on the expression of cross-strain immunity was examined in three ways.
First, the extent of cross-protection between the two strains of E. maxima in both combinations of immunizing and challenge infections was examined in C.B4 and C.B12 sublines of line C chickens (Fig. 4, experiment 4), which differ at the B locus but which have similar genetic backgrounds. Each subline was judged to be equally susceptible to primary infection with either strain of E. maxima; both were completely immune to homologous challenge (Fig. 4), and in neither was there evidence of cross-protection between the H and W strains (Fig. 4; Table 1).
Second, a comparison of the levels of cross-protection achieved by lines of chickens that have the same B locus but that are known to differ at many non-MHC loci was made. Lines 61 and 72 (both B2) were equally susceptible to primary infection with either strain of E. maxima and were completely protected against challenge with the homologous strain (Fig. 5, experiment 5). The H strain conferred partial cross-protection against challenge with the W strain in both lines (65 and 58% for lines 61 and 72, respectively). However, in the reciprocal experiment (in which the W strain preceded challenge with the H strain) the line 72 chickens were substantially protected (81%) but line 61 chickens were not significantly protected (Fig. 5; Table 1).
Third, an examination of the level of cross-protection obtained in line (15I × C.B12) × 15I backcrossed chickens given a primary infection with the H strain and challenged with the W strain was made (Fig. 7) and then a test for an association between oocyst production and the expressed MHC haplotype was performed.
FIG. 7.
Outputs of oocysts of individual line 15I (15I × C.B12) backcross chickens arising from primary infections with the H strain and challenge with the W heterologous strain. Circles, individual cumulative oocyst counts; numbers identify individual birds. The birds were divided into groups based on the expression of the MHC (B-locus) haplotype.
The outputs of oocysts from both the B15/B15 homozygote and the B12/B15 heterozygote chickens showed normal and highly overlapping distributions, and levels of cross-protection within each group were similar. The majority of challenged birds excreted relatively few oocysts (high levels of cross-protection) although two birds representing each of the MHC genotypes (4 of 23) excreted large numbers. Since the birds were tracked individually through both infections, the experiment also demonstrated that there was no association between the numbers of oocysts produced during a primary infection and the subsequent extent of cross-protection after challenge with the heterologous strain.
Taken together, the results of the three experimental approaches suggest that the level of cross-protection was not associated with the expression of any one of four different MHC or B locus (B2, B4, B12, or B15) alleles.
DISCUSSION
Although there are many examples in the literature of diversity within antigen-encoding loci of pathogens, it appears that only a few studies have examined host-pathogen interactions in relation to host genetics, protective immunity, and antigenic diversity. Using two antigenically distinct strains of an apicomplexan parasite (E. maxima) and both outbred and inbred lines of chickens, we demonstrated the dramatic influence of host genetics on the outcomes of challenge infections with heterologous strains of pathogen. The Eimeria-chicken host combination proved to be an excellent model system for this study for reasons that include (i) the highly regulated and self-limiting parasite life cycle, (ii) the high immunogenicity of the parasite, whereby complete protection against homologous challenge is induced, (iii) poor or no protection against heterologous challenge, (iv) a convenient and accurate readout of numbers of excreted oocysts, and (v) the availability of lines of chickens with different genetic backgrounds. As far as we are aware, no natural disease model or host-pathogen relationship that offers such a combination of phenotypes that are so readily measurable experimentally has been reported.
It is clear from our data that line 15I chickens were more susceptible to primary infections with either strain of E. maxima than any other line of chicken tested, and this is in concordance with previously published data (18). All lines of chickens were completely immune to challenge with homologous parasites, which confirms, first, that both strains of E. maxima were equally capable of inducing protective immunity and, second, that none of the lines of chickens had any major defect in immune function. However, the level of protection in the face of heterologous challenge varied considerably, indicating that the capacity to cross-protect is influenced greatly by the genetics of the host. Indeed, with line C.B4, C.B12, and 72 chickens the dichotomy between protection against homologous or heterologous challenge was absolute, i.e., complete protection against the immunizing strain of E. maxima but no protection against challenge with the heterologous strain. Other inbred lines of chickens were associated with levels of cross-protection that were intermediate; e.g., line N chickens were consistently protected to a level of 70 to 90% against heterologous challenge.
Intriguingly, with line 15I chickens the degree of cross-strain immunity was directional and dependent on infection history. Greater than 95% cross-protection was obtained when the H strain of E. maxima preceded the W strain, but no cross-strain protection was observed when the immunizing and challenge strains were reversed (Fig. 2 and 3). Although immunization with the W strain offered no cross-protection to challenge with the H strain, it did not interfere with the capacity of these chickens to develop immunity to tertiary challenge. This result indicates that previous exposure of the chickens to the W strain did not prevent the host from responding appropriately to H strain protective antigens.
The incomplete cross-protection against strains of E. maxima was recently examined with respect to the migration of sporozoites released in the gut from oocysts representative of two other parasite strains that induce incomplete cross-protection to each other (6). Following homologous challenge, an accumulation of sporozoites occurred within the lamina propria of the intestine and only a few were found in the crypts (for asexual development) by 72 h after challenge. In contrast, in birds challenged with the heterologous strain at least four times as many sporozoites migrated to the crypts. Interestingly, the degree of cross protection as measured by the numbers of sporozoites transported to the crypts was not reciprocal. Similarly, there may be some slight differences in the capacities of these strains to protect against each other in terms of pathology (47), but it is unclear whether these differences are significant. These results suggest that the sporozoite may be an important target, but it is not known whether the cross-protective mechanisms also affect later stages of the life cycle. Further work to determine the target stages in different host genetic backgrounds and immune cell types that mediate different types of cross-protection is indicated.
The influence of host genetics on the resolution and/or control of primary infections is well established, and there are many examples with viral (14, 50), bacterial (16, 58), and parasitic pathogens (51, 55), including murine (33, 46) and avian Eimeria spp. (17, 18, 39). Although the loci involved have not been defined, there is strong evidence that the major genetic determinants of resistance lie outside the MHC (41). The biological nature of host resistance to Eimeria spp. is best understood for the murine parasite Eimeria vermiformis (considered a good model for E. maxima infections in the chicken); resistance is mediated by gamma interferon (IFN-γ)-producing T-cell receptor αβ+ (TCRαβ+) CD4+ T cells (66-68, 78). T cells are essential for immunity against E. maxima, and IFN-γ is strongly stimulated by infection with eimerian parasites (67, 87). B lymphocytes play a minor role in limiting primary infection with E. vermiformis and E. maxima, but whether their contribution is mediated by immunoglobulin production or is due to their role as antigen-presenting cells for CD4+ T cells is unknown (77, 78, 84).
The T-cell compartment is essential for the expression of immunity to secondary infection with Eimeria spp. (64, 70, 71, 78, 79). The contribution of host genetics in the expression of a recall (memory) response is much less well defined. For the studies described herein, the level of protection to challenge with heterologous parasites is a measure of the degree of cross-protective immunological memory.
The MHC (B locus) dependence for generating T-cell responses against Eimeria spp. in the chicken in the context of immunization with recombinant antigens has been reported (19, 39, 40). The MHC linkage of vaccination-induced responses with a single recombinant antigen may be ascribed to a lack of peptides that bind to every MHC haplotype. Nonetheless, in these studies we immunized chickens by infection with a live, antigenically complex, pathogen that presents large numbers of MHC-binding peptides to the immune system. The lack of cross-protection indicates that immunological protection may be based on relatively few of the antigens expressed by Eimeria spp. Indeed, T cells and antibodies induced by one species of Eimeria exhibit substantial cross-reactivity with antigens prepared from heterologous species (J. Bumstead, personal communication; P. Hesketh, unpublished observations) that do not cross-protect during natural infections. These observations and the dramatic differences in cross-strain protection reported here reinforce the view that only a few of the estimated 5,000 to 10,000 genes in the Eimeria genome (73) encode antigens that are protective.
Antigenic diversity also characterizes infections with the bovine parasite Theileria parva, with a close association between immunodominance, the strain specificity of a cytotoxic T lymphocyte response, and protection (82, 83). Similarly, a series of CD8 T-cell clones from an individual infected with the malarial parasite differentially recognized naturally occurring immunological variants of Plasmodium falciparum (11). The directional nature of cross-protection seen in our studies with the two strains of E. maxima in line 15I chickens has also been reported for T. parva infections in cattle (49, 82) and Salmonella enterica infections in mice (29).
The MHC of chickens is much less complex than that of mammals such that each haplotype expresses single dominant class I and class II molecules (31, 35). The restricted repertoire of MHC class I-binding peptides in the avian immune response dramatically influences the capacity of chickens to develop protective immunity to a small RNA virus (Rous sarcoma virus) (34). Interestingly, for an antigenically complex DNA virus (Marek's disease virus), the efficacies of different vaccines have been linked to the expression of certain MHC haplotypes (4, 5).
Since all TCRαβ T-cell responses require presentation of a peptide in the context of MHC, antigenic diversity between the strains of E. maxima might be expressed by a change in the capacity of some antigens to bind to a particular MHC. For this reason, we assessed the cross-protective phenotype in various lines of chickens that either share most non-MHC genes but differ at the MHC locus (C.B4 and C.B12 sublines of line C) or have identical MHCs but differ at many non-MHC-linked loci (lines 61 and 72; both B2). The lack of cross-protection between the H and W strains of E. maxima in C.B4 and C.B12 chickens, differences seen with line 61 and 72 chickens, and the results of a limited study with a backcross population (line 15I × [15I × C.B12]) support the hypothesis that the MHC is not a major genetic component of the cross-protective phenotype (Fig. 4, 5, and 7). Some of the most susceptible birds in the backcross study were B15 homozygous (Fig. 7); if the MHC haplotype were a major genetic component of the phenotype, these birds would have been highly protected against heterologous challenge. While it is difficult to propose candidate non-MHC genes that might influence the development of cross-protective immunity, the response is dependent on TCRαβ T cells. Therefore, differences in the TCRαβ T-cell repertoire could be responsible for the differences in the abilities of different inbred lines of chickens to mediate cross-protection. The repertoire could be restricted by differences in the genomic complement of TCR loci, as has been documented in mice (7, 27, 28). Alternatively, the differences could reflect negative selection of T cells in the thymus by, for example, expressed endogenous retrovirus elements (22, 76) that are known to differ among chicken lines (38). Consequently, detailed mapping of the host genetic loci that influence cross-protection is strongly indicated.
In the field, host populations are generally outbred genetically and the induced immune pressures on apicomplexan parasites are likely to be diverse. However, in areas of high parasite endemicity the immune selection pressures on parasites will be more intense and substantial polymorphisms have been found in genes that encode, for example, the circumsporozoite antigen (42) and the merozoite surface protein in P. falciparum (21).
The generation of strong cross-species, nonprotective responses indicates a need to examine the relationship between immunodominance and immunoprotection in relation to the avian responses to E. maxima. Moreover, the influences of host genetics and infection history on the outcome of challenge infection between immunological variants of E. maxima indicate the complexity of the protective immune response. Although further work to determine the functional and genetic basis of phenotypes reported here is necessary, the E. maxima-avian host system has enabled the complexity of the host-pathogen relationship in relation to immune protection and antigenic diversity to be identified. Understanding these aspects of the immunological relationship would provide a theoretical and practical basis for rational development of anticoccidial vaccine strategies. Antigenic diversity is a feature of many pathogens, and similar analysis would support the rational development of vaccines against other antigenically complex pathogens in a wide range of host species.
Editor: J. M. Mansfield
REFERENCES
- 1.Akashi, H., and Y. Inaba. 1997. Antigenic diversity of Akabane virus detected by monoclonal antibodies. Virus Res. 47:187-196. [DOI] [PubMed] [Google Scholar]
- 2.Arnot, D. E., M. J. Stewart, and J. W. Barnwell. 1990. Antigenic diversity in Thai Plasmodium vivax circumsporozoite proteins. Mol. Biochem. Parasitol. 43:147-149. [DOI] [PubMed] [Google Scholar]
- 3.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 4.Bacon, L. D., and R. L. Witter. 1995. Efficacy of Marek's disease vaccines in MHC heterozygous chickens: MHC congenic × inbred line F1 matings. J. Hered. 86:269-273. [DOI] [PubMed] [Google Scholar]
- 5.Bacon, L. D., and R. L. Witter. 1993. Influence of B-haplotype on the relative efficacy of Marek's disease vaccines of different serotypes. Avian Dis. 37:53-59. [PubMed] [Google Scholar]
- 6.Beattie, S. E., M. A. Fernando, and J. R. Barta. 2001. A comparison of sporozoite transport after homologous and heterologous challenge in chickens immunized with the Guelph strain or the Florida strain of Eimeria maxima. Parasitol. Res. 87:116-121. [DOI] [PubMed] [Google Scholar]
- 7.Behlke, M. A., H. S. Chou, K. Huppi, and D. Y. Loh. 1986. Murine T-cell receptor mutants with deletions of beta-chain variable region genes. Proc. Natl. Acad. Sci. USA 83:767-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner, B., S. Bonhoeffer, and M. A. Nowak. 1997. Virus load and antigenic diversity. Bull. Math. Biol. 59:881-896. [DOI] [PubMed] [Google Scholar]
- 9.Blitz, L., F. H. Pujol, P. D. Swenson, L. Porto, R. Atencio, M. Araujo, L. Costa, D. C. Monsalve, J. R. Torres, H. A. Fields, S. Lambert, C. Van Geyt, H. Norder, L. O. Magnius, J. M. Echevarria, and L. Stuyver. 1998. Antigenic diversity of hepatitis B virus strains of genotype F in Amerindians and other population groups from Venezuela. J. Clin. Microbiol. 36:648-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolad, A., and K. Berzins. 2000. Antigenic diversity of Plasmodium falciparum and antibody-mediated parasite neutralization. Scand. J. Immunol. 52:233-239. [DOI] [PubMed] [Google Scholar]
- 11.Bonelo, A., D. Valmori, F. Triponez, J. M. Tiercy, G. Mentha, J. Oberholzer, P. Champagne, J. F. Romero, F. Esposito, I. Nebie, C. Barbey, P. Romero, S. Herrera, G. Corradin, and J. A. Lopez. 2000. Generation and characterization of malaria-specific human CD8(+) lymphocyte clones: effect of natural polymorphism on T cell recognition and endogenous cognate antigen presentation by liver cells. Eur. J. Immunol. 30:3079-3088. [DOI] [PubMed] [Google Scholar]
- 12.Borst, P., G. Rudenko, P. A. Blundell, F. van Leeuwen, M. A. Cross, R. McCulloch, H. Gerrits, and I. M. Chaves. 1997. Mechanisms of antigenic variation in African trypanosomes. Behring Inst. Mitt. 99:1-15. [PubMed] [Google Scholar]
- 13.Borst, P., G. Rudenko, M. C. Taylor, P. A. Blundell, F. Van Leeuwen, W. Bitter, M. Cross, and R. McCulloch. 1996. Antigenic variation in trypanosomes. Arch. Med. Res. 27:379-388. [PubMed] [Google Scholar]
- 14.Brownstein, D. G. 1983. Genetics of natural resistance to Sendai virus infection in mice. Infect. Immun. 41:308-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bumstead, J. M., P. P. Dunn, and F. M. Tomley. 1995. Nitrocellulose immunoblotting for identification and molecular gene cloning of Eimeria maxima antigens that stimulate lymphocyte proliferation. Clin. Diagn. Lab. Immunol. 2:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bumstead, N., and P. A. Barrow. 1988. Genetics of resistance to Salmonella typhimurium in newly hatched chicks. Br. Poult. Sci. 29:521-529. [DOI] [PubMed] [Google Scholar]
- 17.Bumstead, N., and B. Millard. 1987. Genetics of resistance to coccidiosis: response of inbred chicken lines to infection by Eimeria tenella and Eimeria maxima. Br. Poult. Sci. 28:705-715. [DOI] [PubMed] [Google Scholar]
- 18.Bumstead, N., and B. J. Millard. 1992. Variation in susceptibility of inbred lines of chickens to seven species of Eimeria. Parasitology 104:407-413. [DOI] [PubMed] [Google Scholar]
- 19.Clare, R. A., R. L. Taylor, Jr., W. E. Briles, and R. G. Strout. 1989. Characterization of resistance and immunity to Eimeria tenella among major histocompatibility complex B-F/B-G recombinant hosts. Poult. Sci. 68:639-645. [DOI] [PubMed] [Google Scholar]
- 20.Deitsch, K. W., A. del Pinal, and T. E. Wellems. 1999. Intra-cluster recombination and var transcription switches in the antigenic variation of Plasmodium falciparum. Mol. Biochem. Parasitol. 101:107-116. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira, M. U., O. Kaneko, M. Kimura, Q. Liu, F. Kawamoto, and K. Tanabe. 1998. Allelic diversity at the merozoite surface protein-1 (MSP-1) locus in natural Plasmodium falciparum populations: a brief overview. Mem. Inst. Oswaldo Cruz 93:631-638. [DOI] [PubMed] [Google Scholar]
- 22.Ferrick, D. A., K. Cho, L. Gemmell-Hori, and D. W. Morris. 1992. Genetic analysis of the effects of Mtv-2 on the T cell repertoire in the WXG-2 mouse strain. Int. Immunol. 4:805-810. [DOI] [PubMed] [Google Scholar]
- 23.Fitz-Coy, S. H. 1992. Antigenic variation among strains of Eimeria maxima and E. tenella of the chicken. Avian Dis. 36:40-43. [PubMed] [Google Scholar]
- 24.Gilsdorf, J. R. 1998. Antigenic diversity and gene polymorphisms in Haemophilus influenzae. Infect. Immun. 66:5053-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta, S., K. Trenholme, R. M. Anderson, and K. P. Day. 1994. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science 263:961-963. [DOI] [PubMed] [Google Scholar]
- 26.Handunnetti, S. M., K. N. Mendis, and P. H. David. 1987. Antigenic variation of cloned Plasmodium fragile in its natural host Macaca sinica. Sequential appearance of successive variant antigenic types. J. Exp. Med. 165:1269-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haqqi, T. M., S. Banerjee, G. D. Anderson, and C. S. David. 1989. An inbred mouse strain with a massive deletion of T cell receptor VB genes. J. Exp. Med. 169:1903-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haqqi, T. M., S. Banerjee, W. L. Jones, G. Anderson, M. A. Behlke, D. Y. Loh, H. S. Luthra, and C. S. David. 1989. Identification of T-cell receptor V beta deletion mutant mouse strain AU/ssJ (H-2q) which is resistant to collagen-induced arthritis. Immunogenetics 29:180-185. [DOI] [PubMed] [Google Scholar]
- 29.Hormaeche, C. E., H. S. Joysey, L. Desilva, M. Izhar, and B. A. Stocker. 1991. Immunity conferred by Aro− Salmonella live vaccines. Microb. Pathog. 10:149-158. [DOI] [PubMed] [Google Scholar]
- 30.Howard, R. J., L. J. Panton, K. Marsh, I. T. Ling, E. J. Winchell, and R. J. Wilson. 1986. Antigenic diversity and size diversity of Plasmodium falciparum antigens in isolates from Gambian patients. Parasite Immunol. 8:39-55. [DOI] [PubMed] [Google Scholar]
- 31.Jacob, J. P., S. Milne, S. Beck, and J. Kaufman. 2000. The major and a minor class II beta-chain (B-LB) gene flank the Tapasin gene in the B-F/B-L region of the chicken major histocompatibility complex. Immunogenetics 51:138-147. [DOI] [PubMed] [Google Scholar]
- 32.Jongejan, F., M. J. Thielemans, C. Briere, and G. Uilenberg. 1991. Antigenic diversity of Cowdria ruminantium isolates determined by cross-immunity. Res. Vet. Sci. 51:24-28. [DOI] [PubMed] [Google Scholar]
- 33.Joysey, H. S., D. Wakelin, and M. E. Rose. 1988. Resistance to infection with Eimeria vermiformis in mouse radiation chimeras is determined by donor bone-marrow cells. Infect. Immun. 56:1399-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman, J. 2000. The simple chicken major histocompatibility complex: life and death in the face of pathogens and vaccines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman, J., S. Milne, T. W. Gobel, B. A. Walker, J. P. Jacob, C. Auffray, R. Zoorob, and S. Beck. 1999. The chicken B locus is a minimal essential major histocompatibility complex. Nature 401:923-925. [DOI] [PubMed] [Google Scholar]
- 36.Khusmith, S., P. Tapchaisri, S. Tharavanij, and D. Bunnag. 1998. Antigenic diversity of Plasmodium vivax and their geographic distribution in Thailand. Southeast Asian J. Trop. Med. Public Health 29:512-518. [PubMed] [Google Scholar]
- 37.Komada, H., S. Kusagawa, C. Orvell, M. Tsurudome, M. Nishio, H. Bando, M. Kawano, H. Matsumura, E. Norrby, and Y. Ito. Antigenic diversity of human parainfluenza virus type 1 isolates and their immunological relationship with Sendai virus revealed by using monoclonal antibodies. J. Gen. Virol. 73:875-884. [DOI] [PubMed]
- 38.Leib-Mosch, C., M. Bachmann, R. Brack-Werner, T. Werner, V. Erfle, and R. Hehlmann. 1992. Expression and biological significance of human endogenous retroviral sequences. Leukemia 6:72S-75S. [PubMed] [Google Scholar]
- 39.Lillehoj, H. S. 1988. Influence of inoculation dose, inoculation schedule, chicken age, and host genetics on disease susceptibility and development of resistance to Eimeria tenella infection. Avian Dis. 32:437-444. [PubMed] [Google Scholar]
- 40.Lillehoj, H. S., M. C. Jenkins, and L. D. Bacon. 1990. Effects of major histocompatibility genes and antigen delivery on induction of protective mucosal immunity to E. acervulina following immunization with a recombinant merozoite antigen. Immunology 71:127-132. [PMC free article] [PubMed] [Google Scholar]
- 41.Lillehoj, H. S., M. D. Ruff, L. D. Bacon, S. J. Lamont, and T. K. Jeffers. 1989. Genetic control of immunity to Eimeria tenella. Interaction of MHC genes and non-MHC linked genes influences levels of disease susceptibility in chickens. Vet. Immunol. Immunopathol. 20:135-148. [DOI] [PubMed] [Google Scholar]
- 42.Lockyer, M. J., and R. T. Schwarz. 1987. Strain variation in the circumsporozoite protein gene of Plasmodium falciparum. Mol. Biochem. Parasitol. 22:101-108. [DOI] [PubMed] [Google Scholar]
- 43.Long, P. L. 1974. Experimental infection of chickens with two species of Eimeria isolated from the Malaysian jungle fowl. Parasitology 69:337-347. [DOI] [PubMed] [Google Scholar]
- 44.Long, P. L., and B. J. Millard. 1979. Immunological differences in Eimeria maxima: effect of a mixed immunizing inoculum on heterologous challenge. Parasitology 79:451-457. [DOI] [PubMed] [Google Scholar]
- 45.Long, P. L., B. J. Millard, L. P. Joyner, and C. C. Norton. 1976. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 6:201-217. [PubMed] [Google Scholar]
- 46.Mahrt, J. L., and Y. F. Shi. 1988. Murine major histocompatibility complex and immune response to Eimeria falciformis. Infect. Immun. 56:270-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin, A. G., H. D. Danforth, J. R. Barta, and M. A. Fernando. 1997. Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int. J. Parasitol. 27:527-533. [DOI] [PubMed] [Google Scholar]
- 48.McDonald, V., M. W. Shirley, and M. A. Bellatti. 1986. Eimeria maxima: characteristics of attenuated lines obtained by selection for precocious development in the chicken. Exp. Parasitol. 61:192-200. [DOI] [PubMed] [Google Scholar]
- 49.McKeever, D. J., E. L. Taracha, W. I. Morrison, A. J. Musoke, and S. P. Morzaria. 1999. Protective immune mechanisms against Theileria parva: evolution of vaccine development strategies. Parasitol. Today 15:263-267. [DOI] [PubMed] [Google Scholar]
- 50.Meruelo, D., and R. Bach. 1983. Genetics of resistance to virus-induced leukemias. Adv. Cancer Res. 40:107-188. [DOI] [PubMed] [Google Scholar]
- 51.Mulvey, M., and D. S. Woodruff. 1985. Genetics of Biomphalaria glabrata: linkage analysis of genes for pigmentation, enzymes, and resistance to Schistosoma mansoni. Biochem. Genet. 23:877-889. [DOI] [PubMed] [Google Scholar]
- 52.Newbold, C. I. 1999. Antigenic variation in Plasmodium falciparum: mechanisms and consequences. Curr. Opin. Microbiol. 2:420-425. [DOI] [PubMed] [Google Scholar]
- 53.Neyrolles, O., I. Chambaud, S. Ferris, M. C. Prevost, T. Sasaki, L. Montagnier, and A. Blanchard. 1999. Phase variations of the Mycoplasma penetrans main surface lipoprotein increase antigenic diversity. Infect. Immun. 67:1569-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norton, C. C., and H. E. Hein. 1976. Eimeria maxima: a comparison of two laboratory strains with a fresh isolate. Parasitology 72:345-354. [DOI] [PubMed] [Google Scholar]
- 55.Ogunremi, O., and H. Tabel. 1995. Genetics of resistance to Trypanosoma congolense in inbred mice: efficiency of apparent clearance of parasites correlates with long-term survival. J. Parasitol. 81:876-881. [PubMed] [Google Scholar]
- 56.Park, S. M., S. I. Hong, H. Y. Jung, S. K. Yang, H. R. Kim, Y. I. Min, and W. S. Hong. 1998. Antigenic diversity and serotypes of Helicobacter pylori associated with peptic ulcer diseases. Korean J. Intern. Med. 13:104-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips, R. S., L. R. Brannan, P. Balmer, and P. Neuville. 1997. Antigenic variation during malaria infection--the contribution from the murine parasite Plasmodium chabaudi. Parasite Immunol. 19:427-434. [DOI] [PubMed] [Google Scholar]
- 58.Plant, J., and A. A. Glynn. 1976. Genetics of resistance to infection with Salmonella typhimurium in mice. J. Infect. Dis. 133:72-78. [DOI] [PubMed] [Google Scholar]
- 59.Reeder, J. C., and G. V. Brown. 1996. Antigenic variation and immune evasion in Plasmodium falciparum malaria. Immunol. Cell Biol. 74:546-554. [DOI] [PubMed] [Google Scholar]
- 60.Rich, S. M., M. U. Ferreira, and F. J. Ayala. 2000. The origin of antigenic diversity in Plasmodium falciparum. Parasitol. Today 16:390-396. [DOI] [PubMed] [Google Scholar]
- 61.Rich, S. M., R. R. Hudson, and F. J. Ayala. 1997. Plasmodium falciparum antigenic diversity: evidence of clonal population structure. Proc. Natl. Acad. Sci. USA 94:13040-13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson, N. P., N. Burman, S. E. Melville, and J. D. Barry. 1999. Predominance of duplicative VSG gene conversion in antigenic variation in African trypanosomes. Mol. Cell. Biol. 19:5839-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose, M. E. 1973. Immunity, p. 295-341. In D. M. Hammond and P. L. Long (ed.), The coccidia. University Park Press and Butterworth and Co. Publishers Ltd., Baltimore, Md..
- 64.Rose, M. E., H. S. Joysey, P. Hesketh, R. K. Grencis, and D. Wakelin. 1988. Mediation of immunity to Eimeria vermiformis in mice by L3T4+ T cells. Infect. Immun. 56:1760-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rose, M. E., and P. L. Long. 1962. Immunity to four species of Eimeria in fowls. Immunology 5:79-92. [PMC free article] [PubMed] [Google Scholar]
- 66.Rose, M. E., A. L. Smith, and D. Wakelin. 1991. Gamma interferon-mediated inhibition of Eimeria vermiformis growth in cultured fibroblasts and epithelial cells. Infect. Immun. 59:580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rose, M. E., D. Wakelin, and P. Hesketh. 1989. Gamma interferon controls Eimeria vermiformis primary infection in BALB/c mice. Infect. Immun. 57:1599-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rose, M. E., D. Wakelin, and P. Hesketh. 1991. Interferon-gamma-mediated effects upon immunity to coccidial infections in the mouse. Parasite Immunol. 13:63-74. [DOI] [PubMed] [Google Scholar]
- 69.Rudenko, G. 1999. Mechanisms mediating antigenic variation in Trypanosoma brucei. Mem. Inst. Oswaldo Cruz 94:235-237. [DOI] [PubMed] [Google Scholar]
- 70.Schito, M. L., B. Chobotar, and J. R. Barta. 1998. Cellular dynamics and cytokine responses in BALB/c mice infected with Eimeria papillata during primary and secondary infections. J. Parasitol. 84:328-337. [PubMed] [Google Scholar]
- 71.Schito, M. L., B. Chobotar, and J. R. Barta. 1998. Major histocompatibility complex class I- and II-deficient knock-out mice are resistant to primary but susceptible to secondary Eimeria papillata infections. Parasitol. Res. 84:394-398. [DOI] [PubMed] [Google Scholar]
- 72.Shimizu, M. 1990. Current situation of bovine virus diarrhoea-mucosal disease (BVD-MD) virus infections and their antigenic diversity in Hokkaido, Japan. Rev. Sci. Technol. 9:181-194. [DOI] [PubMed] [Google Scholar]
- 73.Shirley, M. W. 2000. The genome of Eimeria spp., with special reference to Eimeria tenella--a coccidium from the chicken. Int. J. Parasitol. 30:485-493. [DOI] [PubMed] [Google Scholar]
- 74.Shirley, M. W. 1980. Maintenance of Eimeria maxima by serial passage of single sporocysts. J. Parasitol. 66:172-173. [PubMed] [Google Scholar]
- 75.Shirley, M. W., and M. A. Bellatti. 1988. Live attenuated coccidiosis vaccine: selection of a second precocious line of Eimeria maxima. Res. Vet. Sci. 44:25-28. [PubMed] [Google Scholar]
- 76.Simpson, E. 1993. T cell repertoire selection by mouse mammary tumour viruses. Eur. J. Immunogenet. 20:137-149. [DOI] [PubMed] [Google Scholar]
- 77.Smith, A. L., and A. C. Hayday. 1998. Genetic analysis of the essential components of the immunoprotective response to infection with Eimeria vermiformis. Int. J. Parasitol. 28:1061-1069. [DOI] [PubMed] [Google Scholar]
- 78.Smith, A. L., and A. C. Hayday. 2000. Genetic dissection of primary and secondary responses to a widespread natural pathogen of the gut, Eimeria vermiformis. Infect. Immun. 68:6273-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stiff, M. I., and J. P. Vasilakos. 1990. Effect of in vivo T-cell depletion on the effector T-cell function of immunity to Eimeria falciformis. Infect. Immun. 58:1496-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 81.Sullender, W. M. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taracha, E. L., B. M. Goddeeris, S. P. Morzaria, and W. I. Morrison. 1995. Parasite strain specificity of precursor cytotoxic T cells in individual animals correlates with cross-protection in cattle challenged with Theileria parva. Infect. Immun. 63:1258-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taracha, E. L., B. M. Goddeeris, A. J. Teale, S. J. Kemp, and W. I. Morrison. 1995. Parasite strain specificity of bovine cytotoxic T cell responses to Theileria parva is determined primarily by immunodominance. J. Immunol. 155:4854-4860. [PubMed] [Google Scholar]
- 84.Wakelin, D., M. E. Rose, P. Hesketh, K. J. Else, and R. K. Grencis. 1993. Immunity to coccidiosis: genetic influences on lymphocyte and cytokine responses to infection with Eimeria vermiformis in inbred mice. Parasite Immunol. 15:11-19. [DOI] [PubMed] [Google Scholar]
- 85.Walker, D. H., Q. H. Liu, X. J. Yu, H. Li, C. Taylor, and H. M. Feng. 1992. Antigenic diversity of Rickettsia conorii. Am. J. Trop. Med. Hyg. 47:78-86. [DOI] [PubMed] [Google Scholar]
- 86.Yakoleff-Greenhouse, V., A. Flisser, A. Sierra, and C. Larralde. 1982. Analysis of antigenic variation in cysticerci of Taenia solium. J. Parasitol. 68:39-47. [PubMed] [Google Scholar]
- 87.Yun, C. H., H. S. Lillehoj, and K. D. Choi. 2000. Eimeria tenella infection induces local gamma interferon production and intestinal lymphocyte subpopulation changes. Infect. Immun. 68:1282-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]