Abstract
Klebsiella pneumoniae is a common cause of gram-negative bacterial nosocomial pneumonia. Two surface polysaccharides, lipopolysaccharide (LPS) O side chain and capsular polysaccharide (CPS), are critical for the microorganism in causing sepsis, but little is known about their role in pneumonia. To investigate their contribution in the pathogenesis of K. pneumoniae pneumonia, we characterized the host response to bacterial challenge with a highly virulent clinical isolate or with isogenic insertion-duplication mutants deficient in CPS or LPS O side chain in a murine model of pneumonia. Animals challenged intratracheally with the wild-type or LPS O side chain-deficient strain developed pneumonia and became bacteremic before death. Extensive lung lesions as well as pleuritis, vasculitis, and edema were observed by histopathological examination, and polymorphonuclear infiltration was also demonstrated. In contrast, none of the animals challenged with the unencapsulated strain developed pneumonia or bacteremia. Examination of tissue from this group did not identify lung lesions, and none of the infected animals died. Analysis of the early host defense mechanisms that contributed to the clearance of the unencapsulated mutant showed that the levels of C3 deposited on the unencapsulated mutant surface were threefold higher than those for the wild-type and LPS O side chain-deficient strains. Furthermore, phagocytosis of the unencapsulated mutant by human alveolar macrophages (AM) was more efficient than that of the wild-type and LPS O side chain-deficient strains. We conclude that CPS, but not LPS O side chain, is essential for Klebsiella pneumonia because it modulates the deposition of C3 and protects the microorganisms against human AM phagocytosis.
Klebsiella pneumoniae is an opportunistic pathogen that frequently causes nosocomial infections, mainly in immunocompromised patients. K. pneumoniae infections range from mild urinary tract infections to severe bacteremia and pneumonia with a high rate of mortality and morbidity (4, 8, 13). Pulmonary infections due to K. pneumoniae are often characterized by a rapid progressive clinical course complicated by lung abscesses and multilobular involvement, which leaves scant time to establish an effective antibiotic treatment. In addition, the increasing emergence of multidrug resistance among K. pneumoniae nosocomial isolates (17) has renewed interest in the investigation of alternative approaches to the treatment or prophylaxis of K. pneumoniae respiratory infections.
Two major factors are essential for the virulence of this pathogen, lipopolysaccharide (LPS) and capsular polysaccharide (CPS) (26). LPS consists of lipid A, core, and O-polysaccharide antigen that are essential for the microorganism to resist complement-mediated killing (1, 16). CPS is the outermost layer of this pathogen and is involved mainly in resistance to phagocytosis by polymorphonuclear cells by acting as a physical barrier (26). Thus, both components are critical for the microorganism to be able to spread through the blood and to cause sepsis. However, little is known about the role of the two components in K. pneumoniae pneumonia.
Experimental evidence suggests that CPS may be important for the establishment of K. pneumoniae pneumonia, since active immunization with purified CPS protected rats against lethal experimentally induced Klebsiella pneumonia (6). Furthermore, in a more recent study, monoclonal antibodies against Klebsiella CPS reduced the severity and hematogenic spread of K. pneumoniae pneumonia (10). In addition to CPS, LPS may play a critical role in the development of necrotic lesions, but its role has been insufficiently investigated (22).
To investigate the role of CPS and LPS O side chain in the pathogenesis of K. pneumoniae pneumonia, we used insertion-duplication mutagenesis to derive acapsular and LPS O side chain-deficient mutants from a highly virulent strain and tested their ability to cause pneumonia in a mouse model and to resist the early host defenses of the lower respiratory tract.
MATERIALS AND METHODS
Bacteria, plasmids, and media.
K. pneumoniae strain 52145 is a clinical isolate (serotype O1:K2) previously described (18). A spontaneous rifampin-resistant mutant derived from strain 52145, designated 52145R, was used in this study. Escherichia coli strains used in the cloning experiments were DH5α and strain S17-1 λpir, which encodes protein π from the pir gene essential for replication of plasmid pFS100 (11). Plasmid pFS100 was used to create insertion-duplication mutations by homologous recombination (21). Bacterial cells were grown in Luria-Bertani broth at 37°C with shaking or solidified with 1.5% agar. When necessary, media were supplemented with ampicillin, kanamycin, and rifampin at concentrations of 50, 50, and 40 μg/ml, respectively.
DNA manipulation.
Plasmid DNA was isolated using the Wizard Miniprep kit (Promega) according to the manufacturer's instructions. Isolation of genomic DNA, transformation, and electroporation were carried out by standard techniques (3). T4 DNA ligase and restriction endonucleases were used following the recommendations of the manufacturer (Pharmacia). DNA fragments prepared by restriction enzyme digestion were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. For Southern blot analysis and probe labeling and detection we used the ECL kit (Amersham) according to the manufacturer's protocol. DNA sequencing was performed using an automated sequencing apparatus (Applied Biosystems).
PCRs were performed according to the standard technique (3). Primers RFBF (5′-CATTGAACTCTAGAAGCAAGC-3′) and RFBR (5′-CGGTACCCCATGGTTTGTTC-3′) include XbaI and KpnI sites, respectively. They are complementary to sequences located 34 bp upstream and 1,200 bp downstream of the wbbM start codon required for LPS O side chain synthesis (14). Primers ORF6F (5′-CCAATCTAGAATGATTCTAGG-3′) and ORF6R (5′-GCATTAGGACTGGTACCGGAA-3′) include XbaI and KpnI sites, respectively. They are complementary to sequences located 276 and 1,618 bp upstream of the orf6 start codon from the K. pneumoniae cps operon required for CPS synthesis (2). Oligonucleotide primers, Taq DNA polymerase, and deoxynucleoside triphosphates were purchased from Pharmacia.
Generation of capsule and LPS O side chain mutants from K. pneumoniae strain 52145R.
To derive a CPS mutant from K. pneumoniae strain 52145R, a 1,341-bp internal fragment of orf6, a gene from the cps operon required for capsule synthesis (2), was amplified by PCR from K. pneumoniae genomic DNA with primers ORF6F and ORF6R. The PCR product was cloned in the π protein-dependent shuttle vector pFS100 digested with XbaI and KpnI to give plasmid pFKI, which was introduced into K. pneumoniae 52145R by conjugation. To select integrants of plasmid pFKI into the K. pneumoniae chromosome, thereby disrupting expression of orf6, an aliquot of the conjugant was spread on kanamycin-containing agar. One integrant designated K. pneumoniae 52K10 was further investigated.
To generate an LPS O side chain mutant, a 1,936-bp fragment including 1,900 bp of the 5′ region of wbbM, a gene required for LPS O side chain synthesis (14), was amplified by PCR from K. pneumoniae 52145R genomic DNA with primers RFBF and RFBR. The PCR product was digested with HincII and KpnI and cloned in the π protein-dependent shuttle vector pFS100 digested with HincII and KpnI to give plasmid pFOI, which was introduced into K. pneumoniae 52145R by conjugation. To select integrants of plasmid pFOI into the K. pneumoniae chromosome, thereby disrupting expression of wbbM, an aliquot of the conjugant was spread on kanamycin-containing agar. One integrant designated K. pneumoniae 52O21 was further investigated.
Determination of CPS and LPS O side chain expression.
CPS and LPS expression was quantified by an inhibition enzyme-linked immunosorbent assay (iELISA). For this purpose, plates were coated with 1 μg of either purified CPS type 2 or LPS O side chain type 1 per well. After a blocking step with phosphate-buffered saline (PBS; pH 7.4) containing 1% bovine serum albumin (BSA), plates were incubated with serial dilutions of CPS extracts or LPS extracts and antisera against CPS type 2 or LPS O side chain type 1, respectively. Bound antibodies were detected with alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G and developed with p-nitrophenyl phosphate. Incubations with antisera diluted in PBS-1% BSA were carried out at 37°C for 1 h and were always followed by PBS washes. Known amounts of CPS and LPS purified by the methods of Wilkinson and Sutherland (25) and Westphal and Jann (24) were used to construct a standard curve.
LPS O side chain expression was also analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). LPS was obtained by the method of Hitchcock and Brown (12), run in a 15% polyacrylamide resolving gel with 25 mM Tris-0.1% SDS-0.19 M glycine (pH 8.3) as the running buffer, and silver stained as described by Tsai and Frasch (23).
Murine model of pneumonia.
Male (16 to 20 g) ICR-CD1 mice (Harlan Ibérica, S.L.) were anesthetized and intubated intratracheally with a blunt-ended feeding needle. Approximately 106 CFU of K. pneumoniae from an early log-phase broth culture was suspended in 50 μl of sterile physiologic serum and inoculated through the blunt-ended needle. The animals were observed daily, and bacteremia was assessed at days 2, 4, and 6 by culturing 10 to 30 μl of tail vein blood on Luria-Bertani agar plates. Lung and spleen tissues from animals surviving to the end of the experiment (7 days) or animals that died were aseptically removed and homogenized for quantitative bacterial cultures.
Microscopy.
A block of lung tissue from representative animals was dissected. Tissues were fixed in 10 volumes of 10% neutral buffered formalin, embedded in paraffin, and sectioned at 4 to 6 μm. Tissue sections were stained with hematoxylin and eosin by standard techniques (20). Bacteria were detected by Gram staining of the tissue sections. For electron microscopy, a block of lung tissue from animals infected as described above was washed, fixed with glutaraldehyde, and processed for transmission electron microscopy as described previously (15).
Analysis of complement C3 deposition.
A bacterial suspension (2 × 108 CFU/ml) was opsonized with nonimmune human sera (NHS) diluted in PBS (25% final concentration) at 37°C for 15 min. Cells were washed three times with PBS-1% SDS by centrifugation. Pellets were resuspended in 50 mM carbonate-bicarbonate buffer (pH 9.0) containing 1 M NH4OH to disrupt ester bonds between C3 fragments and the bacterial surface (9). After 2 h at 37°C, the C3 fragment suspension was reduced and alkylated as described previously (9). Aliquots of C3 fragment suspension were diluted 1:1 in SDS-PAGE sample buffer and subjected to SDS-PAGE and Western blotting. Filters were blocked with PBS-1% BSA, incubated sequentially with anti-human C3 (1:1,000) and alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G, and developed with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium. Finally, the filters were analyzed by densitometry with a Bio Image densitometer and Whole Band 3.1 software (Millipore).
Opsonophagocytosis assay.
Human alveolar macrophages (AM) were isolated from healthy volunteers by bronchoalveolar lavage with 50 ml of saline. The lavage samples were centrifuged, and the cell pellet was washed twice with saline and resuspended in RPMI medium supplemented with 10% fetal bovine serum. An aliquot of the total cells was stained with Giemsa stain to confirm that 95% of the total bronchoalveolar lavage cells were AM. AM were plated at 5 × 105 cells per well in 24-well tissue culture trays. After 1 h of adherence at 37°C in 5% CO2, wells were washed with PBS to remove nonadherent cells and incubated with 106 bacterial cells that were either nonopsonized or opsonized for 15 min in ice with 25% NHS. After 30 min of incubation, wells were washed with PBS and then incubated for 1 h with fresh RPMI medium containing gentamicin (100 μg/ml) to kill extracellular bacteria. Phagocytosed bacteria were quantified by plating them on Luria-Bertani agar plates after AM lysis with a 0.5% Triton X-100 solution. Killing of phagocytosed bacteria was quantified as described above at 2 h after initial inoculation.
RESULTS
Characterization of murine pneumonia caused by wild-type K. pneumoniae strain 52145R.
To investigate the role of CPS and LPS O side chain, we derived two isogenic mutants from wild-type strain 52145R. We selected this strain to cause pneumonia in a mouse model due to its high virulence (50% lethal dose ≈ 1 to 10 CFU in a mouse model [data not shown]). Although we were able to develop pneumonia with 103 CFU of the wild-type strain, we used approximately 107 CFU in order to show that the differences observed between the wild-type strain and the isogenic mutants were not due to the inoculum. Mice were challenged intratracheally with strain 52145R and studied over a week. Macroscopic analysis of the lungs of these animals revealed an important increase of the weight and size over those of lungs from healthy control animals due to the presence of abscesses and edema (Table 1). Histopathologic examination of the lung lesions demonstrated extensive polymorphonuclear infiltration, with pleuritis, vasculitis, and edema. Tissue Gram staining detected gram-negative rods throughout the inflamed tissue.
TABLE 1.
K. pneumoniae pneumonia in micea
| Strain | Phenotype | No. of animals | No. of expts | Mean inoculum (range) | Mean lung wt (g) | Mean log CFU/g | No. blood or spleen culture positive/ total no. (%) | No. dead/total no. (%) |
|---|---|---|---|---|---|---|---|---|
| 52145R | Wild type | 36 | 4 | 1.3 × 107 (3.0 × 106 to 2.7 × 107) | 0.29 ± 0.11 | 6.11 | 17/32b (52) | 21/36 (58.3) |
| 52K10 | CPS− | 18 | 2 | 2.0 × 107 (1.0 × 105 to 6 × 107) | 0.15 ± 0.06 | 0.41 | 0/18 (0) | 0/18 (0) |
| 52O21 | LPS O− | 22 | 2 | 7.9 × 106 (7.8 × 106 to 8.0 × 106) | 0.41 ± 0.16 | 6.61 | 10/18b (55.6) | 14/22 (60.7) |
P < 0.01 for all comparisons (lung weight, log CFU per gram of lung, blood or spleen culture positive, and mortality) between the unencapsulated mutant and the wild type or the LPS O− mutant, two-tailed t test.
Blood or spleen culture was not obtained from four animals.
To determine the capacity of K. pneumoniae strain 52145R to disseminate from lungs and cause a fatal infection after intratracheal instillation in mice, we monitored animals for development of a positive blood culture and recorded their survival. A total of 17 of 32 animals inoculated with 52145R developed bacteremia (Table 1). Analysis of survival indicated that bacteremia preceded fatal infection by 24 to 48 h and that the majority of the deaths occurred between days 3 and 4. The mortality rate was 58.3%.
Role of CPS in the murine model of pneumonia.
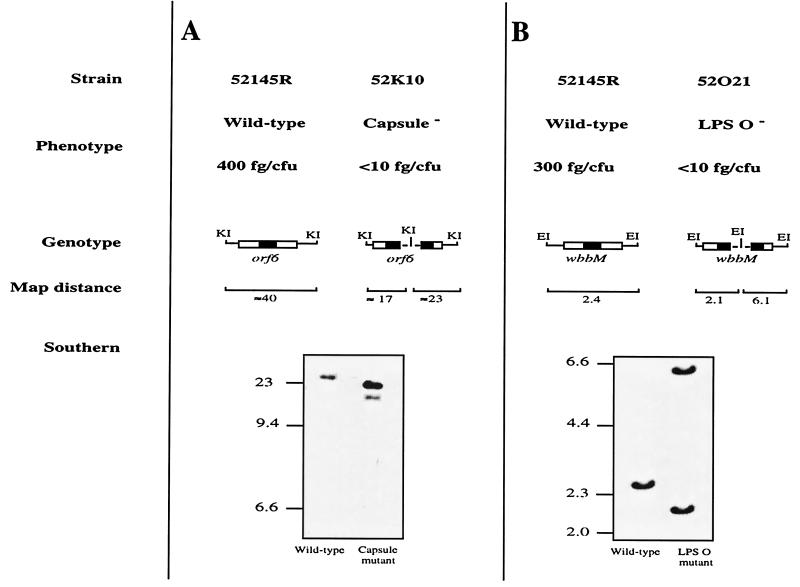
To derive an acapsular mutant from the mouse-virulent K. pneumoniae strain 52145R, we used insertion-duplication mutagenesis to interrupt the cps operon required for the synthesis of CPS. A schematic representation of the wild-type 52145R and the acapsular mutant 52K10 chromosome is shown in Fig. 1A. We demonstrated by an iELISA with capsule extracts from the mutant that this insertion completely abolished capsule expression in the mutant. Furthermore, LPS O side chain expression in the mutant 52K10 was identical to that for wild-type strain 52145R (data not shown).
FIG. 1.
Schematic representation of the K. pneumoniae chromosome in the virulent strain 52145R and the isogenic CPS mutant 52K10 (A) and in the LPS O side chain mutant 52O21 (B). The amount of CPS (A) and LPS O side chain (B) produced by the strains and determined by iELISA is expressed in femtograms per CFU (fg/CFU). DNA fragment size in the schematic is not to scale. The dashed line in the mutant genome represents the DNA plasmid integrated into the chromosome. The black boxes indicate the probes used in the Southern blot analysis. KI and EI indicate KpnI and EcoRI sites, respectively. Southern blot analysis of K. pneumoniae wild-type and isogenic mutant chromosomes digested with KpnI (A) and EcoRI (B) is shown. The expected sizes of the fragments that hybridize with the probes described above are indicated in kilobases. Molecular size markers (in kilobases) are shown to the left of the blots.
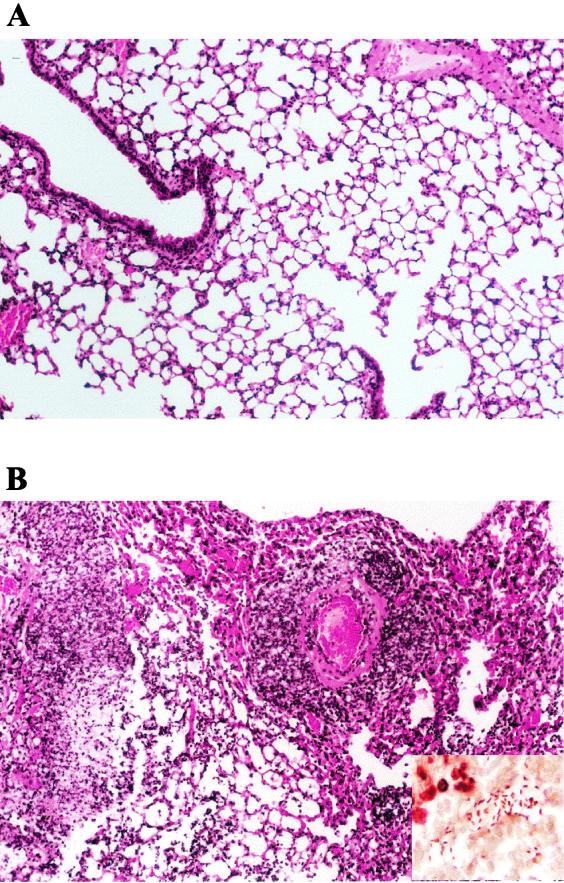
To investigate the role of CPS in the development of pneumonia, we inoculated mice intratracheally with the capsule-deficient mutant 52K10. In contrast with the lesions observed in the lungs of the animals infected with the wild-type strain 52145R, the lungs of the mice infected with the capsule-deficient mutant were apparently normal. Histopathological study of the lungs did not identify any difference from those of the healthy control animals (Fig. 2A). Tissue Gram staining did not detect bacteria in the lungs.
FIG. 2.
Histopathology of the lung in mice after intratracheal instillation of K. pneumoniae. The figure shows hematoxylin and eosin staining of lung sections from mice inoculated with unencapsulated strain 52K10 (A) or LPS O side chain mutant 52O21 (B). Tissue infected with the unencapsulated mutant is normal, but the tissue infected with the LPS O side chain mutant shows extensive infiltration of polymorphonuclear cells, vasculitis, and edema. Gram staining demonstrating the presence of K. pneumoniae 52O21 in the inflamed tissue is shown in the inset in panel B.
The local defenses of the lung were sufficient to contain the acapsular mutant infection; thus, none of the animals infected with strain 52K10 had bacteria in blood or spleen. Furthermore, no bacteria were recovered from the lung homogenates at day 7 (Table 1).
Interestingly, in one experiment only, not included in Table 1, small numbers of bacteria were recovered at day 7 from the lungs of 16 animals inoculated with the acapsular mutant. PCR amplification of the orf6 gene of these isolates generated a product of a size similar to that of the PCR product obtained with the wild-type strain 52145R chromosome as template. In addition, these isolates produced amounts of capsular polysaccharide similar to those observed for the wild-type strain 52145R. None of the animals inoculated with the acapsular mutant 52K10 died.
Role of LPS O side chain in the murine model of pneumonia.
To derive an LPS O side chain mutant from the mouse-virulent strain 52145R, we use insertion-duplication mutagenesis to interrupt the LPS operon required for the synthesis of the LPS O polysaccharide chain. A schematic representation of the wild-type 52145R and the LPS O side chain mutant 52O21 chromosome is shown in Fig. 1B. We verified by SDS-PAGE and silver staining and by an iELISA with LPS extracts of the mutant that this insertion-duplication completely abolished O side chain expression in the derived mutant designated 52O21. Furthermore, CPS expression in the mutant 52O21 was identical to that in the wild-type strain 52145R (data not shown).
Animals challenged intratracheally with the LPS O side chain mutant 52O21 developed pneumonia with features similar to those observed with pneumonia caused by the wild-type strain. Infected animals developed abscesses in their lungs, which increased in size and weight (Table 1). Histopathological study of the lesions demonstrated vasculitis, pleuritis, edema, and inflammatory infiltrates composed of polymorphonuclear cells. Gram staining revealed bacteria in inflamed tissues (Fig. 2B).
Most of the animals had positive blood cultures 24 to 48 h before death. The mortality rate was 60.7% (Table 1).
We verified by SDS-PAGE analysis that bacteria isolated from either lung, spleen, or blood were identical to original LPS O side chain mutant 52O21.
Opsonization and phagocytosis of the K. pneumoniae clinical isolate 52145R and its derived isogenic mutants by human AM.
AM and complement are the early host defenses against infections of the lower respiratory tract. To investigate the host defense mechanisms that cleared the unencapsulated mutant from the lungs, we characterized the ability of the wild-type strain and the derived isogenic mutants to deposit complement component C3.
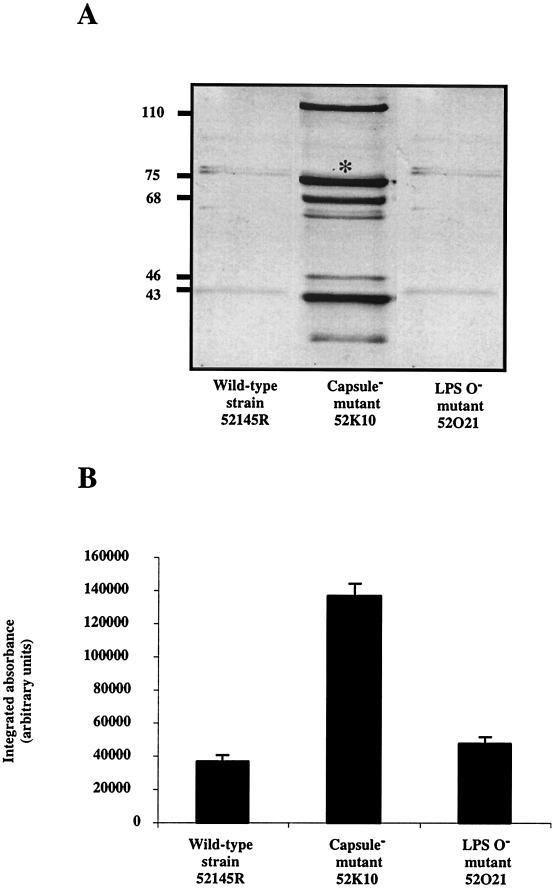
After 15 min of incubation in NHS the acapsular mutant bound threefold more C3 than did the wild-type strain and the LPS O side chain mutant (Fig. 3). We did not detect differences in C3 binding between wild-type strain 52145R and the LPS O side chain mutant 52O21. These results indicate that CPS is critical for modulating the levels of C3 deposited on the bacterial surface.
FIG. 3.
Analysis of complement C3 deposition on K. pneumoniae cell surfaces. Cells of the wild-type strain 52145R and the derived isogenic mutants 52K10 and 52O21 were incubated in NHS. C3 fragments deposited on the bacterial surface were released and identified by Western blotting with anti-human C3 serum and by comparison with purified C3 fragments (A). Numbers at left indicate molecular mass in kilodaltons. Quantification of the 75-kDa band of the β chain (indicated with an asterisk in panel A) common to C3b and iC3b was carried out by densitometric analysis (B). Data are means ± standard deviations of three independent experiments. Control values for each strain, i.e., C3 fragment depositions with heat-inactivated serum, were subtracted from experimental results to give the data shown in panel B (P < 0.01, for comparison between CPS− mutant and wild-type strain or LPS O− mutant, Scheffé's test).
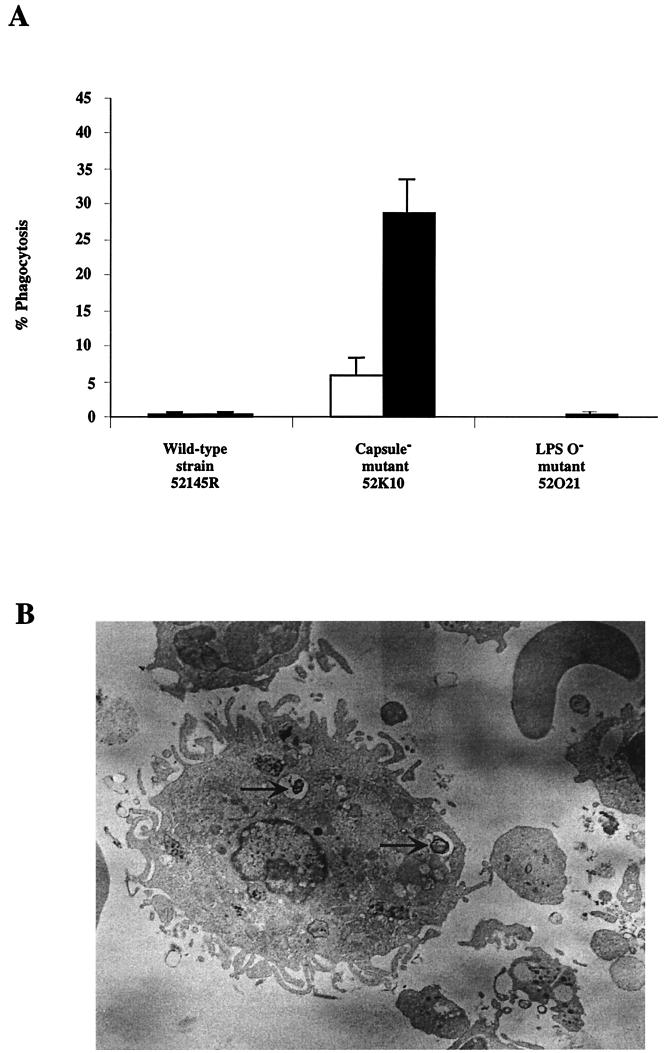
Bacterial opsonization by C3 was crucial to enabling the AM to recognize the pathogen. Phagocytosis of the unencapsulated mutant opsonized with NHS sera was almost threefold more effective than was phagocytosis of nonopsonized cells. AM did not phagocytose strain 52145R or the LPS O− mutant (Fig. 4). Furthermore, after 2 h of phagocytosis 50% of the phagocytosed bacteria were eliminated. Thus, these results indicate that CPS is required for resistance to the early host defense mechanisms of the lung, including complement and AM. For this reason, the unencapsulated mutant was eliminated from mouse lungs in the first 2 days while the wild-type strain and the LPS O side chain mutant resisted lung local defenses and were able to cause systemic infection.
FIG. 4.
(A) Phagocytosis of K. pneumoniae strain 52145R and its derived isogenic mutants by human AM. Human AM were incubated with bacterial cells previously opsonized with NHS (filled bars) or nonopsonized (open bars). After 1 h of incubation, monolayers of AM were washed, and phagocytosed bacteria were quantified. Data are means ± standard deviations of three independent experiments (P < 0.01, for comparison between CPS− mutant and wild-type strain or LPS O− mutant and for comparison between opsonized and nonopsonized unencapsulated cells, Scheffé's test). (B) Transmission electron micrograph of unencapsulated K. pneumoniae strain 52K10 cells (indicated by arrows) phagocytosed in vivo by a murine AM.
DISCUSSION
CPS and LPS O side chain are two of the most important virulence factors of K. pneumoniae.
The roles of CPS and LPS O side chain in resistance to complement and opsonophagocytosis by polymorphonuclear cells and in sepsis are well known (16, 26). However, their contribution to pneumonia has been insufficiently investigated. To investigate the cellular events leading to pneumonia caused by Klebsiella and to study the contribution of both components in this process, we characterized the host response to bacterial challenge in a murine model of pneumonia by using a highly virulent clinical isolate and its derived isogenic insertion-duplication CPS and LPS O side chain-deficient mutants.
Loss of LPS O side chain expression had no apparent effect on the ability of K. pneumoniae to cause pneumonia. Animals challenged with the LPS O side chain mutant developed vasculitis, pleuritis, and edema, as occurred with the parent strain. After 2 to 4 days of the initial lung infection most of the animals became bacteremic and subsequently died. Analysis of the organisms isolated from blood or spleen of infected animals confirmed the original mutant phenotype, indicating that the LPS O side chain mutant was fully virulent in mice and that virulence was not due to a reversion to the wild-type phenotype.
In contrast, our results clearly demonstrated that CPS is crucial for establishing pneumonia in a mouse model. None of the animals inoculated with the unencapsulated mutant developed pneumonia or showed bacterial dissemination from the lungs, and there were no fatal infections. Our results indicated that early host defenses were sufficient to eliminate this mutant efficiently, since at day 2 none of the animals inoculated with the unencapsulated mutant showed bacteria in their lungs (data not shown). Furthermore, these results are supported by the fact that in some animals the mutant with an unencapsulated phenotype, which is highly stable in vitro, reverted to the wild-type phenotype. This suggests that during the development of pneumonia there are strong selective pressures for the expression of CPS.
AM and complement are the first lung defenses against lower respiratory tract infections. Our data demonstrate that human AM were able to phagocytose K. pneumoniae cells, particularly if the bacterial cells were previously opsonized with complement-derived opsonic fragments. Thus, in the early stages of the lung infection, complement levels are critical to facilitating the macrophage-mediated killing of the pathogen. Although complement levels in the lung are low, the unencapsulated mutant bound complement C3 with high efficiency compared with that for the wild-type strain and the LPS O side chain mutant. Thus, during infection, CPS modulates the amount of C3 deposited on the bacterial surface, an experimental observation suggested by other studies (7). Previous studies have shown that CPS also modulates the interaction between surfactant protein D (SP-D) and Klebsiella (19). SP-D mediates aggregation and enhances phagocytic clearance by AM. Altogether CPS but not the LPS O side chain impedes the attachment of two humoral lung components, C3 and SP-D, crucial for the clearance of the microorganism from the lower respiratory tract. Consequently, the amount of CPS produced by K. pneumoniae is critical to determining the progress of the pneumonia.
Broug-Holub et al. showed in vivo that elimination of rat AM by administration of dichloromethylene diphosphonate-encapsulated liposomes decreased bacterial clearance and survival, although it also increased neutrophil recruitment (5). Our experiments performed in vitro with human AM support those results. The wild-type strain and the LPS O side chain mutant were not recognized by AM independently whether they were opsonized or not. Thus, the main host defensive barrier against pulmonary infections failed to eliminate the pathogen. In contrast, the CPS mutant was ingested efficiently by human AM when it was opsonized by complement-derived fragments. Interestingly, nonopsonized unencapsulated bacterial cells were also recognized by the human AM. This result suggests that, in addition to the complement receptors that allow the AM to recognize and ingest complement-opsonized bacterial cells, human AM display a receptor(s) that interacts with K. pneumoniae external components. This bacterial component(s) is unknown, but CPS, which impedes this interaction, masks it.
As occurs in blood with the polymorphonuclear cells (26), human AM are able to phagocytose K. pneumoniae but CPS reduces interaction between bacterial cells and AM by reducing the amount of C3 deposited on the bacteria and by acting as a physical barrier that impedes the interactions between the macrophage receptors and their ligands on the bacterial surface. In summary, our results indicate that the absence of CPS, but not of the LPS O side chain, profoundly affects the ability of K. pneumoniae to cause pneumonia, mainly because its absence increases the bacterial sensitivity to complement deposition and to AM killing.
Acknowledgments
We thank C. Saus and M. Pocoví for assistance in electron microscopy, F. Vivanco for purified C3, and V. J. Benedí for critical reading of the manuscript.
Editor: R. N. Moore
REFERENCES
- 1.Albertí, S., D. Álvarez, S. Merino, M. T. Casado, F. Vivanco, J. M. Tomás, and V. J. Benedí. 1996. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect. Immun. 64:4726-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa, Y., R. Wacharotayankun, T. Nagatsuka, H. Ito, N. Kato, and M. Ohta. 1995. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. Infect. Immun. 177:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. Greene Publishing and Wiley Interscience, New York, N.Y.
- 4.Bartlett, J. G., P. O'Keefe, F. P. Tally, T. J. Louie, and S. L. Gorbach. 1986. Bacteriology of hospital-acquired pneumonia. Arch. Intern. Med. 146:868-871. [PubMed] [Google Scholar]
- 5.Broug-Holub, E., G. B. Toews, F. Van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine III, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryz, S. J., Jr., E. Fürer, and R. Germanier. 1986. Immunization against fatal experimental Klebsiella pneumoniae pneumonia. Infect. Immun. 54:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domenico, P., J. M. Tomás, S. Merino, X. Rubires, and B. A. Cunha. 1999. Surface antigen exposure by bismuth dimercaprol suppression of Klebsiella pneumoniae capsular polysaccharide. Infect. Immun. 67:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García de la Torre, M., J. Romero-Vivas, J. Martínez-Beltrán, A. Guerrero, M. Messeguer, and E. Bouza. 1985. Klebsiella bacteremia: an analysis of 100 episodes. Rev. Infect. Dis. 7:143-150. [DOI] [PubMed] [Google Scholar]
- 9.Gordon, D. L., J. Rice, J. J. Finlay-Jones, P. J. MacDonald, and M. K. Hostetter. 1988. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J. Infect. Dis. 157:697-704. [DOI] [PubMed] [Google Scholar]
- 10.Held, T. K., M. Trautmann, M. E. Mielke, H. Neudeck, S. J. Cryz, and A. Cross. 1992. Monoclonal antibody against Klebsiella capsular polysaccharide reduces severity and hematogenic spread of experimental Klebsiella pneumoniae pneumonia. Infect. Immun. 60:1771-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis, W. R., V. P. Munn, A. K. Highsmith, D. H. Culver, and J. M. Hughes. 1985. The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect. Control 6:68-74. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, R. F., and C. Whitfield. 1996. Clonally diverse rfb gene clusters are involved in expression of a family of related d-galactan O antigens in Klebsiella species. J. Bacteriol. 178:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruskal, B. A., K. Sastry, A. B. Warner, C. E. Mathieu, and R. A. B. Ezekowitz. 1992. Phagocyte chimeric receptors require both transmembrane and cytoplasmic domains from the mannose receptor. J. Exp. Med. 176:1673-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merino, S., S. Camprubí, S. Albertí, V. J. Benedí, and J. M. Tomás. 1992. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect. Immun. 60:2529-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer, K. S., C. Urban, J. A. Eagan, B. J. Berger, and J. J. Rahal. 1993. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann. Intern. Med. 119:353-358. [DOI] [PubMed] [Google Scholar]
- 18.Nassif, X., J. M. Fournier, J. Arondel, and P. J. Sansonetti. 1989. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect. Immun. 57:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ofek, I., A. Mesika, M. Kalina, Y. Keisari, R. Podschun, H. Sahly, D. Chang, D. McGregor, and E. Crouch. 2001. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect. Immun. 69:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prophet, E. B., B. Mills, J. B. Arrington, and L. H. Sobin (ed.). 1992. Laboratory methods in histotechnology. American Registry of Pathology, Washington, D.C.
- 21.Rubirés, X., F. Saigi, N. Piqué, N. Climent, S. Merino, S. Albertí, J. M. Tomás, and M. Regué. 1997. A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J. Bacteriol. 179:7581-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straus, D. C., D. L. Atkisson, and C. W. Garner. 1985. Importance of lipopolysaccharide-containing extracellular toxic complex in infections produced by Klebsiella pneumoniae. Infect. Immun. 50:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 24.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 25.Wilkinson, J. F., and I. W. Sutherland. 1971. Chemical extraction methods of microbial cells. Methods Microbiol. 5B:345-383. [Google Scholar]
- 26.Williams, P., and J. M. Tomás. 1990. The pathogenicity of Klebsiella pneumoniae. Rev. Med. Microbiol. 1:196-204. [Google Scholar]