Abstract
Listeria epitope-specific helper T (Th) cells were able to be primed and induced in vivo by immunization with a plasmid carrying an invariant chain (Ii) gene whose class II-associated invariant chain peptide (CLIP) region was replaced by a Listeria Th epitope. Immunization of C3H/He mice with an Ii-LLO 215-226 plasmid induced specific interferon-γ- and interleukin 2-producing Th cells and conferred significant protective immunity against listerial infection.
Listeria monocytogenes is a gram-positive intracellular bacterium. A murine model of L. monocytogenes infection has been well studied and is considered a good model for exploring immunity against intracellular bacteria (reviewed in references 2 and 12). Cellular immunity has been considered to play a pivotal role in protection against intracellular bacteria (15). A variety of effector cells have been reported or suggested to resolve infection. These include neutrophils, macrophages, NK cells, and γδ T cells, as well as αβ T cells (9, 15, 19, 24). Among these, αβ CD8+ and CD4+ T cells have been shown to play critical roles in protective immunity through experiments with the depletion and adoptive transfer of specific T-cell subsets (1, 3, 4, 10, 27) and analyses of mutant mice with a genetic defect in β2-microglobulin or the H2-Aβ chain gene (13, 25). CD8+ cytotoxic T lymphocytes (CTL) have been reported to play a superior role in protective immunity (14, 18, 25). However, several papers have demonstrated a significant role for the CD4+-T-cell subset in protective immunity (11, 16, 23). Helper T (Th) cells play an important role in many aspects of immunity, especially for modulating immune responses by producing special sets of cytokines. For protection against intracellular bacteria, the activation of macrophages is indispensable, and antigen (Ag)-specific type 1 Th (Th1) cells have been reported to play a pivotal role in the activation (reviewed in reference 12). To investigate the roles of Th cells in protective immunity, we attempted to induce Th cells by immunization with an expression plasmid for a single Th epitope of L. monocytogenes, amino acid residues 215 to 226 of listeriolysin O (LLO 215-226; SQLIAKFGTAFK), an H2-Ek-restricted Th epitope (26, 37). The attempt, however, was unsuccessful (see Fig. 2A, p215), although immunization with plasmids encoding a single CTL epitope was able to induce specific CTL (20, 28, 34). In support of this result, we also showed in a previous work that immunization with an expression plasmid for a single Th epitope of ovalbumin (OVA 323-336) failed to induce specific lymphocyte proliferation (21).
FIG. 2.
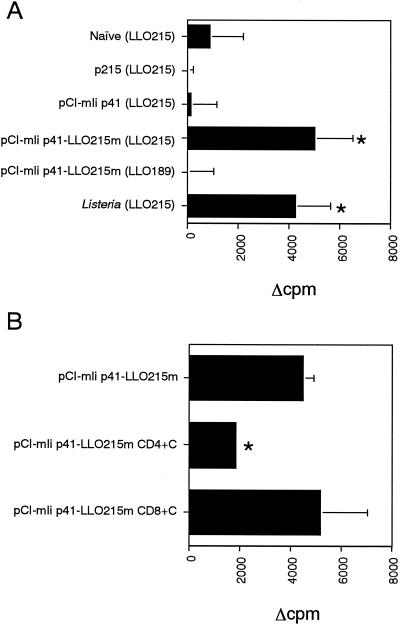
Ag-specific proliferation of splenocytes from mice immunized with an Ii plasmid expressing LLO 215-226, which replaces CLIP. Mice were immunized with p215, pCI-mIi p41, or pCI-mIi p41-LLO215m by gene gun bombardment four times at weekly intervals. (A) Splenocytes of the immunized mice were harvested 3 weeks after the last immunization and cultured in vitro in the presence or absence of 1 μM LLO 215-226 peptide (LLO215) or LLO 189-200 control peptide (LLO189) for 2 days and pulsed with 0.5 μCi of [methyl-3H]thymidine for the last 12 h. The values represent the mean and standard deviation of Δcpm (counts per minute in the presence of peptide minus counts per minute in the absence of the peptide) of quintuplicate determinations in a representative experiment. The asterisks indicate statistical significance (P < 0.001) compared with the values in naive mice. One-factor analysis of variance followed by Fischer's protected least-significant difference test was used in all statistical analyses. (B) Inhibition of LLO 215-226-specific splenocyte proliferation by CD4+-T-cell subset depletion. The splenocytes from pCI-mIi p41-LLO215m-immunized mice were treated with an anti-CD4 or anti-CD8 MAb and rabbit complement. Then, the lymphocyte proliferation assay was performed. The mean and standard deviation of Δcpm of quintuplicate determinations of a representative experiment are shown. The asterisks indicate statistical significance (P < 0.005) compared with the value for untreated splenocytes (pCI-mIi p41-LLO215m).
The invariant chain (Ii) molecule plays a central role in major histocompatibility complex (MHC) class II-mediated Ag presentation. It associates with MHC class II molecules in the endoplasmic reticulum so as to block premature loading of peptides on the molecules there. The Ii molecule works as a molecular chaperone for MHC class II transport to the endosomal compartment, where antigenic peptides are replaced with the class II-associated Ii peptide (CLIP) region of the molecule (reviewed in reference 8). Several groups have reported that MHC class II-positive cultured cells transfected with Ii cDNA whose CLIP region was replaced with a Th epitope of interest efficiently stimulate specific Th lines in vitro (5, 17, 29). In the present study, we investigated the effect of a single epitope-specific Th on protective immunity against L. monocytogenes, using immunization by gene gun bombardment with Ii plasmid DNA expressing a Th epitope that replaces the CLIP region.
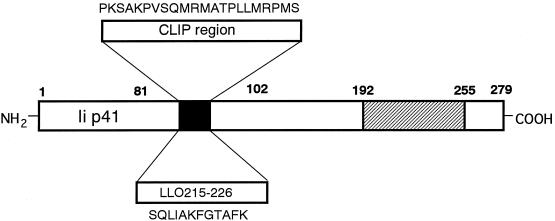
We constructed the plasmid for genetic immunization based on murine Ii p41 isoform cDNA. The EcoRI fragment containing Ii p41 cDNA was inserted into the EcoRI site in the pCI eukaryotic expression plasmid (Promega, Madison, Wis.). The CLIP region in the Ii cDNA was removed and replaced by a synthetic double-stranded oligonucleotide coding for LLO 215-226, resulting in pCI-mIi p41-LLO215m (Fig. 1). The oligonucleotide was designed so as to be adapted to the codon usage most frequent in mouse and human (22).
FIG. 1.
Schema of murine Ii molecule whose CLIP is replaced by LLO 215-226 (mIi p41-LLO215) deduced from the cDNA construct. The nucleotides encoding the CLIP region in the murine Ii p41 cDNA were replaced with the oligonucleotide coding for LLO 215-226 H2-Ek binding peptide. The cDNA was subcloned into the EcoRI site of pCI. The deduced amino acid sequences of the replaced CLIP region and the antigenic peptide LLO 215-226 are shown.
In order to examine whether pCI-mIi p41-LLO215m induces specific T cells in vivo, we immunized C3H/He mice (H2k; Japan SLC, Hamamatsu, Japan) with the plasmid by gene gun bombardment. We chose this immunization method because, based on our previous experience, it is a very reliable and reproducible method (36). All animal experiments were performed according to the animal care guidelines of our university. The plasmid DNA immunization was performed with the Helios gene gun system (Bio-Rad Laboratories, Hercules, Calif.). The preparation of a DNA-coated gold particle cartridge was performed following the manufacturer's instruction manual. Finally, 0.5 mg of 1.0-μm-diameter gold particles were coated with 1 μg of plasmid DNA, and the injection was carried out with 0.5 mg of gold/shot. Then, the mice were injected in the abdomen with 1 μg of plasmids at a helium discharge pressure of 400 lb/in2 four times at weekly intervals.
Three weeks after the last immunization, a lymphocyte proliferation assay was performed with splenocytes from the immunized mice. After treatment with Tris-buffered 0.83% ammonium chloride to lyse erythrocytes, splenocytes (5 × 105/well) from pCI-mIi p41-LLO215m-immunized mice were incubated for 48 h at 37°C in 96-well round-bottom tissue culture plates in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) in the presence or absence of 1 μM LLO 215-226 peptide. Then, DNA synthesis was assessed by adding 0.5 μCi of [methyl-3H]thymidine (6.7 Ci/mmol; ICN Biochemicals, Irvine, Calif.)/well for the last 14 h of culture. The cultures were harvested onto glass fiber filters, and the radioactivity was counted by liquid scintillation. As shown in Fig. 2A, immunization with the plasmid allowed splenocytes to proliferate after incubation in the presence, but not in the absence, of LLO 215-226 peptide at a level comparable to that of viable Listeria immunization. We could not detect any significant LLO 215-226-specific lymphocyte proliferation by immunization with p215 (LLO 215-226 expression plasmid) or pCI-mIi p41 (wild-type Ii p41 expression plasmid). We could not detect nonspecific proliferative responses with pCI-mIi p41-LLO215m when accompanied by incubation with an irrelevant peptide (LLO 189-200; Fig. 2A).
Furthermore, the CD4-CD8 specificity of proliferative lymphocytes was tested by depletion studies with the anti-murine CD4 monoclonal antibody (MAb) GK1.5 or the anti-murine CD8α MAb 53-6.7 (PharMingen, San Diego, Calif.). These MAbs were added to the immune splenocytes, at 1 μg/ml, and the splenocytes were incubated for 1 h at 4°C. They were then centrifuged, and the supernatants were discarded. The cells were resuspended in cytotoxicity medium (RPMI-1640 medium with 25 mM HEPES buffer and 0.3% FCS) containing rabbit complement (Cedarlane, Hornby, Ontario, Canada) and incubated for 1 h at 37°C. The dead cells were removed by Lympholite-M reagent (Cedarlane). The recovered cells were used for the lymphocyte proliferation assay described above. The LLO 215-226-specific proliferative responses of splenocytes from the immunized mice was reduced significantly by CD4+-T-cell subset depletion but not by CD8+-T-cell subset depletion, indicating that LLO 215-226-specific T cells generated by pCI-mIi p41-LLO215m plasmid DNA immunization belong to the CD4+-T-cell subset (Fig. 2B).
Next, we examined specific gamma interferon (IFN-γ), interleukin-2 (IL-2), and IL-4 production by splenocytes from mice immunized with the pCI-mIi p41-LLO215m plasmid. Splenocytes from the immunized mice were plated in 24-well plates at 2 × 106/well in RPMI 1640 medium supplemented with 10% FCS in the presence or absence of 1 μM LLO 215-226 peptide for 4 days in the case of IFN-γ and IL-4 and for 1 day in the case of IL-2. The concentrations of cytokines in the culture supernatants were determined by sandwich enzyme-linked immunosorbent assay, as described elsewhere (35). All of the MAbs used were purchased from PharMingen. As shown in Table 1, we observed the production of significant amounts of IFN-γ and IL-2 by splenocytes from mice immunized with pCI-mIi p41-LLO215m, but not with pCI-mIi p41, at a level comparable with that from mice immunized with viable Listeria after in vitro culture in the presence of LLO 215-226 peptide. In addition, splenocytes of pCI-mIi p41-LLO215m-immunized mice after incubation with an irrelevant MHC class II binding peptide did not produce significant amounts of IFN-γ and IL-2. We could not detect significant levels of IL-4 by using the same culture supernatants of splenocytes from all mice examined (Table 1).
TABLE 1.
Cytokine production by splenocytes from C3H/He mice immunized with pCI-mIip41-LLO 215m plasmid
| Immunization | Stimulationa | Cytokine production (pg/ml)b
|
||
|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | ||
| pCI-mIi p41 | − | 163 | 416 | 62 |
| LLO215 | 140 | 638 | 19 | |
| pCl-mIi p41-LLO 215m | − | 266 | 32 | 49 |
| LLO215 | 2,535 | 2,046 | 35 | |
| LLO189 | 66 | 226 | 40 | |
| Listeria | − | 287 | 377 | 73 |
| LLO215 | 2,034 | 1,053 | 49 | |
Spleen cells of immunized mice (2 × 106 per well) were cultured in the presence of 1 μM LLO 215-226 peptide (LLO215) or control peptide LLO 189-200 (LLO189) or in the absence of any peptides (−).
After 4 (IFN-γ and IL-4) or 1 (IL-2) day, cytokine concentrations in culture supernatants were quantified by sandwich enzyme-linked immunosorbent assay. The mean of duplicate wells of representative data is shown.
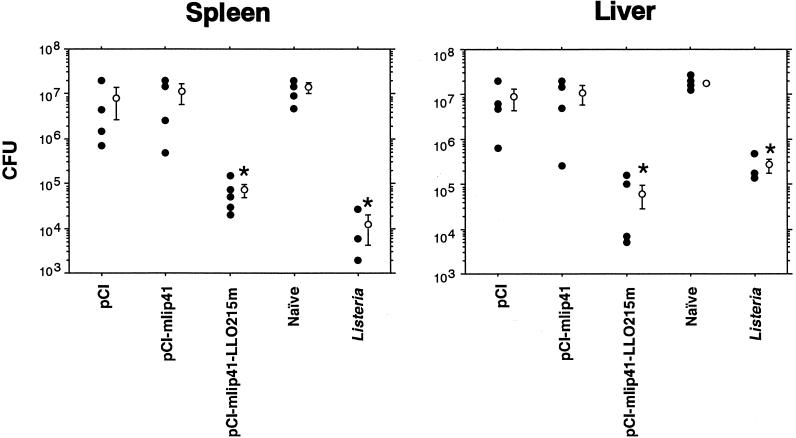
To ascertain if Th cell effectors evoked by plasmid immunization are associated with an increased resistance to infection by the virulent L. monocytogenes EGD strain, in vivo protection experiments were carried out. The bacterium was kept virulent by in vivo passage. For inoculation, a seed of L. monocytogenes was cultured overnight in trypticase soy broth (BBL, Sparks, Md.) at 37°C in a bacterial shaker and suitably diluted with phosphate-buffered saline. The exact infection dose was assessed retrospectively by plating. Mice were immunized with pCI-mIi p41-LLO215m four times at weekly intervals or were immunized by a single intraperitoneal injection with a sublethal dose of L. monocytogenes (104 CFU) as a positive control. The immunized mice were challenged intraperitoneally with 2 × 105 CFU of Listeria 3 weeks after the last immunization. Bacterial numbers in the spleens and livers were determined 72 h after the challenge infection by plating 10-fold dilutions of tissue homogenates on trypticase soy agar. As shown in Fig. 3, immunization with pCI-mIi p41-LLO215m dramatically decreased the bacterial numbers in the spleens and livers of the immunized mice. Verma et al. (32) also demonstrated by using a Salmonella carrier system that induction of a CD4+-T-cell population responsive to LLO 215-226 elicits partial protective immunity. In their system, reduction in the number of Listeria cells was more significant in the livers of LLO 215-226-immunized mice than in the spleens. Our data also show that LLO 215-226-immunized mice were somewhat better protected against Listeria challenge in the liver than in the spleen compared with Listeria-immunized mice (Fig. 3). On the other hand, Geginat et al. (6) reported enhanced protection by p60-specific CD4+-T-cell clones in the spleen compared with the liver in their adoptive-transfer system of the CD4+-T-cell clones. This discrepancy might be attributable to differences in the experimental design, including intraperitoneal listerial challenge (this work and Verma et al. [32]) versus intravenous listerial challenge (Geginat et al. [6]). In addition, other reports also demonstrated a role for CD4+ T cells in protective immunity against listerial challenge (11, 16, 23). The mechanisms of the protective immunity elicited by CD4+ T cells have been speculated upon. Listeria-specific CD4+ T cells may act by direct lysis of the infected target cells (11). Alternatively, the cells may show the bystander effect by secretion of cytokines, especially IFN-γ. IFN-γ will enhance the killing activity of macrophages or augment induction of CD8+ CTL (33).
FIG. 3.
Protective immunity induced by immunization with pCI-Ii p41-LLO215m. C3H/He mice were immunized with pCI-Ii p41-LLO215m four times at weekly intervals. Three weeks after the last immunization, the mice were challenged with 2 × 105 CFU of L. monocytogenes. Three to five mice were used for each group. The bacterial numbers in the spleens and livers were determined 72 h after challenge infection by plating 10-fold dilutions of tissue homogenates on trypticase soy agar plates. The results for pCI-immunized, pCI-mIi p41-immunized, naïve, and Listeria-immunized mice are also shown as controls. The numbers of bacteria recovered from the spleen and liver of each immunized mouse are shown. The mean and standard deviation of each group are also shown. The asterisks indicate statistical significance (P < 0.03) compared with the values in naïve mice.
We report here that DNA immunization with an Ii expression plasmid whose encoded CLIP region has been replaced by a Listeria-derived Th epitope successfully induces T cells specific to the epitope in vivo. Attempts to induce specific Th cells by using Ii plasmids in the cell line system (5, 17, 29; reviewed in reference 30) or, recently, in vivo (21, 31) have been reported. Here, we showed that a similar system can be applied successfully for DNA vaccination against infectious diseases. This is the first report showing that a single immunization with Ii plasmid DNA whose encoded CLIP region has been replaced by a Th epitope induces effective protective immunity against a microorganism. One of the advantages of gene immunization with T-cell epitope minigene plasmids is that we can compare the immunogenicities of all of the T-cell epitopes at the same expression level in vivo. We have analyzed the hierarchy of the magnitudes of immunogenicity of three Listeria-derived CTL epitopes by using a gene immunization system with minigenes for three listerial CTL epitopes (34). Using the system discussed here, it is interesting to compare the immunodominance of several Th epitopes, including those recently identified from LLO and p60, in the induction of protective immunity (7). Furthermore, we plan to examine the effect of combinatorial induction of both CTL and Th cell subsets in order to induce more effective protective immunity against the bacterium by using the system discussed here.
Acknowledgments
We thank Ronald N. Germain (National Institutes of Health, Bethesda, Md.) for murine p41 cDNA, Masao Mitsuyama (Kyoto University, Kyoto, Japan) for L. monocytogenes strain EGD, and Naohiro Seo (Hamamatsu University School of Medicine, Hamamatsu, Japan) for providing anti-CD4 and CD8 MAbs.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and Shizuoka Research and Education Foundation (Shizuoka, Japan) and from the Regional Science Promotion Program of the Japan Science and Technology Corporation.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bishop, K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 2.Cossart, P., and J. Mengaud. 1989. Listeria monocytogenes. A model system for the molecular study of intracellular parasitism. Mol. Biol. Med. 6:463-474. [PubMed] [Google Scholar]
- 3.Czuprynski, C. J., and J. F. Brown. 1987. Dual regulation of anti-bacterial resistance and inflammatory neutrophil and macrophage accumulation by L3T4+ and Lyt 2+ Listeria-immune T cells. Immunology 60:287-293. [PMC free article] [PubMed] [Google Scholar]
- 4.Czuprynski, C. J., and J. F. Brown. 1990. Effects of purified anti-Lyt-2 MAb treatment on murine listeriosis: comparative roles of Lyt-2+ and L3T4+ cells in resistance to primary and secondary infection, delayed-type hypersensitivity and adoptive transfer of resistance. Immunology 71:107-112. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii, S., S. Senju, Y.-Z. Chen, M. Ando, S. Matsushita, and Y. Nishimura. 1998. The CLIP-substituted invariant chain efficiently targets an antigenic peptide to HLA class II pathway in L cells. Hum. Immunol. 59:607-614. [DOI] [PubMed] [Google Scholar]
- 6.Geginat, G., M. Lalic, M. Kretschmar, W. Goebel, H. Hof, D. Palm, and A. Bubert. 1998. Th1 cells specific for a secreted protein of Listeria monocytogenes are protective in vivo. J. Immnol. 160:6046-6055. [PubMed] [Google Scholar]
- 7.Geginat, G., S. Schenk, M. Skoberne, W. Goebel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Imunol. 166:1877-1884. [DOI] [PubMed] [Google Scholar]
- 8.Germain, R. N. 1999. Antigen processing and presentation, p. 287-340. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 9.Hiromatsu, K., Y. Yoshikai, G. Matsuzaki, S. Ohga, K. Muramori, K. Matsumoto, J. A. Bluestone, and K. Nomoto. 1992. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann, S. H. E., E. Hug, U. Vath, and I. Muller. 1985. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect. Immun. 48:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann, S. H. E., E. Hug, U. Väth, and G. De Libero. 1987. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur. J. Immunol. 17:237-246. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 13.Ladel, C. H., I. E. A. Flesch, J. Arnoldi, and S. H. E. Kaufmann. 1994. Studies with MHC-deficient knock-out mice reveal impact of both MHC-I- and MHC-II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153:3116-3122. [PubMed] [Google Scholar]
- 14.Lukacs, K., and R. Kurlander. 1989. Lyt-2+ T cell-mediated protection against listeriosis. Protection correlates with phagocyte depletion but not with IFN-γ production. J. Immunol. 142:2879-2886. [PubMed] [Google Scholar]
- 15.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-417. [PubMed] [Google Scholar]
- 16.Magee, D. M., and E. J. Wing. 1988. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-γ. J. Immunol. 141:3203-3207. [PubMed] [Google Scholar]
- 17.Malcherek, G., C. Wirblich, N. Willcox, H.-G. Rammensee, J. Trowsdale, and A. Melms. 1998. MHC class II-associated invariant chain peptide replacement by T cell epitopes: engineered invariant chain as a vehicle for directed and enhanced MHC class II antigen processing and presentation. Eur. J. Immunol. 28:1524-1533. [DOI] [PubMed] [Google Scholar]
- 18.Mielke, M. A., S. Ehlers, and H. Hahn. 1988. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect. Immunol. 56:1920-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mombaerts, P., J. Arnoldi, F. Russ, S. Tonegawa, and S. H. E. Kaufmann. 1993. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature 365:53-56. [DOI] [PubMed] [Google Scholar]
- 20.Nagata, T., M. Uchijima, A. Yoshida, M. Kawashima, and Y. Koide. 1999. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem. Biophys. Res. Commun. 261:445-451. [DOI] [PubMed] [Google Scholar]
- 21.Nagata, T., T. Higashi, T. Aoshi, M. Suzuki, M. Uchijima, and Y. Koide. 2001. Immunization with plasmid DNA encoding MHC class II binding peptide/CLIP-replaced invariant chain (Ii) induces specific helper T cells in vivo: the assessment of Ii p31 and p41 isoforms as vehicles for immunization. Vaccine 20:105-114. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, Y., T. Gojobori, and T. Ikemura. 1999. Codon usage tabulated from the international DNA sequence databases; its status 1999. Nucleic Acids Res. 27:292.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakhmilevich, A. L. 1993. Evidence for a significant role of CD4+ T cells in adoptive immunity to Listeria monocytogenes in the liver. Immunology 82:249-254. [PMC free article] [PubMed] [Google Scholar]
- 24.Rakhmilevich, A. L. 1995. Neutrophils are essential for resolution of primary and secondary infection with Listeria monocytogenes. J. Leukoc. Biol. 57:827-831. [DOI] [PubMed] [Google Scholar]
- 25.Roberts, A. D., D. J. Ordway, and I. M. Orme. 1993. Listeria monocytogenes infection in β2 microglobulin-deficient mice. Infect. Immunol. 61:1113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safley, S. A., P. E. Jensen, P. A. Reay, and H. K. Ziegler. 1995. Mechanisms of T cell epitope immunodominance analyzed in murine listeriosis. J. Immunol. 155:4355-4366. [PubMed] [Google Scholar]
- 27.Sasaki, T., M. Mieno, H. Udono, T. Yamaguchi, K. Usui, K. Hara, H. Shiku, and E. Nakayama. 1990. Roles of CD4+ and CD8+ cells, and the effect of administration of recombinant murine interferon γ in listerial infection. J. Exp. Med. 171:1141-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchijima, M., A. Yoshida, T. Nagata, and Y. Koide. 1998. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J. Immunol. 161:5594-5599. [PubMed] [Google Scholar]
- 29.van Bergen, J., S. P. Schoenbeger, F. Verreck, R. Amons, R. Offringa, and F. Koning. 1997. Efficient loading of HLA-DR with a T helper epitope by genetic exchange of CLIP. Proc. Natl. Acad. Sci. USA 94:7499-7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Bergen, J., F. Ossendorp, R. Jordens, A. M. Mommaas, J.-W. Drijfhout, and F. Koning. 1999. Get into the groove! Targeting antigens to MHC class II. Immunol. Rev. 172:87-96. [DOI] [PubMed] [Google Scholar]
- 31.van Tienhoven, E. A. E., C. T. B. ten Brink, J. van Bergen, F. Koning, W. van Eden, and C. P. M. Broeren. 2001. Induction of antigen specific CD4+ T cell responses by invariant chain based DNA vaccines. Vaccine 19:1515-1519. [DOI] [PubMed] [Google Scholar]
- 32.Verma, N. K., H. K. Ziegler, M. Wilson, M. Khan, S. Safley, B. A. D. Stocker, and G. K. Schoolnik. 1995. Delivery of class I and class II MHC-restricted T-cell epitopes of listeriolysin of Listeria monocytogenes by attenuated Salmonella. Vaccine 13:142-150. [DOI] [PubMed] [Google Scholar]
- 33.Widmann, C., P. Romero, J. L. Maryanski, G. Corradin, and D. Valmori. 1992. T helper epitopes enhance the cytotoxic response of mice immunized with MHC class I-restricted malaria peptides. J. Immunol. Methods 155:95-99. [DOI] [PubMed] [Google Scholar]
- 34.Yamada, T., H. Uchiyama, T. Nagata, K. Chida, M. Uchijima, H. Nakamura, and Y. Koide. 2001. Protective cytotoxic T lymphocyte responses induced by DNA immunization against immunodominant and subdominant epitopes of Listeria monocytogenes are noncompetitive. Infect. Immun. 69:3427-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida, A., Y. Koide, M. Uchijima, and T. O. Yoshida. 1995. Dissection of strain difference in acquired protective immunity against Mycobacterium bovis Calmette-Guérin Bacillus (BCG). Macrophages regulate susceptibility through the cytokine network and the induction of nitric oxide synthase. J. Immunol. 155:2057-2066. [PubMed] [Google Scholar]
- 36.Yoshida, A., T. Nagata, M. Uchijima, T. Higashi, and Y. Koide. 2000. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine 18:1725-1729. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler, H. K., S. A. Safley, and E. Hiltbold. 1994. Definition of T cell epitopes of Listeria monocytogenes and regulation of antigen processing by the bacterial exotoxin listeriolysin-O (LLO), p. 295-307. In R. E. Humphreys and S. K. Pierce (ed.), Antigen processing and presentation. Academic Press, Inc., San Diego, Calif.