Abstract
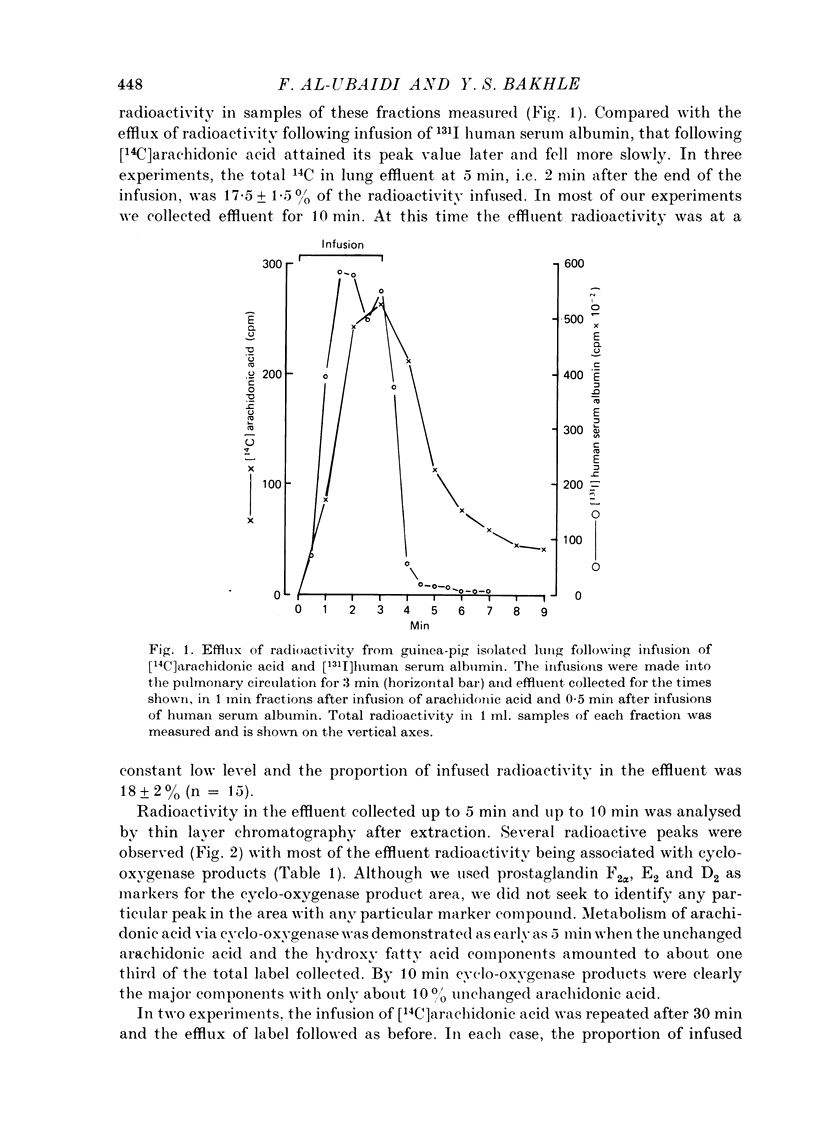
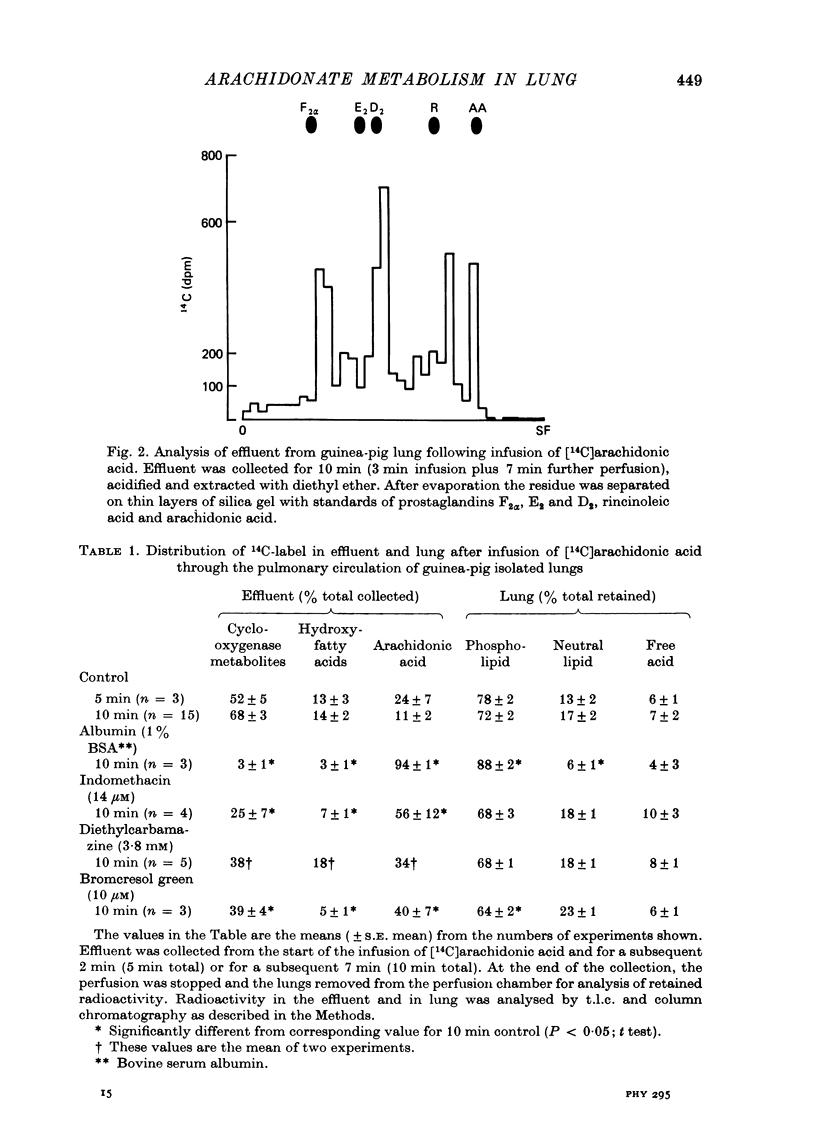
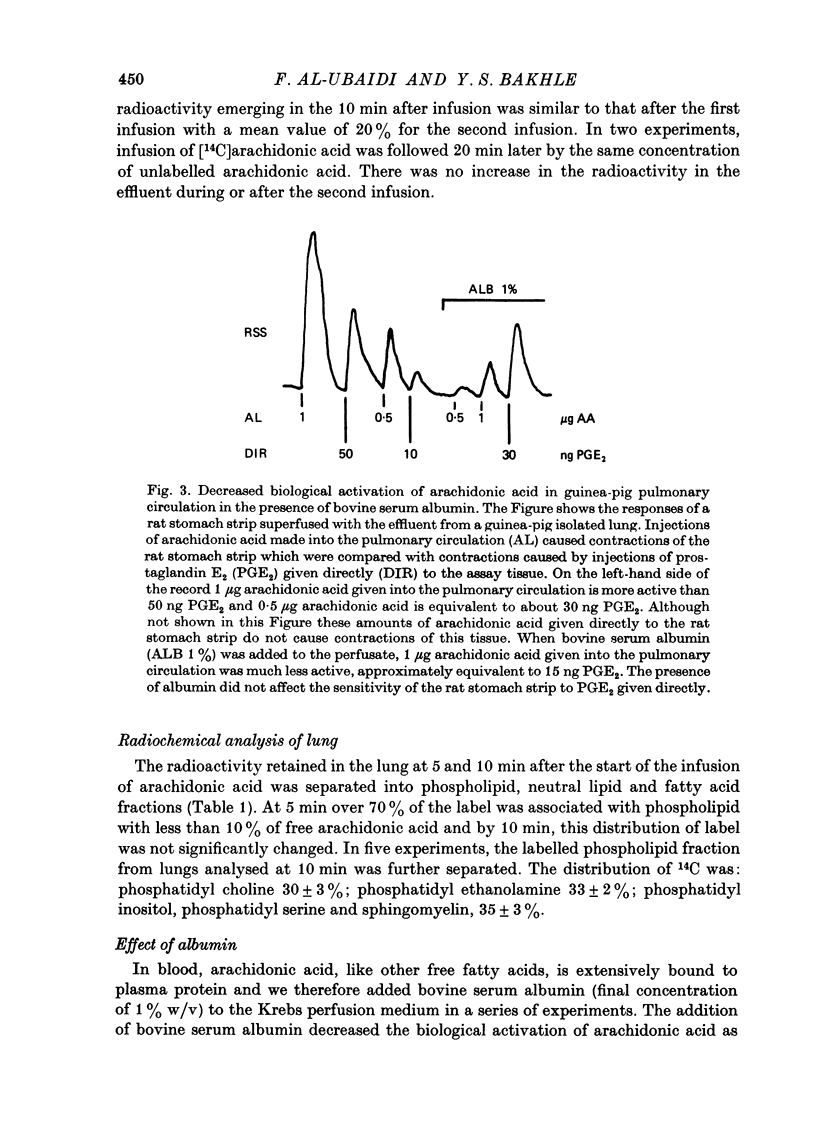
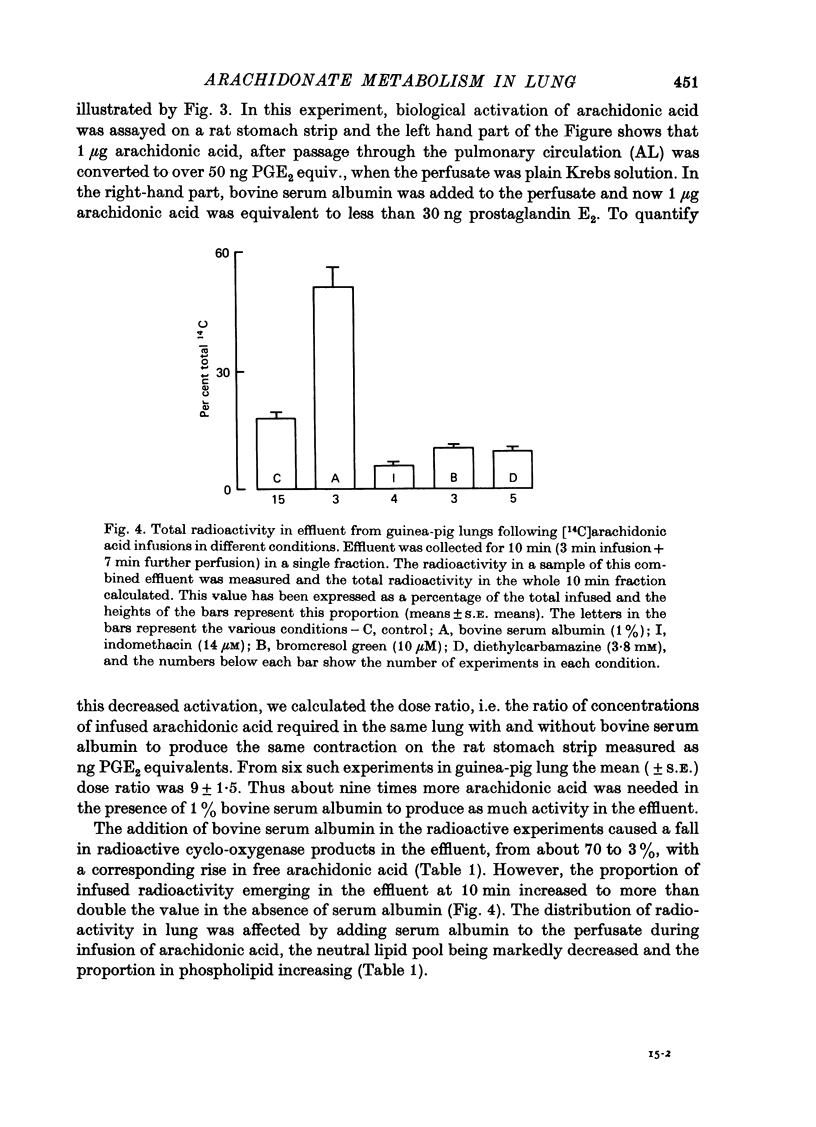
1. The fate of [14C]arachidonic acid perfused through the pulmonary circulation was studied in guinea-pig lungs perfused with Krebs solution. 2. Radioactivity in the lung effluent fell rapidly and by 10 min about 20% of the infused radioactivity had emerged. 3. Most (70%) of the effluent radioactivity was associated with products of cyclo-oxygenase activity, whereas in the lung tissue most of the retained radioactivity was present as phospholipid. 4. Radioactivity in phospholipid was distributed equally between three groups: phosphatidyl choline, phosphatidyl ethanolamine and the other phosphatides. 5. Addition of albumin to the Krebs solution perfusing the lung increased the proportion of effluent radioactivity to 50%, decreased the cyclo-oxygenase products but increased the label in phospholipid in lung. 6. Indomethacin, frusemide, bromcresol green and diethylcarbamazine all decreased biological activation of arachidonic acid. 7. Indomethacin, bromcresol green and diethylcarbamazine also decreased effluent radioactivity and cyclo-oxygenase products with minimal effects on the distribution of radioactivity in lung lipid. 8. It appears that the major metabolic pathway for exogenous arachidonic acid perfused through the pulmonary circulation was incorporation into phospholipid. Metabolism via cyclo-oxygenase only involved about 15% of the total substrate infused.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ubaidi F., Bakhle Y. S., Jose P. J., Seale J. P. Fate of arachidonic acid in guinea-pig isolated lungs [proceedings]. J Physiol. 1978 Aug;281:2P–3P. [PubMed] [Google Scholar]
- Alabaster V. A., Hawkeswood E. PGI2 release from guinea-pig lungs: detection and bioassay [proceedings]. Br J Pharmacol. 1978 Mar;62(3):467P–467P. [PMC free article] [PubMed] [Google Scholar]
- Bakhle Y. S., Reynard A. M., Vane J. R. Metabolism of the angiotensins in isolated perfused tissues. Nature. 1969 Jun 7;222(5197):956–959. doi: 10.1038/222956a0. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Baroody R. A. Inhibition of pulmonary prostaglandin metabolism by inhibitors of prostaglandin biotransport (probenecid and bromcresol green). Prostaglandins. 1975 Oct;10(4):633–639. doi: 10.1016/s0090-6980(75)80010-4. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Derksen A. Comparison of phospholipid and fatty acid composition of human erythrocytes and platelets. Br J Haematol. 1969 Oct;17(4):359–371. doi: 10.1111/j.1365-2141.1969.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Eckenfels A., Vane J. R. Prostaglandins, oxygen tension and smooth muscle tone. Br J Pharmacol. 1972 Jul;45(3):451–462. doi: 10.1111/j.1476-5381.1972.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer D. M., Niederhauser U., Piper P. J., Sirois P. Release of mediators of anaphylaxis: inhibition of prostaglandin synthesis and the modification of release of slow reacting substance of anaphylaxis and histamine. Br J Pharmacol. 1978 Jan;62(1):61–66. doi: 10.1111/j.1476-5381.1978.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. The importance of phospholipase-A2 in prostaglandin biosynthesis. Biochem Pharmacol. 1976 Feb 1;25(3):285–291. doi: 10.1016/0006-2952(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Bunting S., Moncada S., Flower R. J., Vane J. R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins. 1976 Nov;12(5):685–713. doi: 10.1016/0090-6980(76)90047-2. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Hedqvist P., Strandberg K., Samuelsson B. Involvement of endoperoxides and thromboxanes in anaphylactic reactions. Adv Prostaglandin Thromboxane Res. 1976;1:495–501. [PubMed] [Google Scholar]
- Hansen H. S. 15-hydroxyprostaglandin dehydrogenase. A review. Prostaglandins. 1976 Oct;12(4):647–679. doi: 10.1016/0090-6980(76)90044-7. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Raz A., Needleman P. Selective incorporation of 14C-arachidonic acid into the phospholipids of intact tissues and subsequent metabolism to 14C-prostaglandins. Prostaglandins. 1976 Nov;12(5):739–748. doi: 10.1016/0090-6980(76)90049-6. [DOI] [PubMed] [Google Scholar]
- Orange R. P., Valentine M. D., Austen K. F. Inhibition of the release of slow-reacting substance of anaphylaxis in the rat with diethylcarbamazine. Proc Soc Exp Biol Med. 1968 Jan;127(1):127–132. doi: 10.3181/00379727-127-32638. [DOI] [PubMed] [Google Scholar]
- Palmer M. A., Piper P. J., Vane J. R. Release of rabbit aorta contracting substance (RCS) and prostaglandins induced by chemical or mechanical stimulation of guinea-pig lungs. Br J Pharmacol. 1973 Oct;49(2):226–242. doi: 10.1111/j.1476-5381.1973.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S. Pulmonary endothelial cells. Fed Proc. 1977 Dec;36(13):2683–2691. [PubMed] [Google Scholar]
- VANE J. R. THE USE OF ISOLATED ORGANS FOR DETECTING ACTIVE SUBSTANCES IN THE CIRCULATING BLOOD. Br J Pharmacol Chemother. 1964 Oct;23:360–373. doi: 10.1111/j.1476-5381.1964.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


