Abstract
1. Single, isolated, rod photoreceptors were obtained by enzymatic dissociation of the tiger salamander (Ambystoma tigrinum) retina. These solitary cells retained the morphological features of rods of the intact retina and could be maintained in culture for several days. Solitary cells were penetrated with one or two micropipettes and their electrophysiology was studied by the voltage-clamp technique. 2. Intracellular recording with two micropipettes demonstrated that the inner segment of a solitary rod was effectively isopotential with the outer segment. 3. The time course of the voltage response to a flash resembled that of responses observed in rods in the intact retina. At low light intensities the response reached a peak in approximately 0.7 sec and then slowly declined. At high light intensities the time to peak response decreased and an initial transient arose as the response, after reaching the peak, quickly decreased to a less polarized plateau. 4. The normal voltage response could be compared with the current observed during a voltage clamp. At low light intensities the time course of the current response resembled the time course of the voltage response. When light intensity was increased the time course of the current response differed from the voltage response in that the time to peak amplitude remained relatively constant and an initial transient did not occur. It was possible to predict the current response produced by any intensity of light by using (i) an empirical equation which reproduced the time course of a dim response and (ii) the Michaelis-Menten equation. 5. The time course of the voltage-clamp current produced by a flash was the same at different values of maintained voltage. 6. The maximum amplitude of the voltage-clamp current produced by a flash or step of light was a non-linear function of membrane potential. It was relatively constant within the physiological range, decreased as the membrane potential was moved toward 0 mV, reversed polarity between 0 and 10 mV, and rapidly increased in magnitude as membrane potential was made more positive. Although this current was voltage dependent, no time dependence was evident (recording resolution greater than or equal to 5 msec). 7. Voltage-clamp experiments demonstrated an inward current which slowly developed after a hyperpolarizing voltage step. The effect of this voltage and time dependent current was to reduce, after a delay, the polarization initiated by light.
Full text
PDF






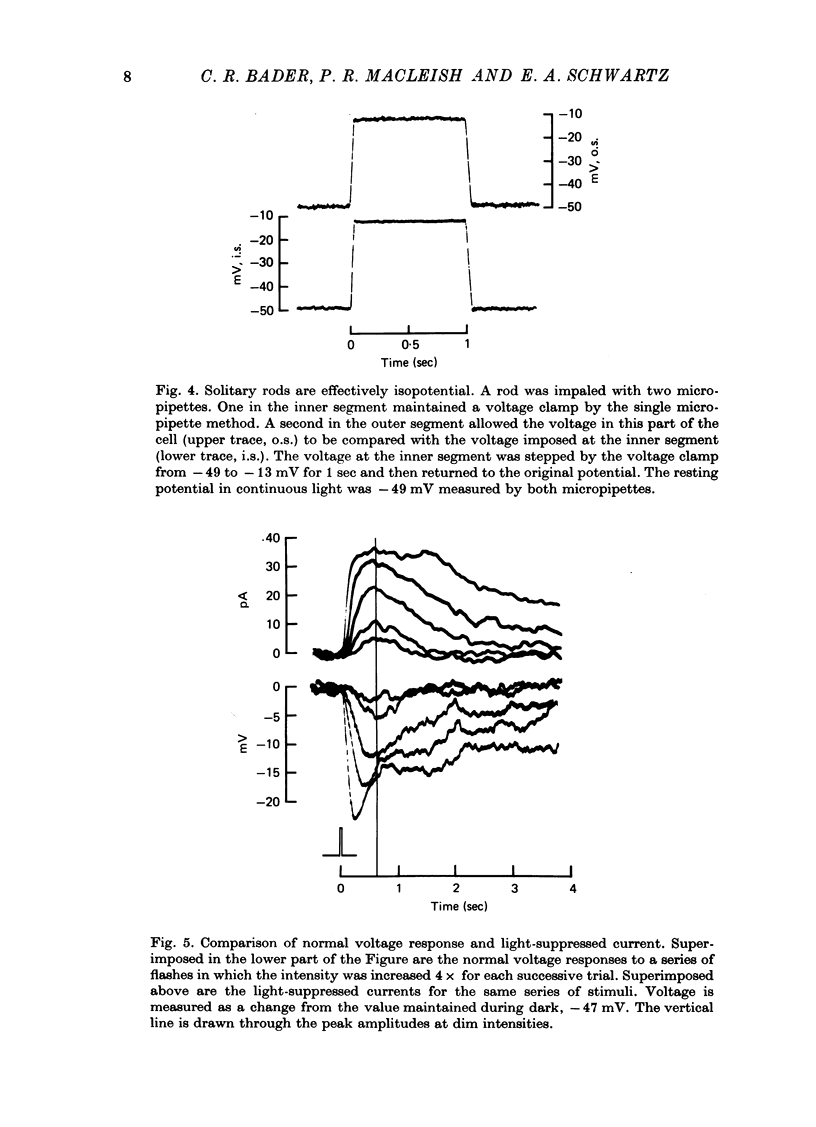

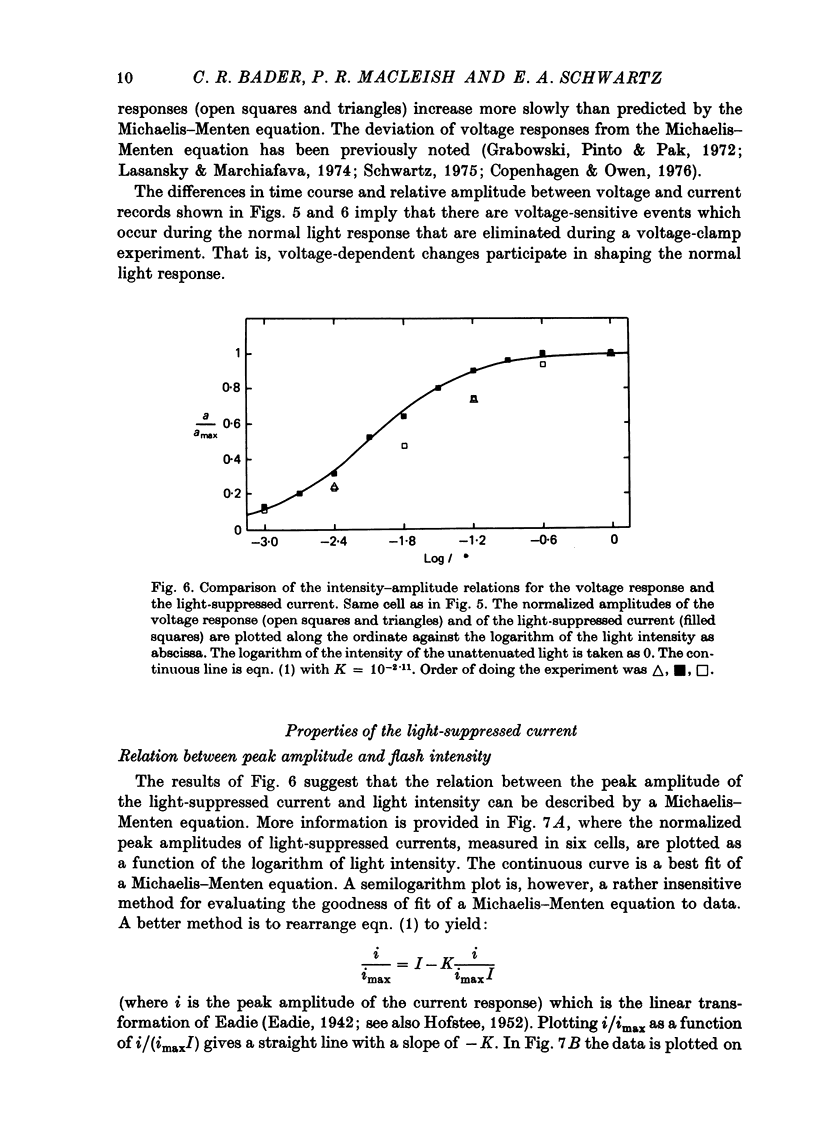
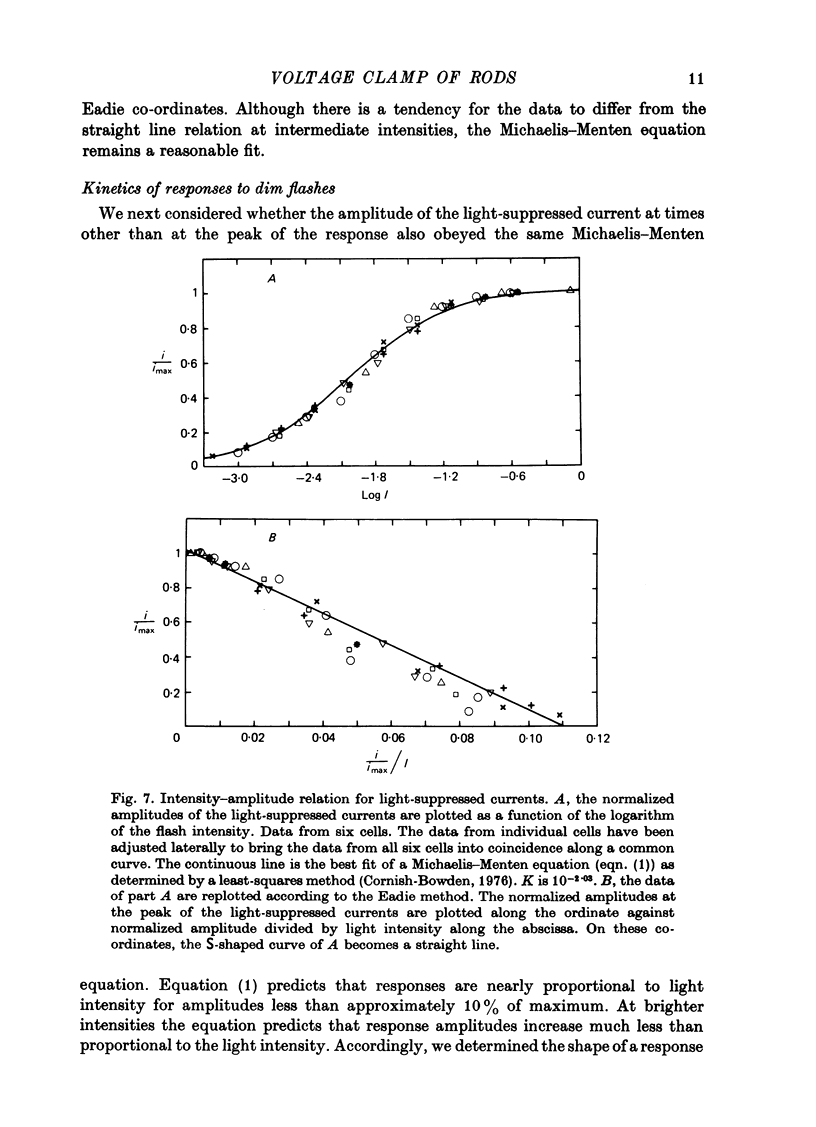




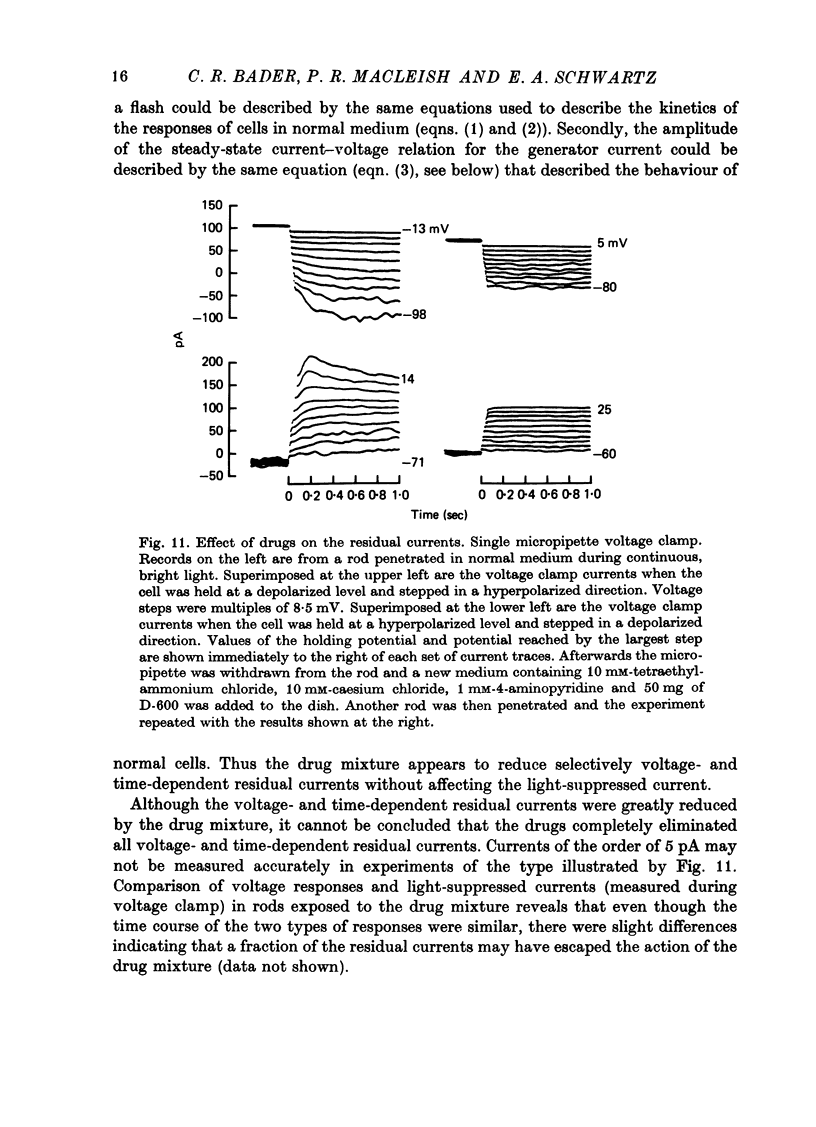










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., MacLeish P. R., Schwartz E. A. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3507–3511. doi: 10.1073/pnas.75.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. A surprising property of electrical spread in the network of rods in the turtle's retina. Nature. 1978 Aug 10;274(5671):562–565. doi: 10.1038/274562a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Gold G. H., Dowling J. E. Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol. 1976;40:547–561. doi: 10.1101/sqb.1976.040.01.051. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Gerschenfeld H. M. Calcium-dependent regenerative responses in rods. Nature. 1977 Oct 20;269(5630):707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- Grabowski S. R., Pinto L. H., Pak W. L. Adaptation in retinal rods of axolotl: intracellular recordings. Science. 1972 Jun 16;176(4040):1240–1243. doi: 10.1126/science.176.4040.1240. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976 Dec;263(2):257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Voltage noise observed in rods of the turtle retina. J Physiol. 1977 Nov;272(2):217–246. doi: 10.1113/jphysiol.1977.sp012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. II. On the basis of the ionic mechanism. Vision Res. 1969 Dec;9(12):1443–1451. doi: 10.1016/0042-6989(69)90060-1. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


