Abstract
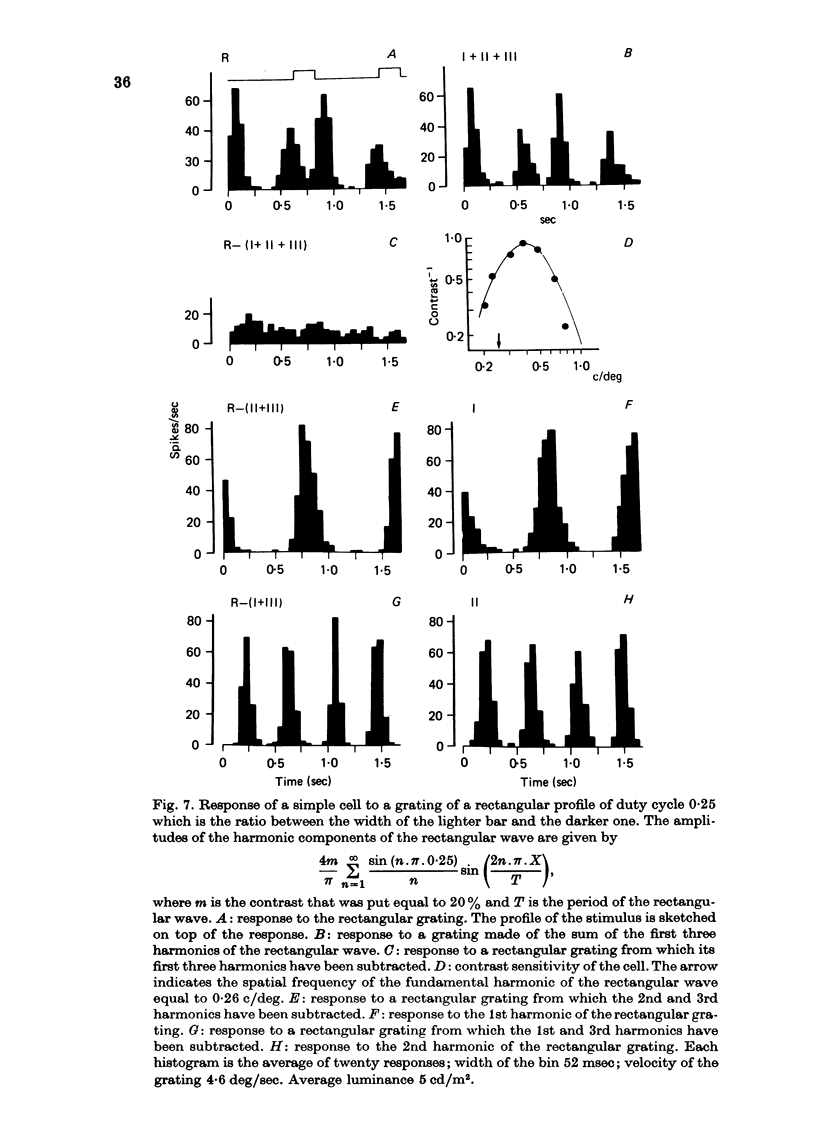
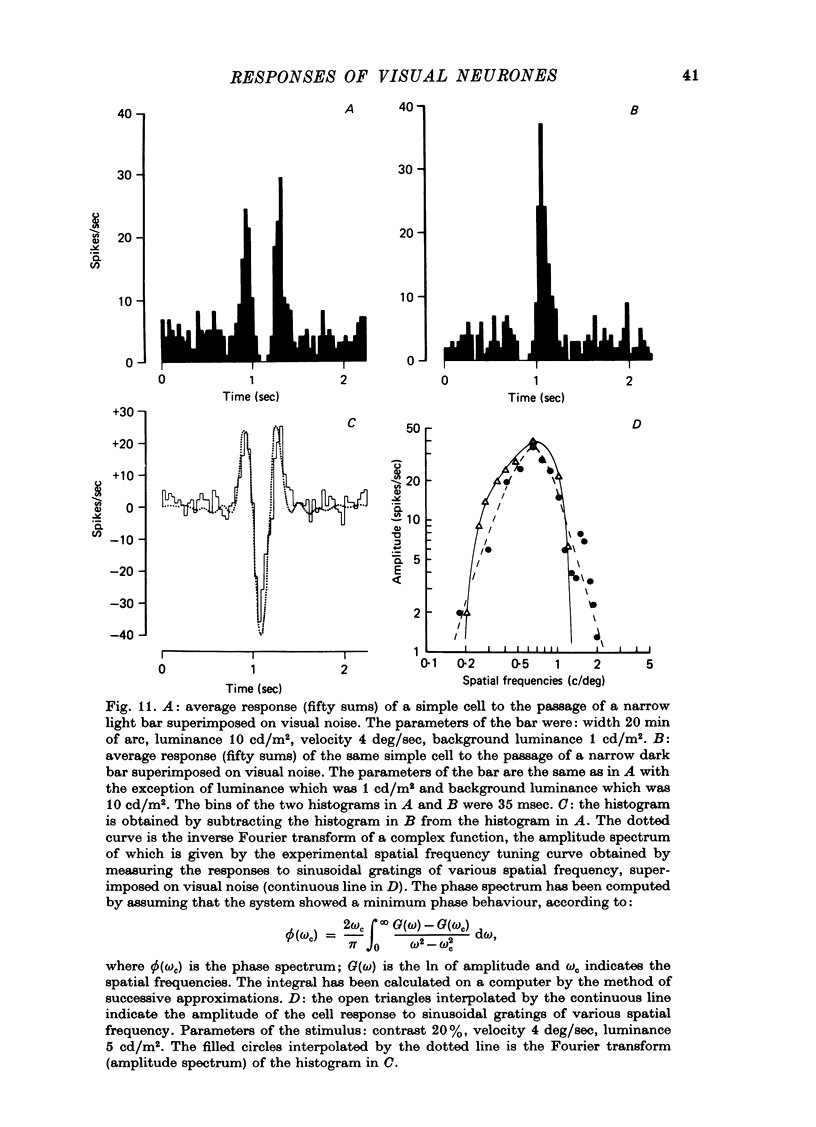
1. The activity of neurones of the visual cortex (area 17) has been recorded in anaesthetized cats in response to gratings of different profile and to single light and dark bars. 2. At very low spatial frequencies, outside the frequency response range to sinusoidal gratings, the response to square-wave drifting gratings is obtainable from a combination of the response to the single bars of the grating presented in isolation. At higher spatial frequencies this is no longer true. 3. At very low spatial frequencies the responses to square-wave gratings and to missing-fundamental gratings (obtained by subtraction from the square-wave grating of its fundamental gratings (obtained by subtraction from the square-wave grating of its fundamental harmonic) are very similar. 4. At spatial frequencies near the peak of the spatial frequency tuning curve of the cell, the responses to square-wave grating and to sinusoidal gratings are very similar. At these spatial frequencies the response to the missing-fundamental grating is practically zero. 5. At spatial frequencies lower than that of best sensitivity for the cell, the response to square-wave gratings is correlated with the 1st and 3rd harmonic of the stimulus. 6. We conclude that at very low spatial frequencies of the grating the response of cortical cells is correlated with the light or dark edges (or light or dark bars) of the stimulus, because the edges contain high frequencies within the range of sensitivity of the cells. At higher spatial frequencies the results are interpreted best by assuming that cortical cells respond to the harmonics of the visual periodic stimulus. 7. When a background of dynamic visual noise is added to increase the spontaneous discharge of simple cells, their response to visual stimuli becomes linear or quasi-linear. The stimuli could be either single bars or gratings.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. W., Pollen D. A. Relationship between spatial frequency selectivity and receptive field profile of simple cells. J Physiol. 1979 Feb;287:163–176. doi: 10.1113/jphysiol.1979.sp012652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Howell E. R., Johnstone J. R. A comparison of threshold and suprathreshold appearance of gratings with components in the low and high spatial frequency range. J Physiol. 1978 Nov;284:193–201. doi: 10.1113/jphysiol.1978.sp012535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L. Contrast and spatial frequency. Sci Am. 1974 Nov;231(5):106–114. doi: 10.1038/scientificamerican1174-106. [DOI] [PubMed] [Google Scholar]
- De Valois K. K., De Valois R. L., Yund E. W. Responses of striate cortex cells to grating and checkerboard patterns. J Physiol. 1979 Jun;291:483–505. doi: 10.1113/jphysiol.1979.sp012827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Sensitivity of neurones in visual cortex (area 17) under different levels of anaesthesia. Exp Brain Res. 1974;20(5):471–484. doi: 10.1007/BF00238014. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Maffei L., Morrone C., Pirchio M., Sandini G. Visual cortical cells as spatial frequency analysers [proceedings]. J Physiol. 1977 Oct;272(1):89P–90P. [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. III. Spatial frequency. J Neurophysiol. 1976 Nov;39(6):1334–1351. doi: 10.1152/jn.1976.39.6.1334. [DOI] [PubMed] [Google Scholar]
- Sullivan G. D., Georgeson M. A. The missing fundamental illusion: variation of spatio-temporal characteristics with dark adaptation. Vision Res. 1977;17(8):977–981. doi: 10.1016/0042-6989(77)90074-8. [DOI] [PubMed] [Google Scholar]