Abstract
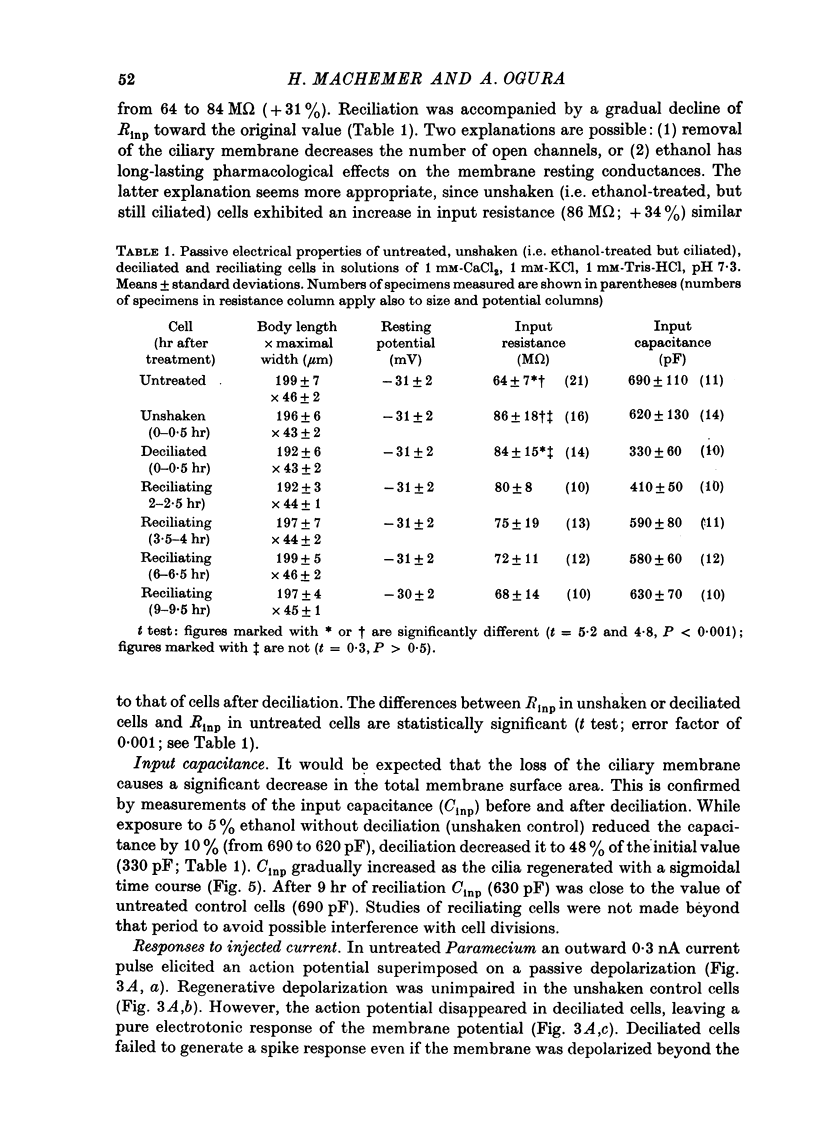
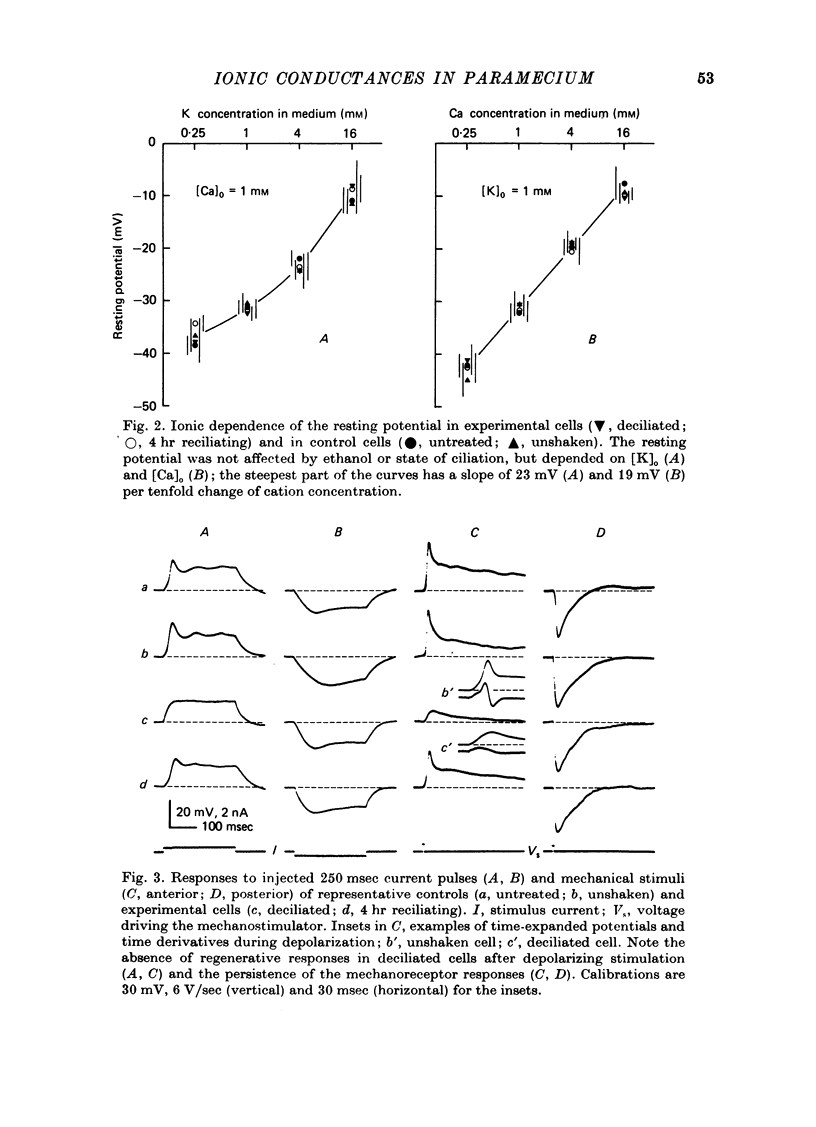
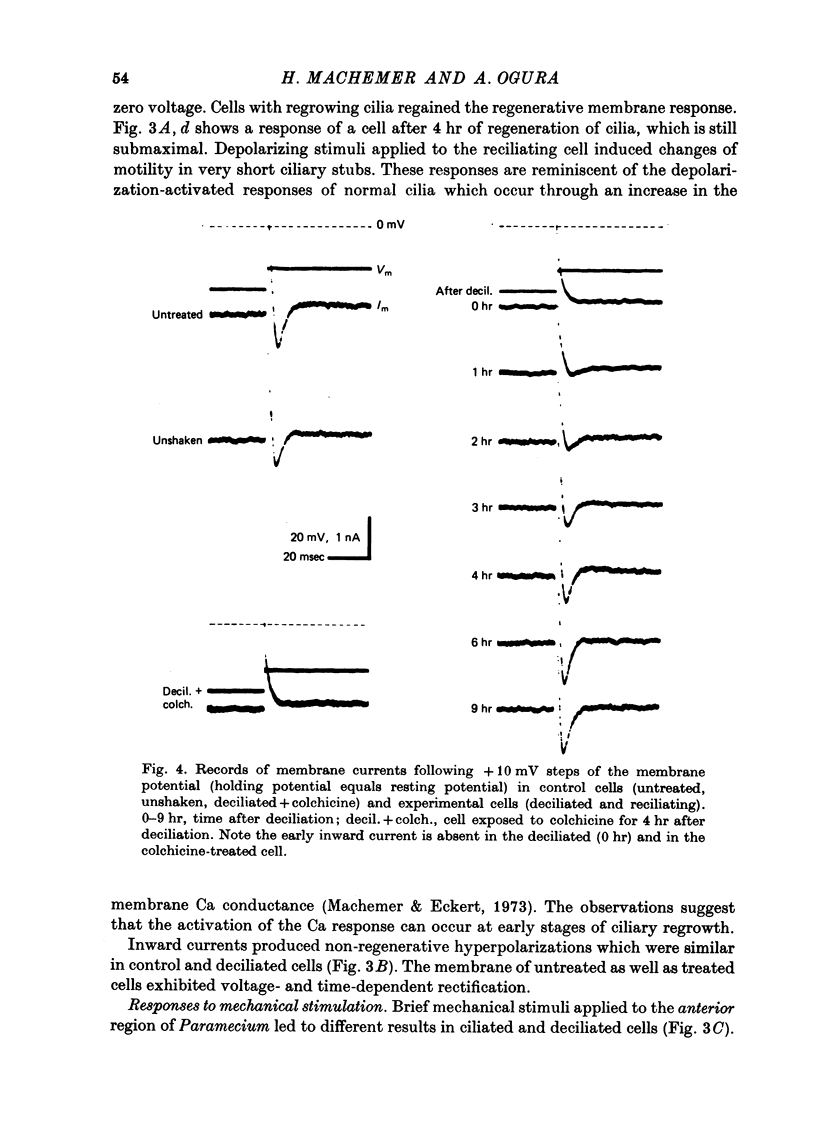
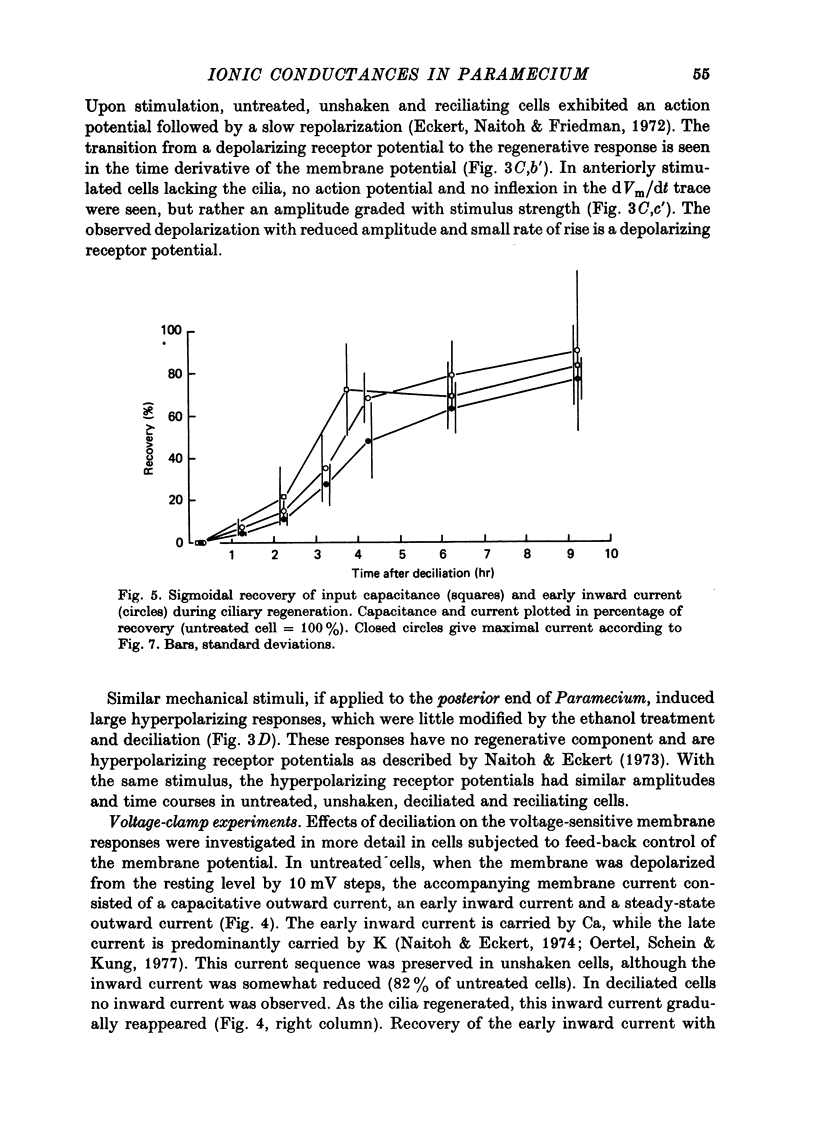
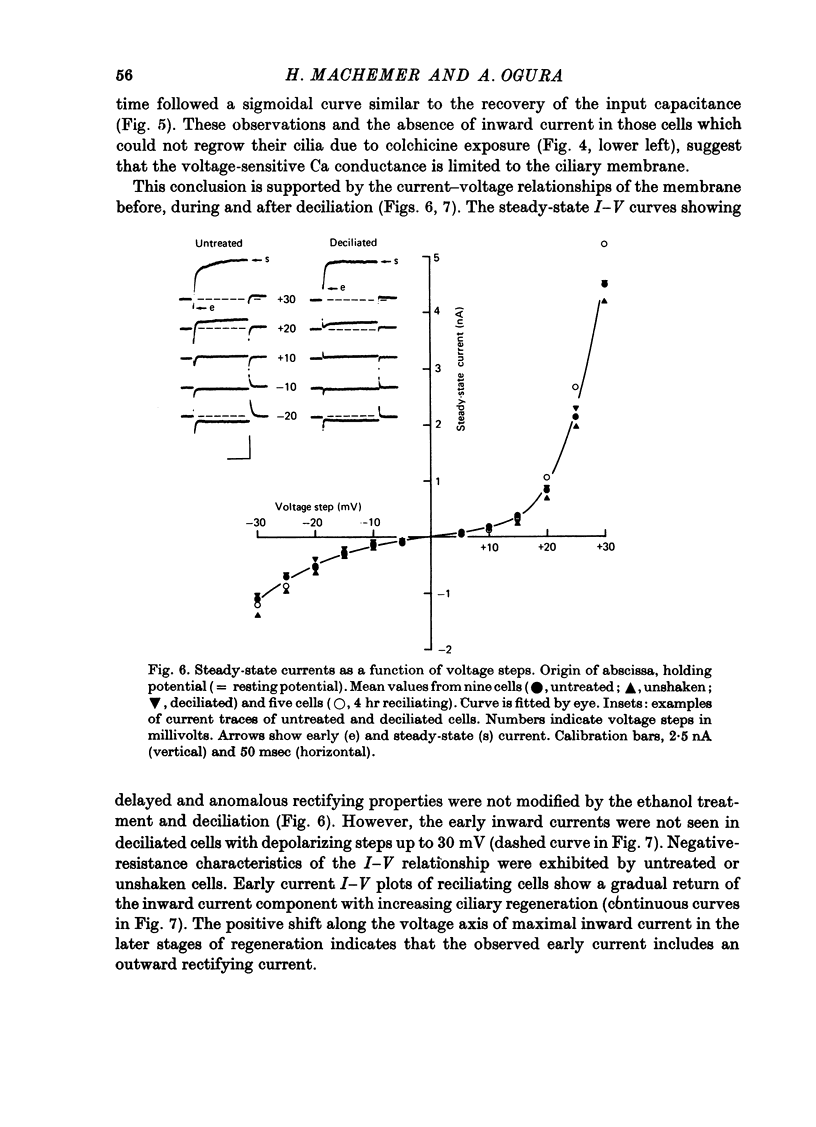
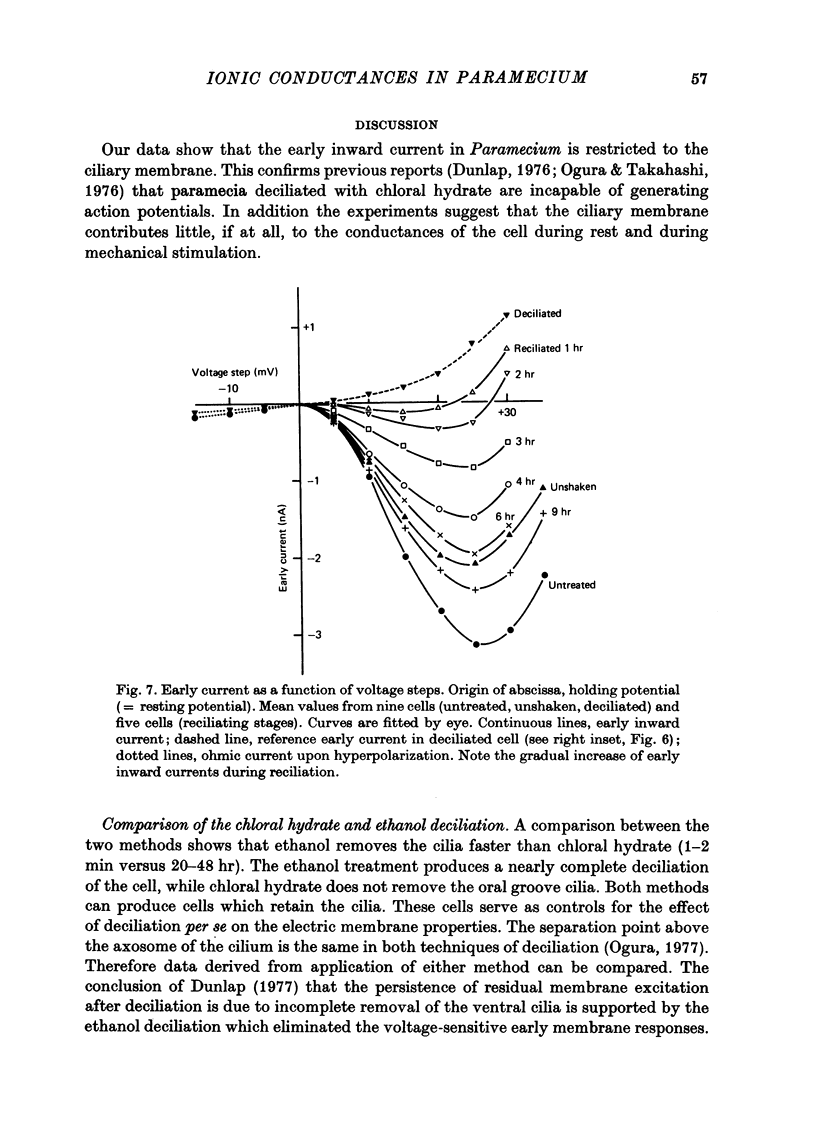
1. Paramecium caudatum was deciliated with ethanol. The ionic conductance of the membrane was investigated with constant current, voltage clamp and mechanical stimuli. 2. The resting potential was not modified by the removal of the cilia. The dependence of the resting potential on the extracellular concentrations of Ca and K was the same in deciliated and control cells. 3. The input resistance in deciliated and ciliated cells increased after the ethanol treatment. 4. The membrane capacitance decreased to 48% after deciliation, suggesting that the ciliary surface area is equal to the somatic surface area. 5. Deciliation completely removed the regenerative response (graded action potential) elicited by depolarizing current pulses or mechanical stimuli. 6. Deciliated cells retained the depolarizing and hyperpolarizing mechanoreceptor responses. 7. Voltage-clamp experiments demonstrated the loss of the early inward current in deciliated cells; it was restored during ciliary regeneration. Steady-state current-voltage relationships were unchanged by deciliation. 8. The time courses of the recovery of the membrane capacitance and of the early inward current were similar, suggesting that the number of voltage-sensitive Ca channels is proportional to the ciliary membrane area. 9. We conclude that the voltage-sensitive Ca channels reside in the ciliary membrane (in confirmation of Dunlap, 1976; Ogura & Takahashi, 1976), while mechanoreceptor channels, rectifier channels and resting conductances are localized in the somatic membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm P., Dunlap K., Eckert R. Calcium-dependent repolarization in Paramecium. J Physiol. 1978 Jan;274:639–654. doi: 10.1113/jphysiol.1978.sp012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eckert R., Naitoh Y., Friedman K. Sensory mechanisms in Paramecium. I. Two components of the electric response to mechanical stimulation of the anterior surface. J Exp Biol. 1972 Jun;56(3):683–694. doi: 10.1242/jeb.56.3.683. [DOI] [PubMed] [Google Scholar]
- Kung C., Eckert R. Genetic modification of electric properties in an excitable membrane (paramecium-calcium conductance-electrophysiological measurements-membrane mutant). Proc Natl Acad Sci U S A. 1972 Jan;69(1):93–97. doi: 10.1073/pnas.69.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machemer H., Eckert R. Electrophysiological control of reversed ciliary beating in Paramecium. J Gen Physiol. 1973 May;61(5):572–587. doi: 10.1085/jgp.61.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machemer H. Motor activity and bioelectric control of cilia. Fortschr Zool. 1977;24(2-3):195–210. [PubMed] [Google Scholar]
- Oertel D., Schein S. J., Kung C. Separation of membrane currents using a Paramecium mutant. Nature. 1977 Jul 14;268(5616):120–124. doi: 10.1038/268120a0. [DOI] [PubMed] [Google Scholar]
- Ogura A., Takahashi K. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature. 1976 Nov 11;264(5582):170–172. doi: 10.1038/264170a0. [DOI] [PubMed] [Google Scholar]
- de Peyer J., Machemer H. Are receptor-activated ciliary motor responses mediated through voltage or current? Nature. 1978 Nov 16;276(5685):285–287. doi: 10.1038/276285a0. [DOI] [PubMed] [Google Scholar]



