Abstract
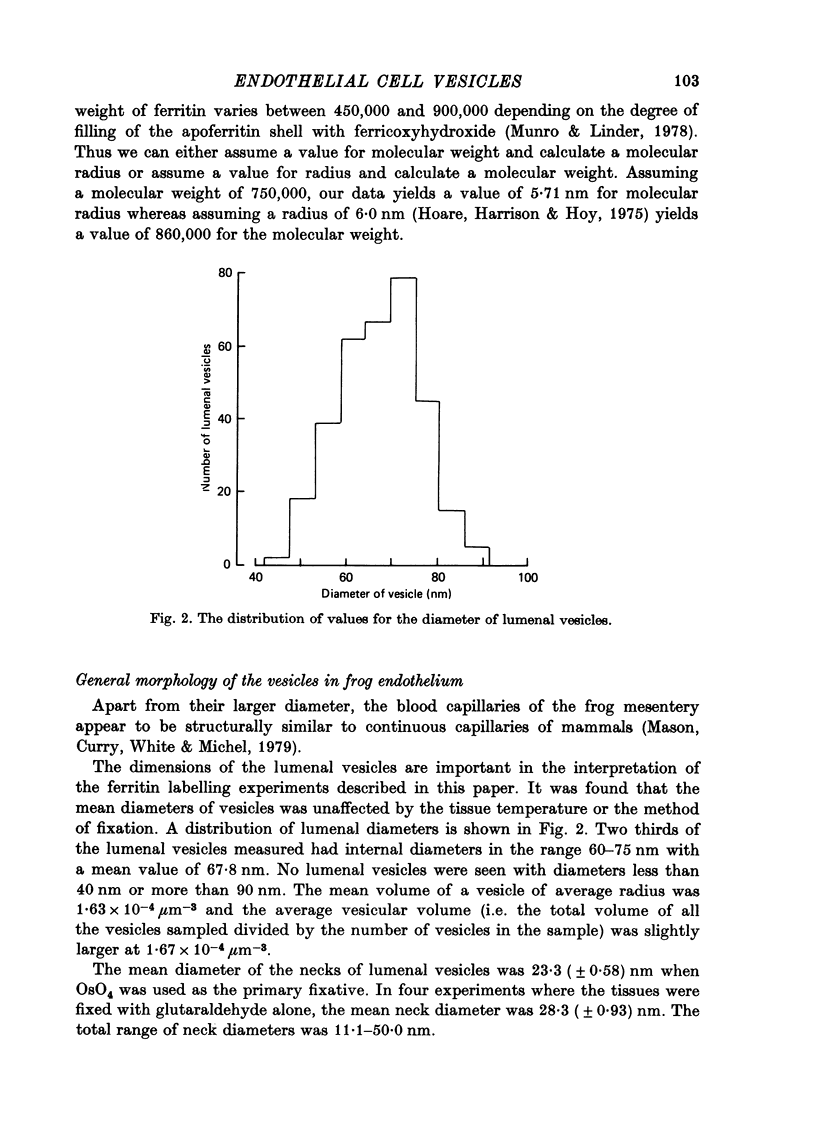
1. The labelling of endothelial cell vesicles with ferritin has been investigated by electron microscopy. Single capillaries in the frog mesentery have been perfused with solutions of known concentrations of ferritin for known periods before fixing the tissue in situ by superfusion with osmium tetroxide. 2. At 17 degrees C, the percentage of lumenal vesicles labelled with ferritin increased as the period of perfusion was increased up to 16 sec prior to fixation. When perfusions were longer than 16 sec, the percentage of vesicles labelled with ferritin remained fairly constant at 70%. 3. At 3 degrees C, no more than 25% of the lumenal vesicles were labelled during the first 30 sec. 4. After correcting the data for losses of ferritin due to sectioning, the distribution of ferritin molecules in the lumenal vesicles was consistent with a Poisson distribution. 5. After perfusions of 16 sec or longer, the number of ferritin molecules per labelled vesicle was roughly three to four times less than would be predicted from the lumenal concentration. 6. At all times there was a gradient of vesicles labelled with ferritin across the endothelial cells, i.e. the percentage of lumenal vesicles labelled was greater than that for cytoplasmic vesicles which in turn was greater than that for vesicles at the ablumenal surface. 7. Whereas the labelling of lumenal vesicles from zero time up to 16-20 sec, the main increase in labelling of cytoplasmic vesicles occurred between 10 and 20 sec. 8. It is concluded that there is a major diffusion barrier to ferritin molecules either close to the endothelial cell surface or across the necks of the lumenal vesicles. It also appears that ferritin molecules do not have access to vesicles during the latter part of their residence at the lumenal surface.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. R., Palade G. E. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968 May;37(2):244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. R., Palade G. E. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968 May;37(2):277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith J. R., Chin J. C. The passage of cytoplasmic vesicles across endothelial and mesothelial cells. J Microsc. 1971 Jun;93(3):167–189. doi: 10.1111/j.1365-2818.1971.tb02280.x. [DOI] [PubMed] [Google Scholar]
- Clementi F., Palade G. E. Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol. 1969 Apr;41(1):33–58. doi: 10.1083/jcb.41.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. S., Casley-Smith J. R. Calculations on the passage of small vesicles across endothelial cells by Brownian motion. J Theor Biol. 1972 Apr;35(1):103–111. doi: 10.1016/0022-5193(72)90195-6. [DOI] [PubMed] [Google Scholar]
- Helander H. F., Rehm W. S., Sanders S. S. Influence of fixation on physiological properties of frog gastric mucosa. Acta Physiol Scand. 1973 May;88(1):109–122. doi: 10.1111/j.1748-1716.1973.tb05438.x. [DOI] [PubMed] [Google Scholar]
- Hoare R. J., Harrison P. M., Hoy T. G. Structure of horse-spleen apoferritin at 6 angstom resolution. Nature. 1975 Jun 19;255(5510):653–654. doi: 10.1038/255653a0. [DOI] [PubMed] [Google Scholar]
- Jennings M. A., Florey L. An investigation of some properties of endothelium related to capillary permeability. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):39–63. doi: 10.1098/rspb.1967.0012. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The permeability of individually perfused frog mesenteric capillaries to T1824 and T1824-albumin as evidence for a large pore system. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):67–85. doi: 10.1113/expphysiol.1973.sp002192. [DOI] [PubMed] [Google Scholar]
- Loundon M. F., Michel C. C., White I. F. Proceedings: Some observations upon the rate of labelling of endothelial vesicles by ferritin in frog mesenteric capillaries. J Physiol. 1975 Nov;252(2):79P–80P. [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. TRANSPORT OF LARGE MOLECULES ACROSS CAPILLARY WALLS. Physiologist. 1964 Feb;60:13–28. [PubMed] [Google Scholar]
- Renkin E. M. Multiple pathways of capillary permeability. Circ Res. 1977 Dec;41(6):735–743. doi: 10.1161/01.res.41.6.735. [DOI] [PubMed] [Google Scholar]
- Shea S. M., Karnovsky M. J., Bossert W. H. Vesicular transport across endothelium: simulation of a diffusion model. J Theor Biol. 1969 Jul;24(1):30–42. doi: 10.1016/s0022-5193(69)80004-4. [DOI] [PubMed] [Google Scholar]
- Shea S. M., Karnovsky M. J. Brownian motion: a theoretical explanation for the movement of vesicles across the endothelium. Nature. 1966 Oct 22;212(5060):353–355. doi: 10.1038/212353a0. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. The role of mucopolysaccharides in vesicle architecture and endothelial transport. An electron microscope study of myocardial blood vessels. J Cell Biol. 1972 Jan;52(1):198–206. doi: 10.1083/jcb.52.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin S. G. Vesicular transport across endothelial cells. Biochim Biophys Acta. 1969;183(3):559–564. doi: 10.1016/0005-2736(69)90169-2. [DOI] [PubMed] [Google Scholar]



