Abstract
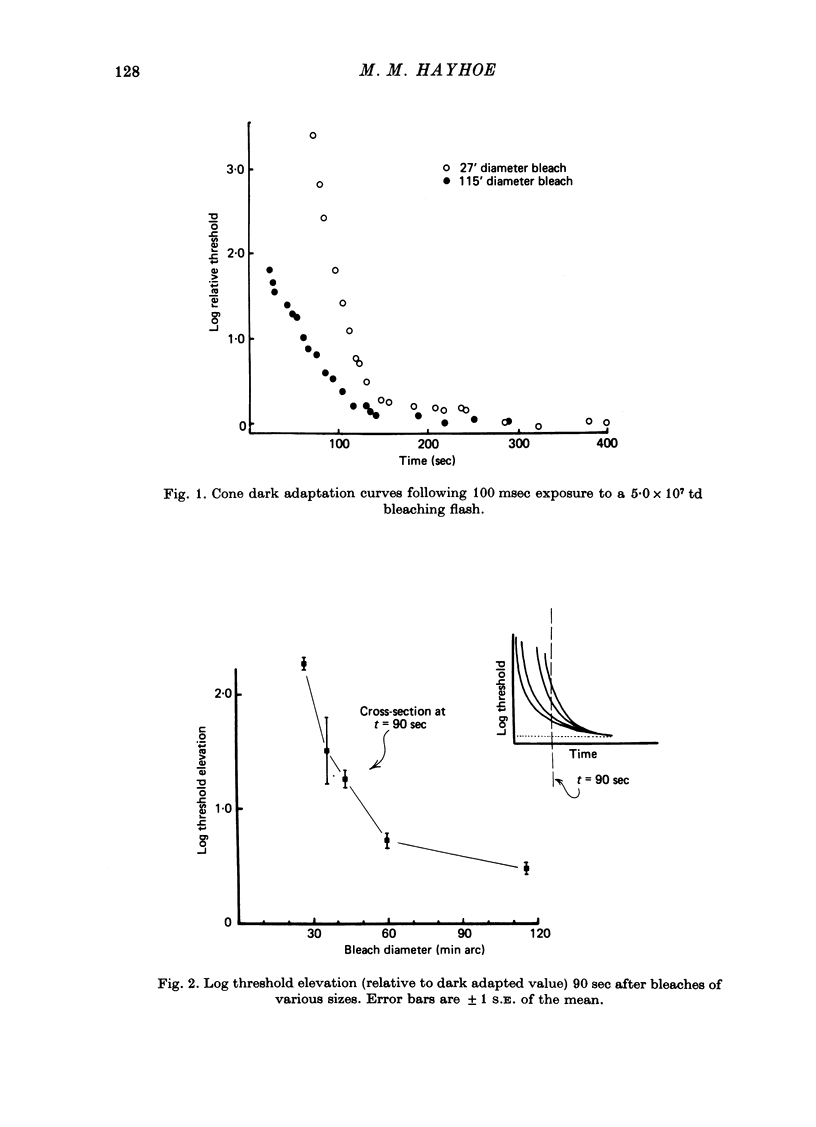
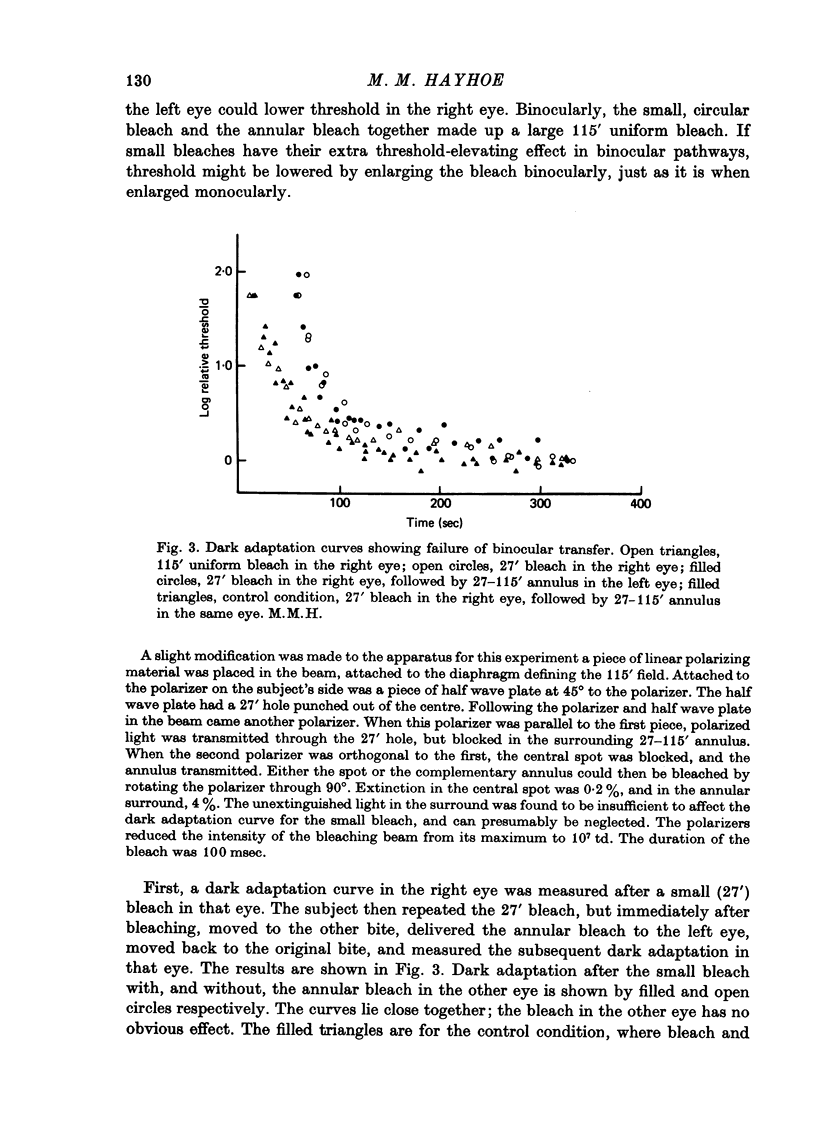
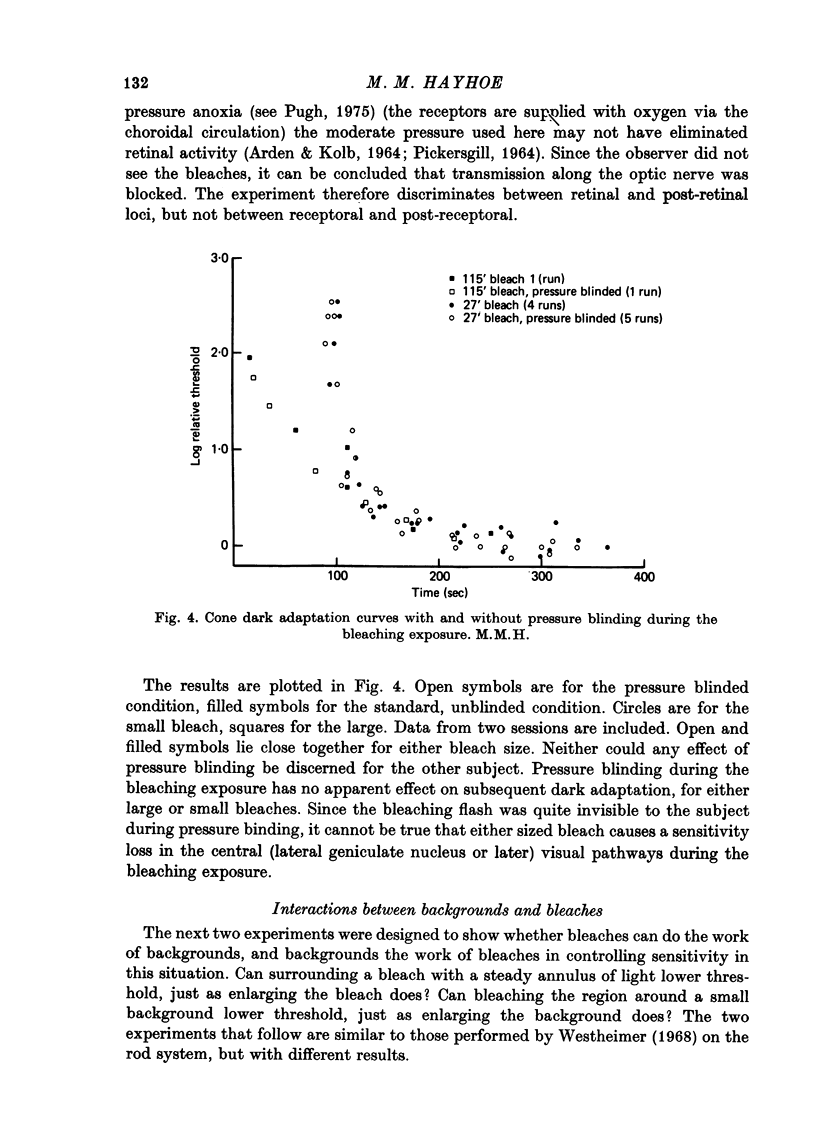
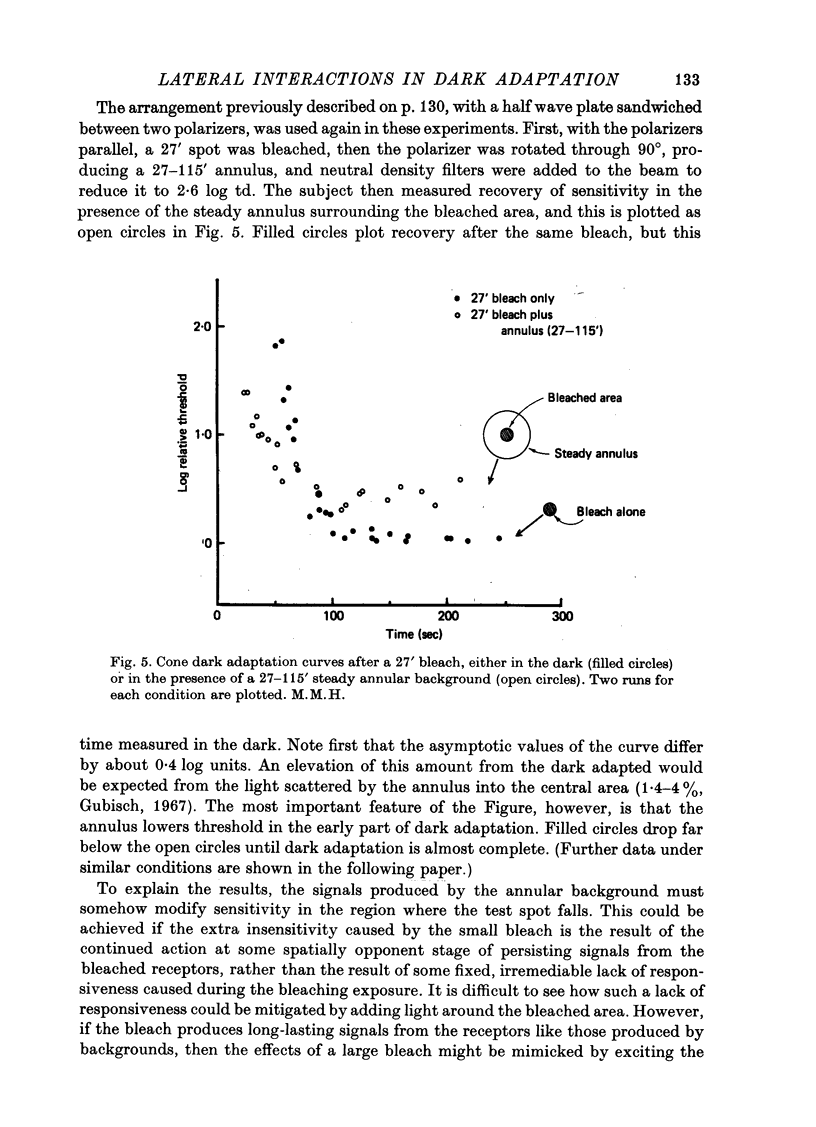
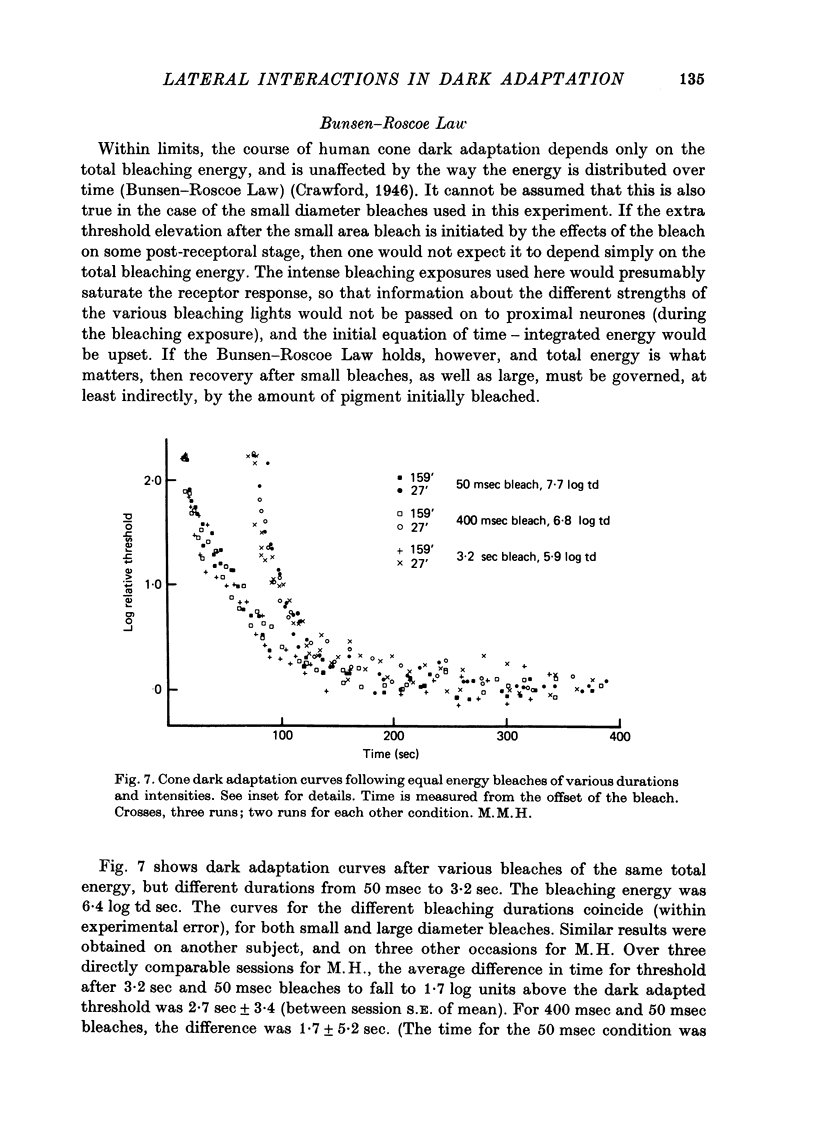
1. The course of cone dark adaptation after exposure to a strong bleaching light depends on the size of the bleached region. Threshold for brief, tiny test flash centred in the bleached region is elevated more, and recovery is retarded by a small bleach. This effect has its parallel in the sensitization effect observed with steady backgrounds. 2. Previous results, that a similar sensitization effect is not observed in rod dark adaptation, are confirmed. 3. This sensitization effect in cone dark adaptation does not transfer binocularly, and is unaffected by pressure blinding during the bleaching exposure. 4. Threshold following a small bleach may be lowered by adding a steady annular background to the region surrounding the bleached patch. Conversely, bleaching the area surrounding a small, steady background can lower threshold for a test flash centred on the background. 5. These interactions between backgrounds and bleaches may be explained if bleaches produce long-lasting signals from neurones in the bleached area, which then lead into a spatially opponent stage of processing. 6. It is likely that the persisting signals come from the cone receptors, since the Bunsen-Roscoe Law (intensity-time reciprocity) holds for small bleaches as well as large, for durations up to about 3 sec.
Full text
PDF




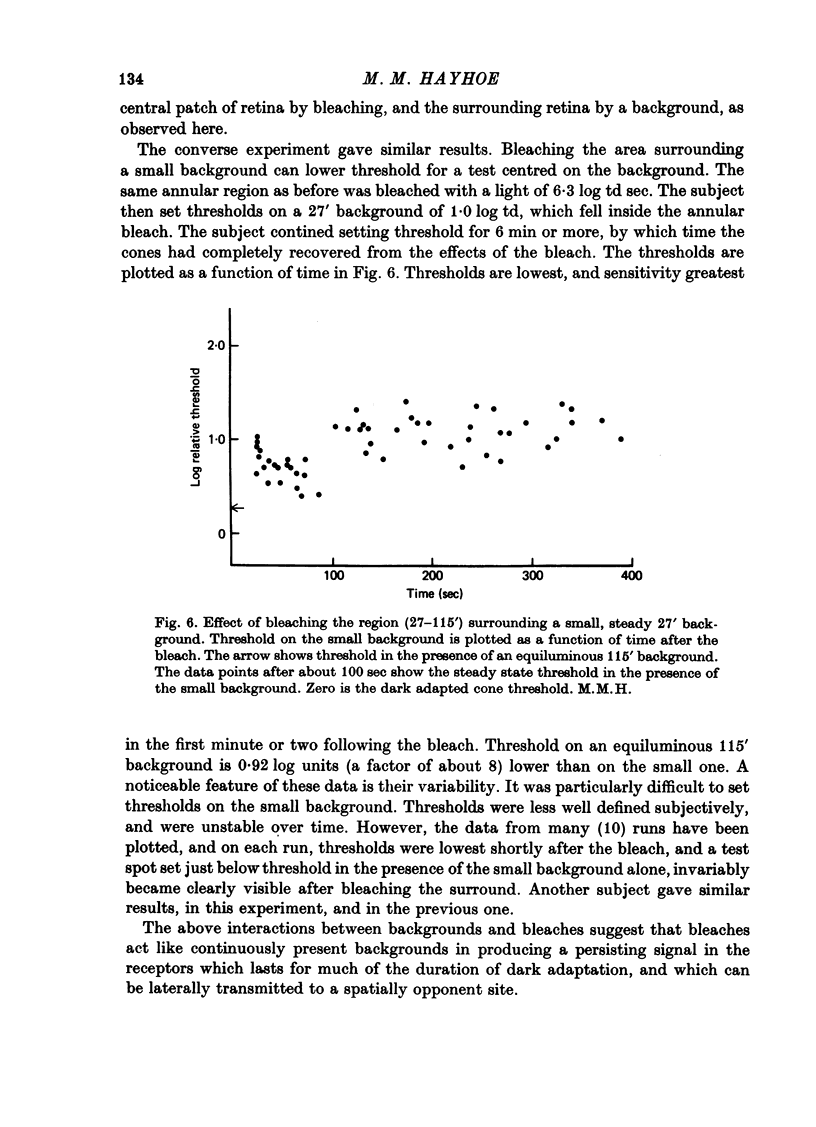





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B. Dark-adaptation: a new hypothesis. Vision Res. 1964 May;4(1):47–58. doi: 10.1016/0042-6989(64)90031-8. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Sensitization and centre-surround antagonism in Necturus retina. J Physiol. 1974 Feb;236(3):593–610. doi: 10.1113/jphysiol.1974.sp010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G., Dowling J. E., Siegel I. M., Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol. 1975 Apr;65(4):483–502. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe M. M. After-effects of small adapting fields. J Physiol. 1979 Nov;296:141–158. doi: 10.1113/jphysiol.1979.sp012996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latch M., Lennie P. Rod-cone interaction in light adaptation. J Physiol. 1977 Aug;269(3):517–534. doi: 10.1113/jphysiol.1977.sp011912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P., MacLeod D. I. Background configuration and rod threshold. J Physiol. 1973 Aug;233(1):143–156. doi: 10.1113/jphysiol.1973.sp010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. I. Visual sensitivity. Annu Rev Psychol. 1978;29:613–645. doi: 10.1146/annurev.ps.29.020178.003145. [DOI] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Specificity of cone mechanisms in lateral interaction. J Physiol. 1970 Jan;206(1):117–128. doi: 10.1113/jphysiol.1970.sp009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D. A., Leibowitz H. W. The fixation point as a stimulus for accommodation. Vision Res. 1975 Oct;15:1161–1163. doi: 10.1016/0042-6989(75)90016-4. [DOI] [PubMed] [Google Scholar]
- PICKERSGILL M. J. AFTER-EFFECT OF MOVEMENT IN THE STIMULATED AND OPPOSITE EYES DURING AND AFTER PRESSURE BLINDING. Nature. 1964 May 23;202:833–834. doi: 10.1038/202833a0. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N. Rushton's paradox: rod dark adaptation after flash photolysis. J Physiol. 1975 Jun;248(2):413–431. doi: 10.1113/jphysiol.1975.sp010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Threshold changes near the light-dark border: a comparison of real and equivalent background lights. Vision Res. 1977;17(1):117–122. doi: 10.1016/0042-6989(77)90209-7. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Gestrin P. J. Sensitization by annular surrounds: sensitization and dark adaptation. Vision Res. 1969 Dec;9(12):1481–1489. doi: 10.1016/0042-6989(69)90064-9. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Matter C., Phillips W. D., Alexander K. Sensitization by annular surrounds: sensitization and masking. Vision Res. 1971 Dec;11(12):1445–1458. doi: 10.1016/0042-6989(71)90065-4. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U., Jones R. M. Spatial sensitization as a function of delay. Vision Res. 1977;17(10):1191–1199. doi: 10.1016/0042-6989(77)90153-5. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U., Vassilev A. Foveal spatial sensitization with stabilized vision. Vision Res. 1974 Jan;14(1):101–105. doi: 10.1016/0042-6989(74)90122-9. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Bleached rhodopsin and retinal interaction. J Physiol. 1968 Mar;195(1):97–105. doi: 10.1113/jphysiol.1968.sp008448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in human cone vision. J Physiol. 1967 May;190(1):139–154. doi: 10.1113/jphysiol.1967.sp008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]


