Abstract
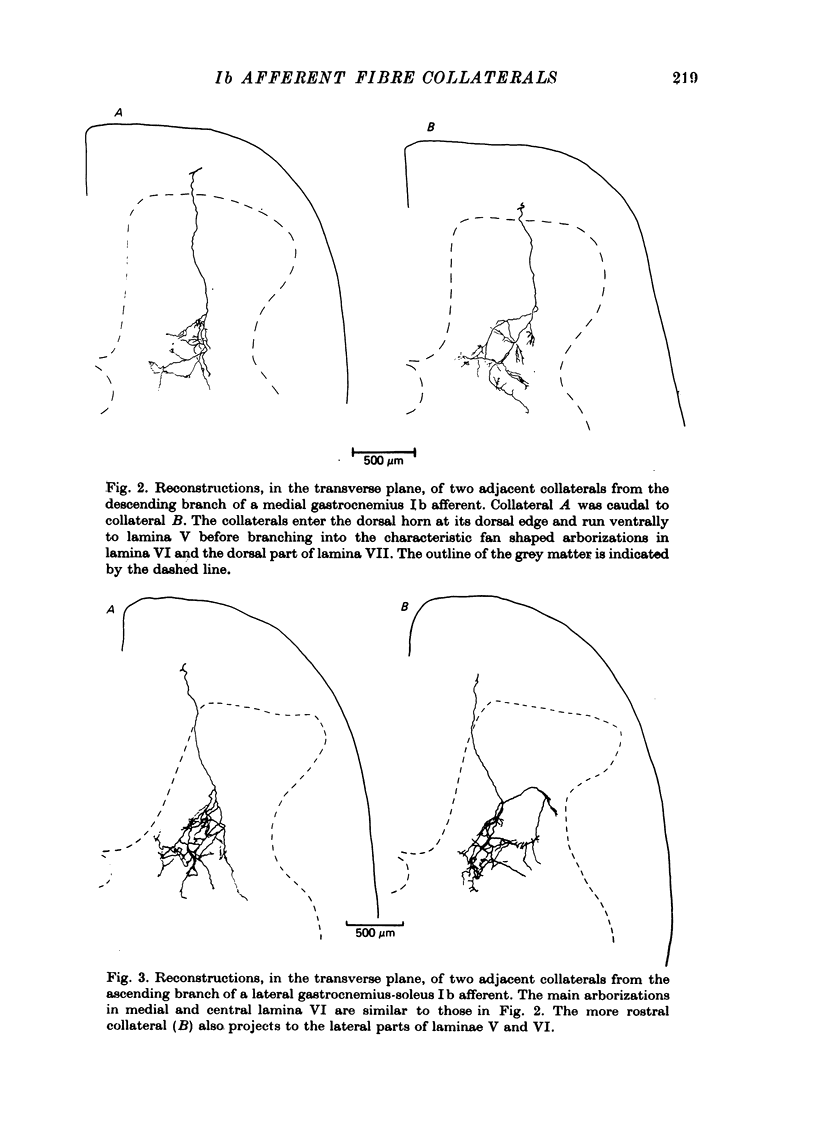
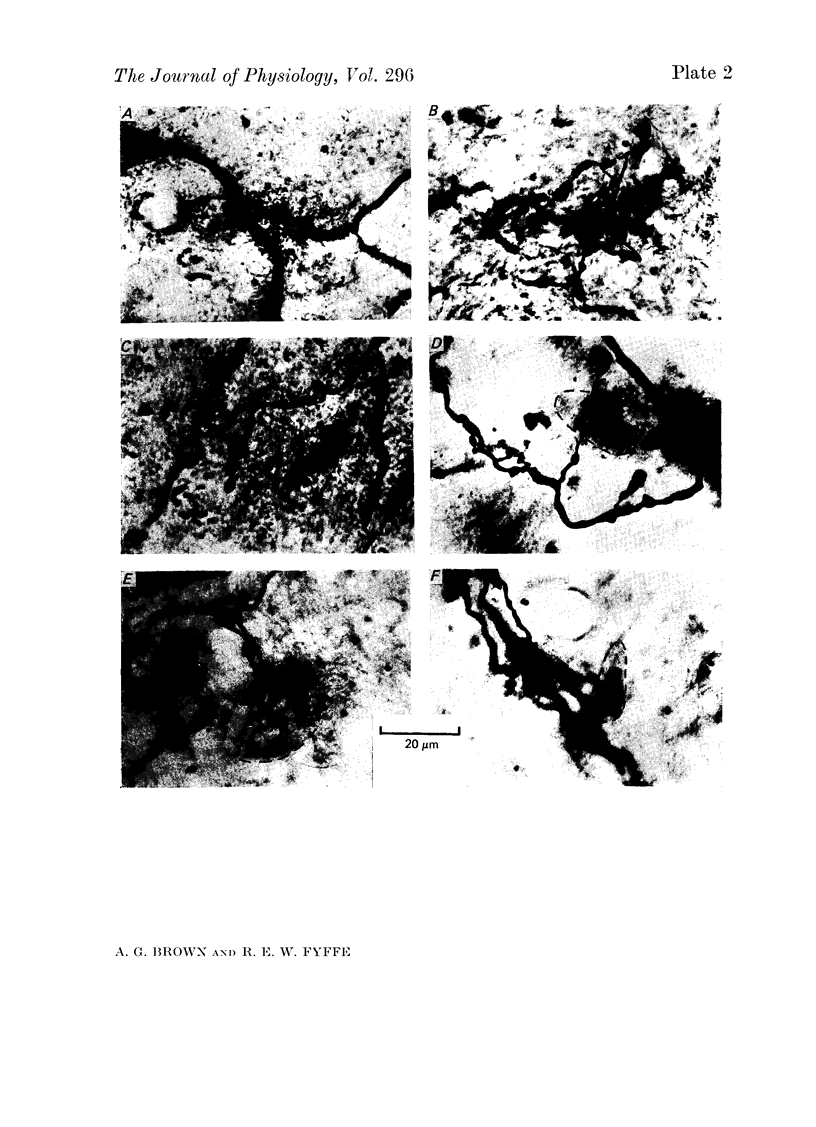
1. The enzyme horseradish peroxidase (HRP) was injected into single Ib muscle afferent fibres in anaesthetized cats. Subsequently, histochemistry allowed the morphology of the axons and their collaterals in the lumbosacral spinal cord to be determined. 2. Eleven Ib axons were stained, seven from lateral gastrocneminus-soleus, one from medial gastrocnemius and three from muscles innervated by the posterior tibial nerve. Ten of the axons were traced into the dorsal roots and all but one (from the posterior tibial nerve) bifurcated upon entering the cord. Between 5.1 and 9.9 mm of each axon was stained and the fibres gave off eighty-four collaterals at intervals of 100-2300 micron, at an average spacing of about 900 micron. The spacing between collaterals on the (finer) descending axon branches was generally less than the intervals between collaterals on ascending branches. 3. All Ib collaterals had a characteristic morphology. The collaterals coursed cranially on a direct path through the dorsal horn to lamina IV or V before branching. They arborized widely in the intermediate region, mainly in lamina VI and in the dorsal part of lamina VII. Occasionally, less extensive arborizations were seen more dorsally in lamina IV and V. The rostro-caudal extent of individual collateral arborizations was limited to 200-400 micron and there was no overlap between adjacent collaterals. Each terminal arborization gave rise to 56-384 boutons, mainly of them 'en passant' type. 4. The results are discussed in relation to previous anatomical and electrophysiological studies.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G. Cutaneous axons and sensory neurones in the spinal cord. Br Med Bull. 1977 May;33(2):109–112. doi: 10.1093/oxfordjournals.bmb.a071409. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Synaptic contacts made by identified Ia afferent fibres upon motoneurones [proceedings]. J Physiol. 1978 Nov;284:43P–44P. [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of Group Ib muscle afferent fibre collaterals [proceedings]. J Physiol. 1978 Apr;277:44P–45P. [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. Morphology and organization of axon collaterals from afferent fibres of slowly adapting type I units in cat spinal cord. J Physiol. 1978 Apr;277:15–27. doi: 10.1113/jphysiol.1978.sp012257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R., Lundberg A., Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):283–294. doi: 10.1007/BF00237921. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., HUBBARD J. I., OSCARSSON O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol. 1961 Oct;158:486–516. doi: 10.1113/jphysiol.1961.sp006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., OSCARSSON O. Localization of the cell bodies of the ventral spino-cerebellar tract in lumbar segments of the cat. J Comp Neurol. 1962 Apr;118:199–204. doi: 10.1002/cne.901180206. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Lindström S. Morphology of interneurones mediating Ia reciprocal inhibition of motoneurones in the spinal cord of the cat. J Physiol. 1972 Nov;226(3):805–823. doi: 10.1113/jphysiol.1972.sp010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- Lucas M. E., Willis W. D. Identification of muscle afferents which activate interneurons in the intermediate nucleus. J Neurophysiol. 1974 Mar;37(2):282–293. doi: 10.1152/jn.1974.37.2.282. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12(3):317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSCARSSON O. FUNCTIONAL ORGANIZATION OF THE SPINO- AND CUNEOCEREBELLAR TRACTS. Physiol Rev. 1965 Jul;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal patterns in cat spinal cord. 3. Primary afferent collaterals. Brain Res. 1969 May;13(3):417–443. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]