Abstract
Although equine diphtheria antitoxin may be an effective therapy for human diphtheria, its use often induces serum sickness. We describe here a strategy for developing an alternative treatment based on the human diphtheria toxin (DT) receptor/heparin-binding epidermal growth factor-like growth factor (HB-EGF) precursor. Recombinant mature human HB-EGF acts as a soluble receptor analog, binding radioiodinated DT and preventing its binding to the cellular DT receptor/HB-EGF precursor. However, the possibility existed that radioiodinated DT-HB-EGF complexes associate with cells due to the binding of the heparin-binding domain of recombinant HB-EGF to cell surface heparan sulfate proteoglycans. This possibility was confirmed by performing DT binding studies in the presence of heparin. A recombinant truncated HB-EGF (residues 106 to 149), which lacks most of the heparin-binding domain, showed an essentially heparin-independent binding of radioiodinated DT to cells. Furthermore, it was a more effective inhibitor of DT binding than was recombinant mature HB-EGF. Since mature HB-EGF is a known ligand for the EGF receptor and is thus highly mitogenic (tumorigenic), we then changed amino acid residues in the EGF-like domain of the recombinant truncated HB-EGF and demonstrated that this DT receptor analog (I117A/L148A) displayed a low mitogenic effect. The truncated (I117A/L148A) HB-EGF protein retained high DT binding affinity, as confirmed by using surface plasmon resonance. Our results suggest that the truncated (I117A/L148A) HB-EGF protein could be an effective, safe antidote for human diphtheria.
Although several years ago it appeared that diphtheria was no longer a major public health threat, the recent resurgence of diphtheria in the New Independent States of the former Soviet Union (8, 9), Ecuador (35), Thailand (7), Algeria, and elsewhere (21) suggests that this old menace remains a significant global health problem. Historically, diphtheria patients have been treated with equine antitoxin, which neutralizes unbound toxin; however, surviving patients have often developed serum sickness, an immune complex-type disease. Thus, a better treatment for diphtheria patients is needed. Diphtheria toxin (DT) is a Corynebacterium diphtheriae exotoxin that inhibits protein synthesis of toxin-sensitive eukaryotic cells. DT is translated as a single polypeptide of 535 amino acid residues (Mr, 58,342). Partial proteolysis yields a disulfide-linked polypeptide composed of two fragments: the enzymatic A fragment (Mr, 21,167) and the receptor-binding/translocation-mediating B fragment (Mr, 37,195) (13, 15, 20, 31, 36).
DT binds to cell surface DT receptors and is internalized by receptor-mediated endocytosis (18). Upon subsequent acidification of the endosome, the A fragment is translocated into the cytosol, where the toxin inhibits protein synthesis by ADP ribosylation of elongation factor 2. Although all elongation factor 2s in mammalian species are sensitive to DT (30), only those cells displaying a specific DT receptor are sensitive to the toxin (32).
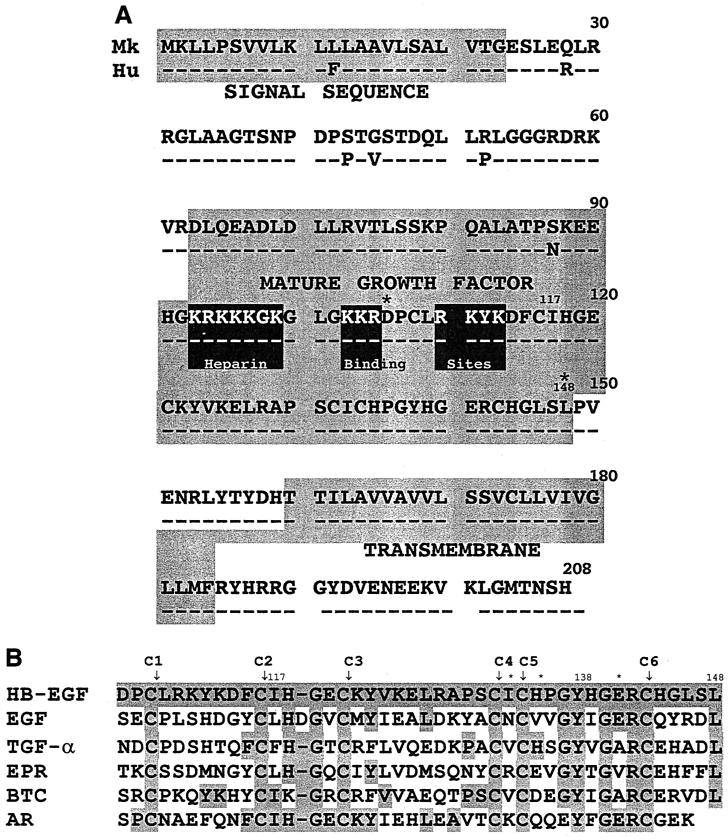
One laboratory previously cloned from highly toxin-sensitive monkey Vero cells the cDNA encoding a DT receptor that conferred DT sensitivity to normally toxin-resistant mouse L-M(TK−) cells (34). The predicted amino acid sequence of the DT receptor was found to be identical to that of the cell surface-expressed heparin-binding epidermal growth factor-like growth factor (HB-EGF) precursor (proHB-EGF) (23). HB-EGF is a member of the EGF growth factor family, which includes EGF (14), transforming growth factor α (TGF-α) (17), epiregulin (44), amphiregulin (AR) (40), betacellulin (BTC) (39), and heregulins (25). Although these members were first purified and identified as secreted mature peptides, subsequent sequencing of their cDNAs revealed that all are derived from membrane-bound precursors that are proteolytically cleaved from the cellular membrane. Although proHB-EGF is cleaved and released as soluble mature HB-EGF (22), a significant amount of proHB-EGF remains on the cell surface and functions as a juxtacrine growth factor (24) and as a DT receptor (27, 34). Soluble mature HB-EGF binds to the EGF receptor and is a more potent mitogen for smooth muscle cells than is EGF (23). In addition to the monkey (Mk) proHB-EGF, the proHB-EGF cDNAs of human (Hu) (23), mouse (1), rat (1, 42), and hamster (11) have been cloned. The predicted structure of proHB-EGFs (Fig. 1A) includes a signal sequence (residues 1 to 23), a pro region (residues 24 to 62), the mature growth factor (residues 63 to 148), a juxtamembrane domain (residues 149 to 159), a transmembrane domain (residues 160 to 184), and a carboxyl-terminal cytoplasmic domain (residues 185 to 208). HB-EGF, like all members of the EGF growth factor family, contains an EGF-like domain, which has 6 conserved cysteine residues that form three disulfide bonds (linked C1—C3, C2—C4, and C5—C6 [Fig. 1B]). This EGF-like motif is important for tertiary structure stabilization, EGF receptor binding, and DT binding.
FIG. 1.
(A) Comparison of Mk (upper line) (34) and human (Hu) (lower line) (23) HB-EGF precursors. Identical amino acid residues are denoted by dashes, and differing residues are denoted by their single-letter amino acid code. The signal sequence, mature growth factor domain, and the transmembrane domain are indicated by shaded boxes and are based on the designations of Abraham et al. (1). Three stretches of basic amino acids in the HB domain (93KRKKKGK, 103KKR, and 110RKYK) are indicated by the three black boxes. Ile117 and Leu148 are indicated by the residue number. The beginning (residue 106) and the end (residue 148) of the EGF-like domain are denoted by asterisks. (B) EGF-like domains sequence comparison of Hu HB-EGF with the other members of the Hu EGF family. EPR, epiregulin. All sequences were obtained from GenBank. The numbers at the top refer to the position of the residues in the Mk HB-EGF precursor, including the signal sequence shown in panel A (34). The shaded boxes indicate residues that are identical to those in Mk HB-EGF. The characteristic six conserved cysteine residues, which form three disulfide bonds (linked C1—C3, C2—C4, and C5—C6), are marked with arrows. Critical residues (I133, H135, and E141) for DT binding are marked with asterisks. The residues noted for Hu EGF correspond to residues 4 to 47 of its mature form, the residues noted for Hu TGF-α correspond to residues 6 to 48 of its mature form, the residues noted for Hu epiregulin correspond to residues 4 to 46 of its mature form, the residues noted for Hu BTC correspond to residues 36 to 78 of its mature form, and the residues noted for Hu AR correspond to residues 44 to 84 of its mature form (41).
The binding of growth factors to cell surfaces and extracellular matrices is often mediated by proteoglycans. Many growth factors are known to interact with heparin and heparan sulfate proteoglycans (HSPGs); these growth factors include HB-EGF, AR, BTC, platelet-derived growth factor, acidic fibroblast growth factor, basic fibroblast growth factor, vascular endothelial growth factor, and granulocyte-macrophage colony-stimulating factor (16). Using a combination of site-directed mutagenesis and synthetic peptide studies, the sequences within HB-EGF that mediate its interaction with heparin were located in the middle of the protein (residues 93 to 113 in the precursor sequence [Fig. 1A]) (3, 43). Three stretches of basic amino acids within this region (93KRKKKGK, 103KKR, and 110RKYK [Fig. 1A]) have been shown to play a critical role in HB; interestingly, the third region is within the EGF-like domain of HB-EGF.
The discovery that the DT receptor is a growth factor precursor raises the possibility that a soluble receptor analog might be used therapeutically to block the binding of toxin to the membrane DT receptor. A laboratory earlier reported that recombinant mature Hu HB-EGF (residues 63 to 148), which is identical to the mature Mk HB-EGF (except that Asn87 of human is replaced with Ser87 in the monkey [Fig. 1A]), strongly inhibits the binding of radiolabeled DT to toxin receptor-bearing cells (26). This result suggested that it would be theoretically possible to treat diphtheria patients with mature HB-EGF, which is a natural growth factor. This approach would have significant advantages over the presently used equine antitoxin, which often causes serum sickness, an immune complex-type disease. However, mature HB-EGF might produce side effects due to its growth factor activity.
We present here a rationale for the design of such a modern antidote for diphtherial disease. The newly designed recombinant protein is a more effective inhibitor of DT binding and is significantly less mitogenic (tumorigenic) than recombinant mature HB-EGF.
MATERIALS AND METHODS
Materials.
The plasmid vector pcDNA3 and the Escherichia coli host strains TOP10F and DH5α were purchased from Invitrogen. The protein expression vector pET21a, the protein expression E. coli host strain BL21(DE3), and the T7 tag purification kit were obtained from Novagen. The recombinant mature Hu HB-EGF and AR were from R&D Systems. Waymouth's medium powder was from Life Technologies, Inc. Insulin-transferrin-selenium-X culture supplement was from Collaborative Biomedical Products (Bedford, Mass.). The BALB/c/3T3 cell line (ATCC CCL-163) was from the American Type Culture Collection. [125I]NaI (IMS 30; 13 to 17 mCi/μg), l-[4,5-3H]leucine (60 Ci/mmol), and [methyl-3H]thymidine (2 Ci/mmol) were obtained from Amersham. Partially purified DT was purchased from Connaught Laboratories (Willowdale, Ontario, Canada) and was purified further by anion-exchange chromatography according to published methods (37). The BIAcore 2000 system and CM5 sensor chips were obtained from Pharmacia. All other reagents were as previously described (2, 4, 10, 12).
Plasmid construction.
The plasmid pcDNA3 is a mammalian expression vector that allows growth in the presence of Geneticin. The wild-type Mk proHB-EGF cDNA (34) was subcloned into the expression vector pcDNA3 using the HindIII and XbaI restriction sites as described previously (26). The resulting plasmid was named pMkHB-EGF. To create pMkHB-EGF/F115A, pMkHB-EGF/I117A, pMkHB-EGF/Y138A, pMkHB-EGF/L148A, pMkHB-EGF/F115A/L148A, pMkHB-EGF/I117A/L148A, and pMkHB-EGF/Y138A/L148A, site-directed mutagenesis was performed using the Quikchange kit (from Stratagene) as described previously (10). Oligonucleotides were synthesized by GIBCO/BRL and were used as primers to generate and to sequence the mutants used in this study. The mutated sequences of the proHB-EGF cDNAs were confirmed by automated sequencing using the ABI PRISM dye terminator cycle-sequencing ready-reaction kit with the ABI Prism 377 DNA Sequencer at the sequencing facilities of our institution. The desired mutants of proHB-EGF cDNA were recloned into pcDNA3 at the HindIII and XbaI restriction sites.
The pET21a plasmid was used for protein expression in E. coli. The coding regions (residues 63 to 149 for wild-type-sized recombinant HB-EGF or residues 106 to 149 for those proteins with a deletion of the HB domain) were amplified by PCR with pMkHB-EGF, pMkHB-EGF/I117A/L148A, or pMkHB-EGF/Y138A/L148A templates and were cloned into pET21a using the BamHI and HindIII sites, resulting in pET21a/WT, pET21a/HBΔ, pET21a/HBΔ/I117A/L148A, and pET21a/HBΔ/Y138A/L148A plasmids. The mutated sequences of HB-EGF were confirmed by automated sequencing as described above.
Transfection.
Electroporation was used to transfect plasmid DNA into LCD9 cells using the BTX ECM 600 System as described previously (10) with the following modifications. The LCD9 cell line is DT resistant and hygromycin resistant; the construction and biochemical characterization of this cell line are described in detail elsewhere (5). LCD9 cells were transfected with the wild-type DT receptor/Mk proHB-EGF plasmid pMkHB-EGF (10) and with the seven DT receptor mutant plasmids (pMkHB-EGF/F115A, pMkHB-EGF/I117A, pMkHB-EGF/Y138A, pMkHB-EGF/L148A, pMkHB-EGF/F115A/L148A, pMkHB-EGF/I117A/L148A, and pMkHB-EGF/Y138A/L148A), and cloned cell lines stably expressing the wild-type or mutant DT receptor were obtained. The cell lines were screened for the presence of cell surface proHB-EGF by the abbreviated cytotoxicity assay.
Abbreviated cytotoxicity assay.
We used the previously described abbreviated cytotoxicity assay (2, 10) to observe the inhibition of protein synthesis by DT. Cells were tested with one concentration of DT (1 μg/ml) and no DT as a negative control. Protein synthesis inhibition was measured by [3H]leucine incorporation into acid-precipitable radioactivity as described previously (19, 38). Each assay included the LCD9/Mk HB-EGF cell line described previously (10) as a positive control and the LCD9 cell line as a negative control. Cell lines in which protein synthesis was inhibited by 50% or more at 1 μg of DT/ml were considered to be DT sensitive. All individual DT-sensitive cell lines were subcloned to obtain pure stable cell populations as described previously (10); the presence of cell surface Mk CD9 was verified by a quantitative whole-cell enzyme-linked immunosorbent assay as described previously (10) and was further analyzed by a radiolabeled DT binding assay described below.
Radiolabeled DT binding assay.
The ability of wild-type and mutant DT receptor-expressing cell lines to bind radioiodinated DT was examined as previously described (5, 34) with modifications. For this assay, 125I-labeled DT (0.4 to 400 nM) was added to the cell monolayers with or without a 100-fold-excess unlabeled DT. Cell-associated radioactivity was determined employing a Tm Analytical Gamma Trac 1290 g counting system. Specific binding was determined as the difference between the total cell-associated radioactivity without excess unlabeled DT and the cell-associated radioactivity obtained when a 100-fold excess of unlabeled toxin was included. An acceptable level of nonspecific binding was 20% or less. Specific binding data were subjected to Scatchard analyses. All radiolabeled DT binding assays were performed in duplicate, and at least two assays were performed for each cell line. Each assay included the same positive and negative control cell lines described above for the abbreviated cytotoxicity assay.
Expression and purification of recombinant HB-EGF proteins.
The recombinant HB-EGF proteins [mature Mk HB-EGF, HBΔ-EGF, HBΔ-EGF(I117A/L148A), and HBΔ-EGF(Y138A/L148A)] were expressed from pET21a/WT, pET21a/HBΔ, pET21a/HBΔ/I117A/L148A, and pET21a/HBΔ/Y138A/L148A, respectively, in E. coli BL21(DE3). All recombinant HB-EGF proteins contained a T7 tag at their amino termini to facilitate the purification step. To prevent the expression of the histidine tag at the carboxyl terminus of the proteins, which might interfere with DT binding, a termination codon was introduced into the plasmids before the histidine tag-coding region. The overexpressed recombinant HB-EGF proteins formed inclusion bodies, which were isolated and dissolved in 8 M urea. The recombinant HB-EGF proteins were refolded by sequential dialytic removal of urea in 25 mM Tris buffer with 4, 2, 1, and 0 M urea steps, in the presence of reduced/oxidized glutathione (2.0/0.2 mM) (29). The refolded recombinant HB-EGF proteins were concentrated and purified by T7 affinity column as described in the manufacturer's instructions.
Assay for 125I-DT binding inhibition.
A LCD9/Mk HB-EGF cell line (n = 15,000) was used for this assay. LCD9/Mk HB-EGF cells are mouse LCD9 cells that were obtained by transfection with pMkHB-EGF and stably express cell surface DT receptor/Mk proHB-EGF (12).
Forty-eight-well plates were seeded at 4 × 104 LCD9/HB-EGF cells per well. After 3 days of incubation at 37°C in 5% CO2, confluent monolayers were washed twice with ice-cold phosphate-buffered saline (PBS) (8.8 mM Na2HPO4, 1.2 mM KH2PO4, 140 mM NaCl, 10 mM KCl, 0.5 mM MgCl2, and 1.0 mM CaCl2 [pH 7.4]), and then ice-cold binding medium (medium 199) (50-μg ml−1 bovine serum albumin, 100-μg ml−1 gelatin, and 20 mM HEPES, pH 7.4) was added to the monolayers and preincubated for 30 min at 4°C. The preincubation medium was removed, and the monolayers were again washed twice with ice-cold PBS. The monolayers were incubated with the recombinant HB-EGF proteins in the absence or in the presence of heparin (10 U/ml) for 5 h at 4°C. Binding medium (in the absence or in the presence of heparin) containing 125I-DT (5 nM, initial saturating concentration) and six various concentrations (0 to 450 nM) of recombinant HB-EGF proteins were incubated with the monolayer of LCD9/Mk HB-EGF cells at 4°C for 5 h. Counts bound in the presence of competitor were compared to those in the absence and reported as a percentage of the latter. In the experiments shown, LCD9/Mk HB-EGF cells bound about 10,000 cpm in the absence of competitor. All 125I-DT binding assays were performed in duplicate, and at least two assays were performed for each cell line.
Assay for mitogenic activity.
The BALB/c/3T3 cell line (ATCC CCL-163) was used for this assay. Twenty-four-well plates were seeded at 105 BALB/c/3T3 cells per well. After 3 days of incubation at 37°C in 5% CO2, about 95% confluent monolayers were obtained and were washed once with PBS and once with assay medium (1:1 Dulbecco's minimal essential medium containing 1.5 g of NaCO3/liter and Waymouth's medium containing 1.5 g of NaCO3/liter, with 0.1% l-glutamine and 1% insulin-transferrin-selenium-X). The cells were then incubated for 24 h in the assay medium to serum starve the cells. Dead cells were removed by washing once with PBS and once with assay medium. The various concentrations of recombinant HB-EGF proteins in assay medium were added to the monolayers and incubated at 37°C in 5% CO2 for 3 days. The cells were then incubated with 5 μCi of [methyl-3H]thymidine/well for 3 h at 37°C in 5% CO2. The radioactive media were aspirated, and the cells were detached with trypsin-EDTA. The contents of the well plus 1 ml of PBS rinse were collected on a glass fiber filter using vacuum manifolds (Hoefer Scientific Instruments). Three 1-ml aliquots of PBS, three 1-ml aliquots of 10% trichloroacetic acid, and three 1-ml aliquots of 95% ethanol, all chilled on ice, were passed through each filter. The filters were dried, and the amount of radioactive thymidine incorporated into DNA was determined by scintillation counting (2). All assays for mitogenic activity were performed in duplicate, and at least two assays were performed for each cell line.
Surface plasmon resonance.
The BIAcore 2000 system with CM5 sensor chips was employed as described previously (4) with modifications. The commercially available mature Hu HB-EGF (as a positive control), the commercially available mature Hu AR (as a negative control), and the recombinant HB-EGF proteins expressed in E. coli and purified by T7 affinity column were immobilized using the standard amine-coupling protocol provided by Pharmacia and using a flow rate of 10 μl/min. A running buffer (modified buffer of tissue culture medium 199 with Hanks salts containing CaCl2, KCl, KH2PO4, MgSO4, NaCl, NaH2PO4, C2H3O2Na, NaHCO3, and Tween 80, pH 7.4) flow rate of 30 μl/min was used in the kinetic analysis experiments. The coupling of HB-EGF and AR proteins onto the chips resulted in a range of 250 to 350 resonance units immobilized on the sensor chips. A range of DT concentrations (20 nM to 1 μM) was injected over the CM5 chip with the immobilized HB-EGFs or AR. The injections were performed at 25°C using the kinject method of injection. Injections of DT at concentrations of 100 to 400 nM were used in the 1:1 analysis of the DT:HB-EGF complex. A blank control used to monitor nonspecific binding to the sensor chip surface was achieved by activating and inactivating the carboxymethylated dextran in the first flow cell without coupling any ligand to this flow cell. No binding was observed when running buffer alone (without DT) was passed over the immobilized HB-EGF, indicating that none of the components in the running buffer bound to HB-EGF (data not shown). Also, no binding was observed when 1 μM DT was allowed to pass over immobilized AR as a negative control (data not shown). Bulk refractive index shifts were removed from the analysis by subtraction of the surface plasmon resonance response observed for running buffer passed over the first flow cell from the binding curves observed for the interaction of DT with HB-EGF. Version 3.0 of the BIAcore software package was used to analyze these curves. The kinetic experiments were performed twice, each time using a different chip.
RESULTS AND DISCUSSION
Recombinant mature Hu HB-EGF (residues 63 to 148 [Fig. 1A]) binds radiolabeled DT and therefore inhibits the binding of radiolabeled DT to toxin receptor-bearing cells (26), suggesting that mature HB-EGF could be used as an antidote for diphtherial disease.
Inhibition of DT binding by mature HB-EGF in the absence or presence of heparin.
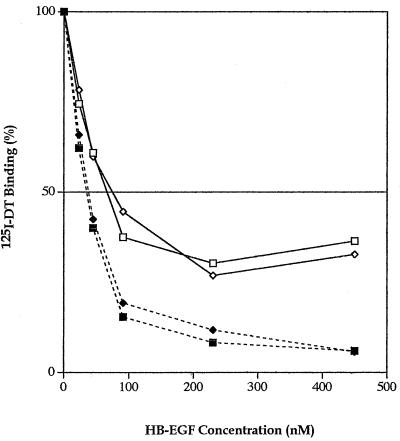
Preliminary experiments showed that wild-type mature Mk HB-EGF and the commercially available mature Hu HB-EGF inhibited the binding of 125I-DT to wild-type receptor-bearing cells (LCD9/Mk HB-EGF); a detailed analysis of the inhibition is shown in Fig. 2. Both proteins showed the same pattern of DT binding inhibition, suggesting that our purified recombinant mature Mk HB-EGF protein refolded correctly. Lanzrein et al. (28) reported that radioiodinated DT-HB-EGF complexes can bind to cells via binding of the HB domain to cell surface HSPGs. To confirm this possibility, we performed the same experiment in the presence of heparin which we expected would bind to the HB domain and block the binding of the HB domain to cell surface HSPGs. In the presence of heparin, there was a dramatic decrease in the heparin-dependent binding of radioiodinated DT to cells (Fig. 2). Therefore, to eliminate the HB effect, we prepared a recombinant truncated HB-EGF protein (residues 106 to 149, HBΔ-EGF) which lacks the amino terminus, including most of the HB domain (i.e., lacks 93KRKKKGK and 103KKR but not 110RKYK [Fig. 1A]).
FIG. 2.
Radiolabeled DT binding inhibition by the recombinant mature Hu and Mk HB-EGF proteins. The specific bound 125I-DT is plotted against the concentration of competitors, the recombinant mature Hu HB-EGF (◊, ⧫) or the recombinant mature Mk HB-EGF (□, ▪), in the absence (◊, □) or the presence (⧫, ▪) of heparin (10 U/ml). The average from duplicate wells was calculated and plotted. Results are representative of at least two separate experiments.
Inhibition of DT binding by truncated HBΔ-EGF.
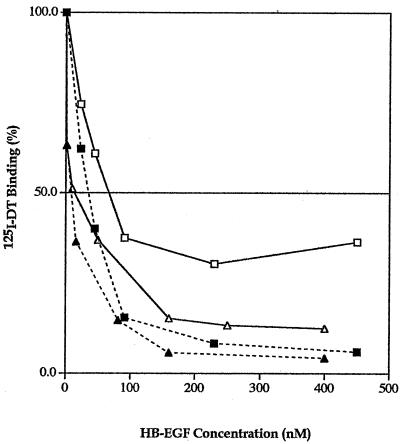
Experiments on the inhibition of radiolabeled DT binding were then performed with the purified recombinant HBΔ-EGF and mature Mk HB-EGF proteins in the absence or the presence of heparin (Fig. 3). The recombinant HBΔ-EGF protein at all concentrations, in the absence of heparin, was a more effective inhibitor of DT binding than was recombinant mature Mk HB-EGF, and addition of heparin showed only a small effect on toxin binding. Thus, HBΔ-EGF appears to be a better candidate for neutralizing DT than wild-type mature HB-EGF.
FIG. 3.
Radiolabeled DT binding inhibition by the recombinant truncated HBΔ-EGF and mature HB-EGF proteins. The specific bound 125I-DT is plotted against the concentration of competitors, the recombinant mature Mk HB-EGF (□, ▪) or the recombinant truncated HBΔ-EGF (▵, ▴), in the absence (□, ▵) or the presence (▪, ▴) of heparin (10 U/ml). The average from duplicate wells was calculated and plotted. Results are representative of at least two separate experiments.
DT binding ability of cells expressing mutant DT receptors.
Since mature HB-EGF is a known ligand of the EGF receptor and is thus highly mitogenic, the mitogenic (tumorigenic) effect would need to be reduced to make the mature HB-EGF safe for in vivo use. Accordingly, we first employed site-directed mutagenesis to destroy the mitogenic activity of HB-EGF without affecting its DT binding ability.
All members of the EGF protein family have an EGF-like domain containing six conserved residues with a characteristic pattern (CX7CX3-5CX10-12CXCX5GXRC) that form three disulfide bonds (linked C1—C3, C2—C4, and C5—C6) (Fig. 1B). All growth factors of the EGF family bind to the EGF receptor through their EGF-like domain, even though they have quite a few nonconserved amino acids within this domain (Fig. 1B). Using site-directed mutagenesis of EGF and TGF-α, a number of conserved amino acid residues have been shown to be involved in activation of the EGF receptor. The positions of these residues (using the numbering system based on the HB-EGF precursor [Fig. 1A]) are 115, 117, 127, 138, 142, and 148 (Fig. 1B); the highly conserved leucine residue at position 148 has been shown to be the most critical residue involved in the interaction with the EGF receptor (6).
The EGF-like domain of HB-EGF, unlike that of the other EGF family members, has the unique capability of binding DT. Our laboratory found that 3 residues in this domain, Ile133, His135, and Glu141, play critical roles in DT binding; Leu127 may also contribute to DT binding (10). Furthermore, the crystal structure study of a complex of DT with part (residues 73 to 147) of the extracellular domain of Hu HB-EGF, which includes the EGF-like domain, demonstrated that these 4 amino acid residues, as well as Arg142, are in close contact with the toxin (29). Based on this rationale, we used site-directed mutagenesis to convert Phe115, Ile117, Tyr138, and Leu148 to alanine, either alone or in combination with L148A. We hoped to find mutations that affect mitogenicity without affecting DT binding.
We first analyzed the DT binding ability of LCD9 cells stably expressing mutant DT receptors to test whether any of the mutations change the receptor's DT binding ability. Table 1 summarizes the data for toxin receptor affinity and the number of DT binding sites per cell for wild-type and seven different mutant DT receptors. The DT receptor affinity was decreased ∼3-fold for the LCD9/HB-EGF(F115A) cell line (Kd = 20.0 nM) and ∼2-fold for the LCD9/HB-EGF(F115A/L148A) cell line (Kd = 15.9 nM), compared to the cell line expressing the wild-type DT receptor (Kd = 7.0 nM). This result suggests that Phe115 may also be involved in DT binding. Mitamura et al. (33) had earlier reported that the DT binding activity of a F115Y mutant was also moderately decreased.
TABLE 1.
Summary of DT binding affinity and the number of DT binding sites per cell
| Cell linea | Kd (nM)b | n (105)b |
|---|---|---|
| LCD9/Mk HB-EGF | 7.0 | 8.7 |
| LCD9/F115A | 20.0 | 5.4 |
| LCD9/I117A | 4.0 | 1.7 |
| LCD9/Y138A | 6.6 | 6.5 |
| LCD9/L148A | 3.6 | 2.4 |
| LCD9/F115A/L148A | 15.9 | 5 |
| LCD9/I117A/L148A | 3 | 4.6 |
| LCD9/Y138A/L148A | 6.8 | 11.8 |
These cell lines are LCD9 cells expressing wild-type or mutant Mk proHB-EGF/DT receptors.
All binding assays were done in duplicate and were performed at least twice for each cell line with the average calculated Kd and number of binding sites per cell (n) being reported.
The DT binding affinity was increased ∼2-fold for both the LCD9/HB-EGF(I117A) cell line (Kd = 4.0 nM) and the LCD9/HB-EGF(I117A/L148A) cell line (Kd = 3.0 nM). The DT binding affinity was also increased ∼2-fold for the LCD9/HB-EGF(L148A) cell line (Kd = 3.6 nM). It is interesting that these mutant DT receptors display a higher affinity for DT than does the wild-type DT receptor. This increased affinity is probably a reflection of the decreased level of expression of cell surface DT receptor/Mk proHB-EGF (Table 1) at a constant level of expression of CD9, which we previously showed results in a higher Mk CD9/Mk proHB-EGF ratio and in an increased receptor affinity (12). The DT receptor affinities of the LCD9/HB-EGF(Y138A) cell line (Kd = 6.6 nM) and of the LCD9/HB-EGF(Y138A/L148A) cell line (Kd = 6.8 nM) are similar to that of the wild-type DT receptor. In summary, these results suggest that residues Ile117, Tyr138, and Leu148 are not involved in DT binding but that residue Phe115 is. Therefore, residues Ile117, Tyr138, and Leu148 are good candidates to mutate in order to attempt to reduce the tumorigenic effect without affecting the toxin binding ability. Furthermore, the double mutants I117A/L148A and Y138A/L148A, which have a mutation in the most critical residue (Leu148), might be even better candidates. Thus, the double mutants I117A/L148A and Y138A/L148A were created in the truncated HBΔ-EGF background, resulting in HBΔ-EGF(I117A/L148A) and HBΔ-EGF(Y138A/L148A) proteins. These proteins were then tested for their mitogenic activity and their ability to inhibit binding of radiolabeled DT to cells.
HBΔ-EGF(I117A/L148A) displays a lower mitogenic activity.
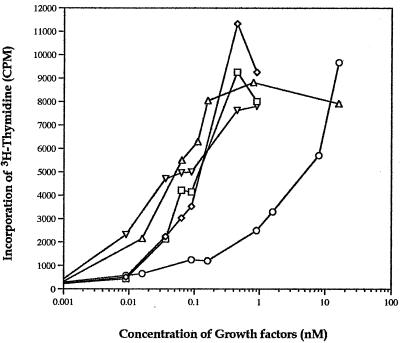
The mitogenic activity of the commercial Hu HB-EGF, the purified Mk HB-EGF, HBΔ-EGF, HBΔ-EGF(I117A/L148A), and HBΔ-EGF(Y138A/L148A) proteins was examined using serum-starved BALB/c/3T3 cells (Fig. 4). As expected, the recombinant mature Hu HB-EGF and the purified recombinant Mk HB-EGF showed similar, high levels of mitogenicity. Interestingly, HBΔ-EGF showed a similar mitogenic effect to that of the wild-type proteins, a result consistent with the fact that the EGF-like domain is the one that interacts with the EGF receptor.
FIG. 4.
Induction of DNA synthesis in BALB/c/3T3 cells by the recombinant mature Hu HB-EGF, mature Mk HB-EGF, HBΔ-EGF, HBΔ-EGF(I117A/L148A), and HBΔ-EGF(Y138A/L148A) proteins. The serum-starved BALB/c/3T3 cells were stimulated with various concentrations of recombinant mature Hu HB-EGF (◊), mature Mk HB-EGF (□), HBΔ-EGF (▵), HBΔ-EGF(I117A/L148A) (○), or HBΔ-EGF(Y138A/L148A) (▿) for 3 days, and the amount of [3H]thymidine incorporation was measured as described in Materials and Methods. The average from duplicate wells was calculated and plotted. Results are representative of at least two separate experiments.
Surprisingly, the mitogenic effect of HBΔ-EGF(Y138A/L148A) was not decreased but was similar to that of the commercial Hu HB-EGF, the purified Mk HB-EGF, and HBΔ-EGF, although the two mutations in this truncated protein are in highly conserved residues in the EGF family which have been shown, in EGF and TGF-α, to be involved in binding to the EGF receptor (Fig. 1B).
More important was the mitogenic effect observed with HBΔ-EGF(I117A/L148A); it was decreased at least 100-fold when the 50% effective dose (ED50) of HBΔ-EGF(I117A/L148A) (ED50 ≈ 10 nM) was compared with those of mature Hu HB-EGF, Mk HB-EGF, HBΔ-EGF, and HBΔ-EGF(Y138A/L148A) (ED50 ≈ ∼0.06 to 0.1 nM). The extremely low mitogenic activity suggested that HBΔ-EGF(I117A/L148A) would be the best candidate for an antidote against diphtherial disease.
HBΔ-EGF(I117A/L148A) inhibits binding of DT as well as HBΔ-EGF.
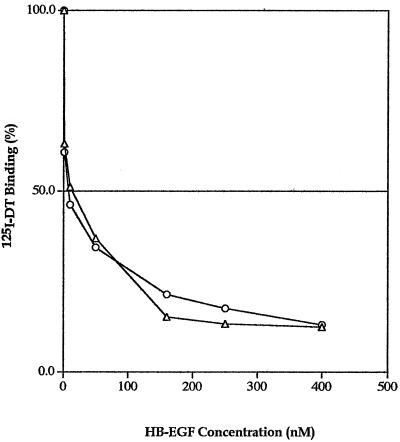
The experiment for inhibition of radiolabeled DT binding was performed with HBΔ-EGF(I117A/L148A) and with HBΔ-EGF proteins; they both showed a similar pattern of inhibition of DT binding (Fig. 5).
FIG. 5.
Radiolabeled DT binding inhibition by the truncated HBΔ-EGF(I117A/L148A) and the truncated HBΔ-EGF proteins. The specific bound 125DT is plotted against the concentrations of competitors, the recombinant truncated HBΔ-EGF (▵) and the recombinant truncated mutant HBΔ-EGF(I117A/L148A) (○), used in the assay. The average from duplicate wells was calculated and plotted. Results are representative of at least two separate experiments.
High DT binding affinity of HBΔ-EGF(I117A/L148A).
We examined the binding of the recombinant HB-EGF, HBΔ-EGF, and HBΔ-EGF(I117A/L148A) to DT, employing surface plasmon resonance. Table 2 summarizes the data for the association rate constant (Ka), the dissociation rate constant (Kd), and the apparent dissociation constant (KD) of the recombinant HB-EGF proteins towards DT. Our purified recombinant wild-type Mk HB-EGF (KD = 3.4 × 10−8 M) showed an apparent KD very similar to that of the commercial recombinant Hu HB-EGF (KD = 3.8 × 10−8 M); these apparent KD values obtained here are in agreement with those previously reported (4). Interestingly, the apparent KD for HBΔ-EGF (KD = 5.2 × 10−8M) was similar to that of the recombinant Hu HB-EGF and of the purified recombinant Mk HB-EGF, in agreement with the DT binding inhibition results described above. High-affinity binding of HBΔ-EGF(I117A/L148A) for DT was also observed (KD = 10 × 10−8 M); however, it was ∼2-fold lower than that obtained with HBΔ-EGF.
TABLE 2.
Interaction of DT with immobilized HB-EGF moleculesa
| Recombinant HB-EGF protein used | Avg Ka (104) (1/Ms) | Avg Kd (10−3) (1/S) | Avg KD (10−8) (M) |
|---|---|---|---|
| Mature Hu HB-EGF | 4.6 | 1.8 | 3.8 |
| Mature Mk HB-EGF | 6.4 | 2.2 | 3.4 |
| HBΔ-EGF | 4.9 | 2.5 | 5.2 |
| HBΔ-EGF(I117A/L148A) | 7.1 | 7.1 | 10 |
The BIAcore 2000 system with CM5 sensor chips was employed. The recombinant HB-EGF proteins were immobilized on CM5 sensor chips by standard amine coupling, and the kinetic experiments were performed as described in Materials and Methods. This table shows values for a representative experiment of two separate experiments.
In this study we demonstrated that the truncated mutant protein HBΔ-EGF(I117A/L148A) resulted in a DT receptor analog that both strongly inhibited binding of radioiodinated DT to cells and displayed a 100-fold-lower mitogenic effect. Therefore, we conclude that the HBΔ-EGF(I117A/L148A) protein may be a suitable candidate for a diphtheria antidote. This protein will now be tested in vivo employing a toxin-sensitive animal model. We propose to start by testing that concentration of HBΔ-EGF(I117A/L148A) that inhibits binding of radioiodinated DT by 50%, ∼10 nM (Fig. 5), a concentration which is expected to result in about a 50%-lower mitogenic effect than that obtained with the truncated and mature HB-EGF molecules (Fig. 4).
Acknowledgments
We thank Robert S. Munford for critical review of the manuscript. We also thank Kathryn Ivey, Leigh Cunningham, and Allen Dyke for technical assistance. The editorial assistance of Eleanor R. Eidels is greatly appreciated.
This research was supported by U.S. Public Health Service Grant AI-16805.
Editor: J. T. Barbieri
REFERENCES
- 1.Abraham, J. A., D. Damm, A. Bajardi, J. Miller, M. Klagsbrun, and R. A. Ezekowitz. 1993. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem. Biophys. Res. Commun. 190:125-133. [DOI] [PubMed] [Google Scholar]
- 2.Almond, B. D., and L. Eidels. 1994. The cytoplasmic domain of the diphtheria toxin receptor (HB-EGF precursor) is not required for receptor-mediated endocytosis. J. Biol. Chem. 269:26635-26641. [PubMed] [Google Scholar]
- 3.Besner, G. E., D. Whelton, M. A. Crissman-Combs, C. L. Steffen, G. Y. Kim, and D. R. Brigstock. 1992. Interaction of heparin-binding EGF-like growth factor (HB-EGF) with the epidermal growth factor receptor: modulation by heparin, heparinase, or synthetic heparin-binding HB-EGF fragments. Growth Factors 7:289-296. [DOI] [PubMed] [Google Scholar]
- 4.Brooke, J. S., J. H. Cha, and L. Eidels. 1998. Diphtheria toxin:receptor interaction: association, dissociation, and effect of pH. Biochem. Biophys. Res. Commun. 248:297-302. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. G., B. D. Almond, J. G. Naglich, and L. Eidels. 1993. Hypersensitivity to diphtheria toxin by mouse cells expressing both diphtheria toxin receptor and CD9 antigen. Proc. Natl. Acad. Sci. USA 90:8184-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter, G., and M. I. Wahl. 1990. The epidermal growth factor family, p. 69-171. In M. B. Sporn and A. B. Roberts (ed.), Handbook of experimental pharmacology, vol. 95. Springer-Verlag, New York, N.Y. [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1996. Diphtheria outbreak—Saraburi Province, Thailand, 1994. Morb. Mortal. Wkly. Rep. 45:271-273. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1993. Diphtheria outbreak—Russian Federation, 1990-1993. Morb. Mortal. Wkly. Rep. 42:840-841, 847. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1995. Diphtheria epidemic--New Independent States of the former Soviet Union, 1990-1994. Morb. Mortal. Wkly. Rep. 44:177-181. [PubMed] [Google Scholar]
- 10.Cha, J. H., J. S. Brooke, and L. Eidels. 1998. Toxin binding site of the diphtheria toxin receptor. Loss and gain of diphtheria toxin binding of monkey and mouse heparin-binding, epidermal growth factor-like growth factor precursors by reciprocal site-directed mutagenesis. Mol. Microbiol. 29:1275-1284. [DOI] [PubMed] [Google Scholar]
- 11.Cha, J. H., J. S. Brooke, and L. Eidels. 1999. Hamster diphtheria toxin receptor: a naturally occurring chimera of monkey and mouse HB-EGF precursors. Biochem. Biophys. Res. Commun. 254:325-329. [DOI] [PubMed] [Google Scholar]
- 12.Cha, J. H., J. S. Brooke, K. N. Ivey, and L. Eidels. 2000. Cell surface monkey CD9 antigen is a coreceptor that increases diphtheria toxin sensitivity and diphtheria toxin receptor affinity. J. Biol. Chem. 275:6901-6907. [DOI] [PubMed] [Google Scholar]
- 13.Choe, S., M. J. Bennett, G. Fujii, P. M. Curmi, K. A. Kantardjieff, R. J. Collier, and D. Eisenberg. 1992. The crystal structure of diphtheria toxin. Nature 357:216-222. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, S., and G. A. Elliot. 1963. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J. Investig. Dermatol. 40:1-5. [DOI] [PubMed] [Google Scholar]
- 15.Collier, R. J. 1975. Diphtheria toxin: mode of action and structure. Bacteriol. Rev. 39:54-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis-Fleischer, K. M., and G. E. Besner. 1998. Structure and function of heparin-binding EGF-like growth factor (HB-EGF). Front. Biosci. 3:d288-d299. [DOI] [PubMed] [Google Scholar]
- 17.De Larco, J. E., and G. J. Todaro. 1978. Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. USA 75:4001-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorland, R. B., J. L. Middlebrook, and S. H. Leppla. 1979. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J. Biol. Chem. 254:11337-11342. [PubMed] [Google Scholar]
- 19.Eidels, L., and D. A. Hart. 1982. Effect of polymers of l-lysine on the cytotoxic action of diphtheria toxin. Infect. Immun. 37:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eidels, L., R. L. Proia, and D. A. Hart. 1983. Membrane receptors for bacterial toxins. Microbiol. Rev. 47:596-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galazka, A. M., S. E. Robertson, and G. P. Oblapenko. 1995. Resurgence of diphtheria. Eur. J. Epidemiol. 11:95-105. [DOI] [PubMed] [Google Scholar]
- 22.Goishi, K., S. Higashiyama, M. Klagsbrun, N. Nakano, T. Umata, M. Ishikawa, E. Mekada, and N. Taniguchi. 1995. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol. Biol. Cell 6:967-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashiyama, S., J. A. Abraham, J. Miller, J. C. Fiddes, and M. Klagsbrun. 1991. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251:936-939. [DOI] [PubMed] [Google Scholar]
- 24.Higashiyama, S., R. Iwamoto, K. Goishi, G. Raab, N. Taniguchi, M. Klagsbrun, and E. Mekada. 1995. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J. Cell Biol. 128:929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes, W. E., M. X. Sliwkowski, R. W. Akita, W. J. Henzel, J. Lee, J. W. Park, D. Yansura, N. Abadi, H. Raab, G. D. Lewis, H. M. Shepard, W.-J. Kuang, W. I. Wood, D. V. Goeddel, and R. L. Vandlen. 1992. Identification of heregulin, a specific activator of p185erbB2. Science 256:1205-1210. [DOI] [PubMed] [Google Scholar]
- 26.Hooper, K. P., and L. Eidels. 1995. Localization of a critical diphtheria toxin-binding domain to the C-terminus of the mature heparin-binding EGF-like growth factor region of the diphtheria toxin receptor. Biochem. Biophys. Res. Commun. 206:710-717. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto, R., S. Higashiyama, T. Mitamura, N. Taniguchi, M. Klagsbrun, and E. Mekada. 1994. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 13:2322-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanzrein, M., O. Garred, S. Olsnes, and K. Sandvig. 1995. Diphtheria toxin endocytosis and membrane translocation are dependent on the intact membrane-anchored receptor (HB-EGF precursor): studies on the cell-associated receptor cleaved by a metalloprotease in phorbol-ester-treated cells. Biochem. J. 310:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louie, G. V., W. Yang, M. E. Bowman, and S. Choe. 1997. Crystal structure of the complex of diphtheria toxin with an extracellular fragment of its receptor. Mol. Cell 1:67-78. [DOI] [PubMed] [Google Scholar]
- 30.Middlebrook, J. L., and R. B. Dorland. 1977. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can. J. Microbiol. 23:183-189. [DOI] [PubMed] [Google Scholar]
- 31.Middlebrook, J. L., and R. B. Dorland. 1984. Bacterial toxins: cellular mechanisms of action. Microbiol. Rev. 48:199-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middlebrook, J. L., R. B. Dorland, and S. H. Leppla. 1978. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J. Biol. Chem. 253:7325-7330. [PubMed] [Google Scholar]
- 33.Mitamura, T., T. Umata, F. Nakano, Y. Shishido, T. Toyoda, A. Itai, H. Kimura, and E. Mekada. 1997. Structure-function analysis of the diphtheria toxin receptor toxin binding site by site-directed mutagenesis. J. Biol. Chem. 272:27084-27090. [DOI] [PubMed] [Google Scholar]
- 34.Naglich, J. G., J. E. Metherall, D. W. Russell, and L. Eidels. 1992. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 69:1051-1061. [DOI] [PubMed] [Google Scholar]
- 35.Pan American Health Organization. 1994. Diphtheria epidemic in Ecuador. EPI Newsl. 16:5-8.12345538 [Google Scholar]
- 36.Pappenheimer, A. M. J. 1977. Diphtheria toxin. Annu. Rev. Biochem. 46:69-94. [DOI] [PubMed] [Google Scholar]
- 37.Pappenheimer, A. M. J., T. Uchida, and A. A. Harper. 1972. An immunological study of the diphtheria toxin molecule. Immunochemistry 9:891-906. [DOI] [PubMed] [Google Scholar]
- 38.Proia, R. L., L. Eidels, and D. A. Hart. 1981. Diphtheria toxin:receptor interaction. Characterization of the receptor interaction with the nucleotide-free toxin, the nucleotide-bound toxin, and the B-fragment of the toxin. J. Biol. Chem. 256:4991-4997. [PubMed] [Google Scholar]
- 39.Shing, Y., G. Christofori, D. Hanahan, Y. Ono, R. Sasada, K. Igarashi, and J. Folkman. 1993. Betacellulin: a mitogen from pancreatic beta cell tumors. Science 259:1604-1607. [DOI] [PubMed] [Google Scholar]
- 40.Shoyab, M., V. L. McDonald, J. G. Bradley, and G. J. Todaro. 1988. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc. Natl. Acad. Sci. USA 85:6528-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strachan, L., J. G. Murison, R. L. Prestidge, M. A. Sleeman, J. D. Watson, and K. D. Kumble. 2001. Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J. Biol. Chem. 276:18265-18271. [DOI] [PubMed] [Google Scholar]
- 42.Temizer, D. H., M. Yoshizumi, M. A. Perrella, E. E. Susanni, T. Quertermous, and M. E. Lee. 1992. Induction of heparin-binding epidermal growth factor-like growth factor mRNA by phorbol ester and angiotensin II in rat aortic smooth muscle cells. J. Biol. Chem. 267:24892-24896. [PubMed] [Google Scholar]
- 43.Thompson, S. A., S. Higashiyama, K. Wood, N. S. Pollitt, D. Damm, G. McEnroe, B. Garrick, N. Ashton, K. Lau, and N. Hancock. 1994. Characterization of sequences within heparin-binding EGF-like growth factor that mediate interaction with heparin. J. Biol. Chem. 269:2541-2549. [PubMed] [Google Scholar]
- 44.Toyoda, H., T. Komurasaki, D. Uchida, Y. Takayama, T. Isobe, T. Okuyama, and K. Hanada. 1995. Epiregulin: a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J. Biol. Chem. 270:7495-7500. [DOI] [PubMed] [Google Scholar]