Abstract
1. The effects of Ca2+ deprivation upon mechanical and electrophysiological parameters of single muscle fibres from the m. semitendinosus and the m. iliofibularis of the frog were investigated. 2. When the external free Ca concentration was reduced in steps of one order of magnitude from 10(-3) to 10(-9) M, using up to 10 mM-EGTA and in the presence of 3 mM-Mg2+, the maximum force of K contractures declined by 5-15%, the plateau of maximum force shortened, and in most cases the phase of spontaneous relaxation lengthened. 3. In Ringer solution containing 10(-9) M-Ca2+ and 1 mM-Mg2+ 85% of maximum tetanic force could be maintained for at least 15 sec (5 Hz; 3 degrees C). 4. The reduction of external Ca2+ from 10(-3) to 10(-9) M and its replacement by Mg2+ induced a 20-30 mV shift towards more negative potentials of the 'steady state' inactivation curve (which relates maximum force upon full depolarization to the logarithm of the K concentration or the corresponding membrane potential during the conditioning period). 5. The same alteration in concentrations of divalent cations caused little or no change in the shape and potential dependence of the activation curve (which relates maximum force to the logarithm of the external K concentration of the corresponding membrane potential). 6. The threshold potential for the onset of delayed rectification (point voltage clamp) and that for the initiation of an action potential did not change when external Ca2+ was reduced to 10(-9) M and replaced by Mg2+. 7. When the concentration of EGTA2- was increased to 80 mM (in the presence of 5 mM-Mg2+) twitch height dropped to very small values within a few minutes. However, tetanic force (50 Hz) reaching 20-85% of the original value could still be induced after 1 hr in high EGTA2-. 8. The experiments show that external Ca2+ acts upon excitation-contraction coupling mainly by impeding 'inactivation'. A hypothesis is proposed in which the plateau of maximum force during a contracture is the consequence of a regenerative Cai2+-dependent shift of the inactivation curve towards more positive potentials.
Full text
PDF



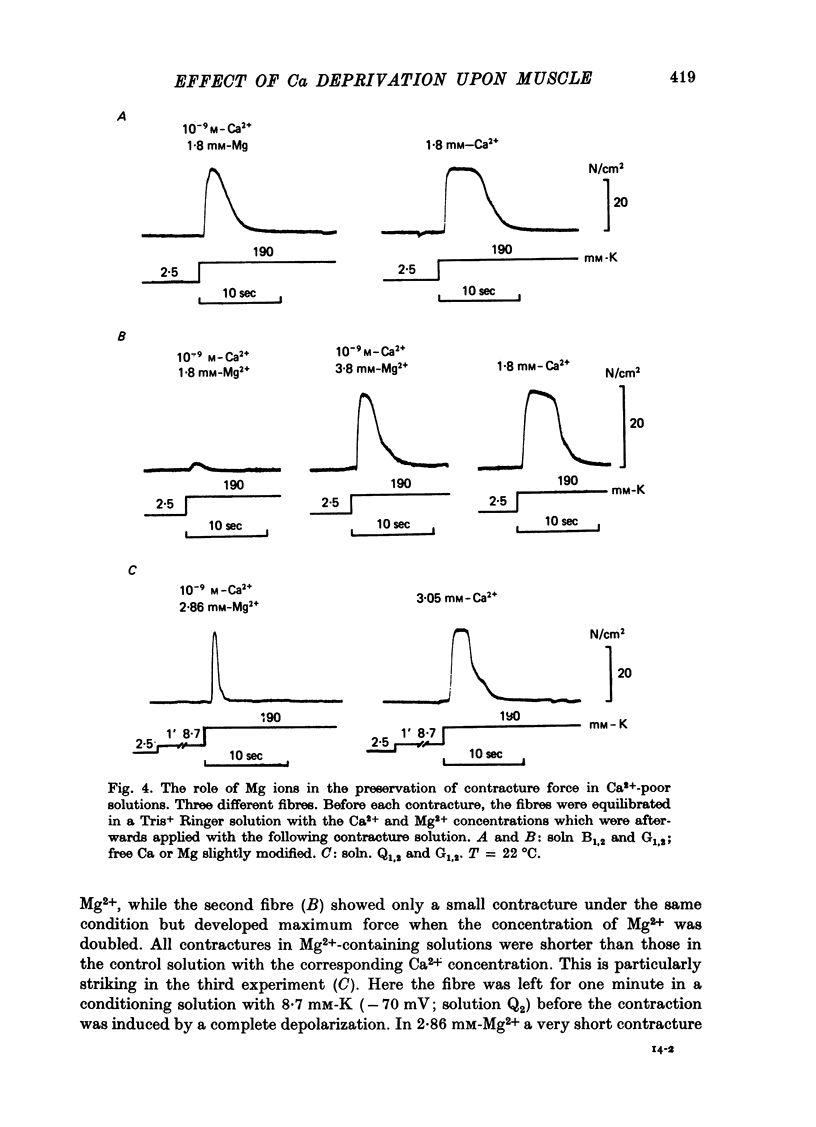

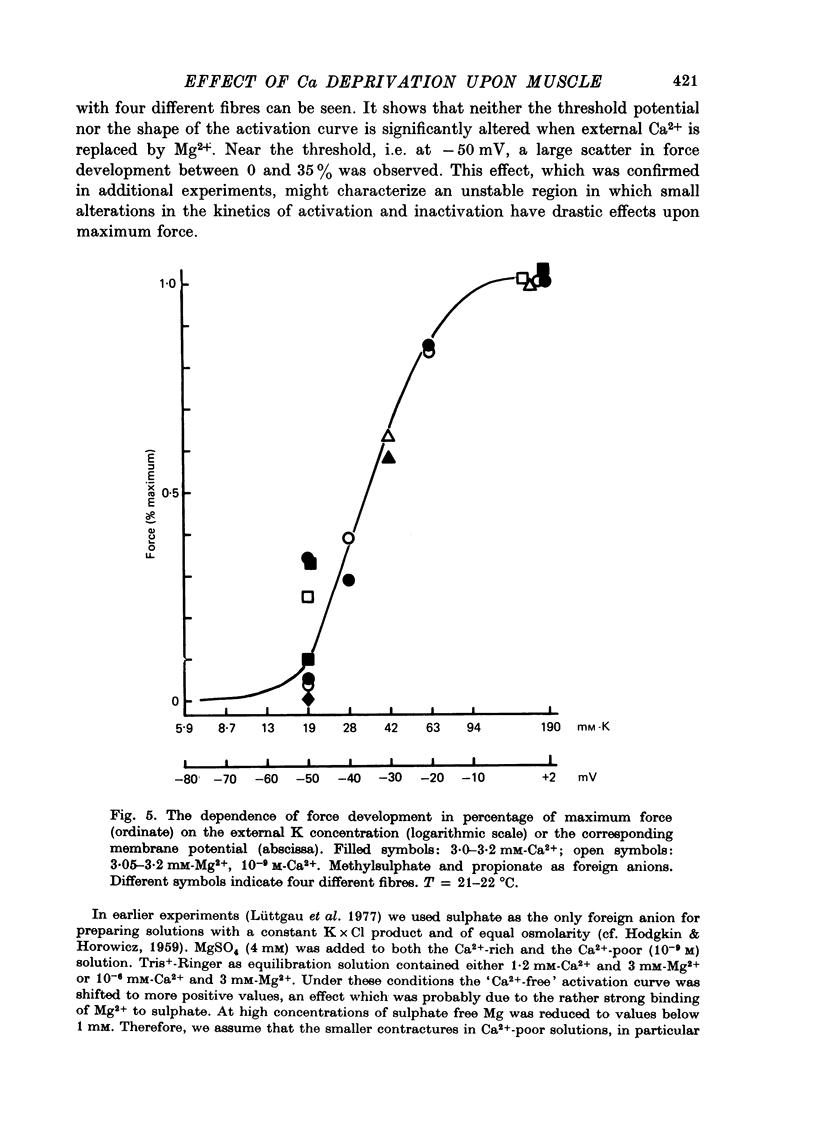
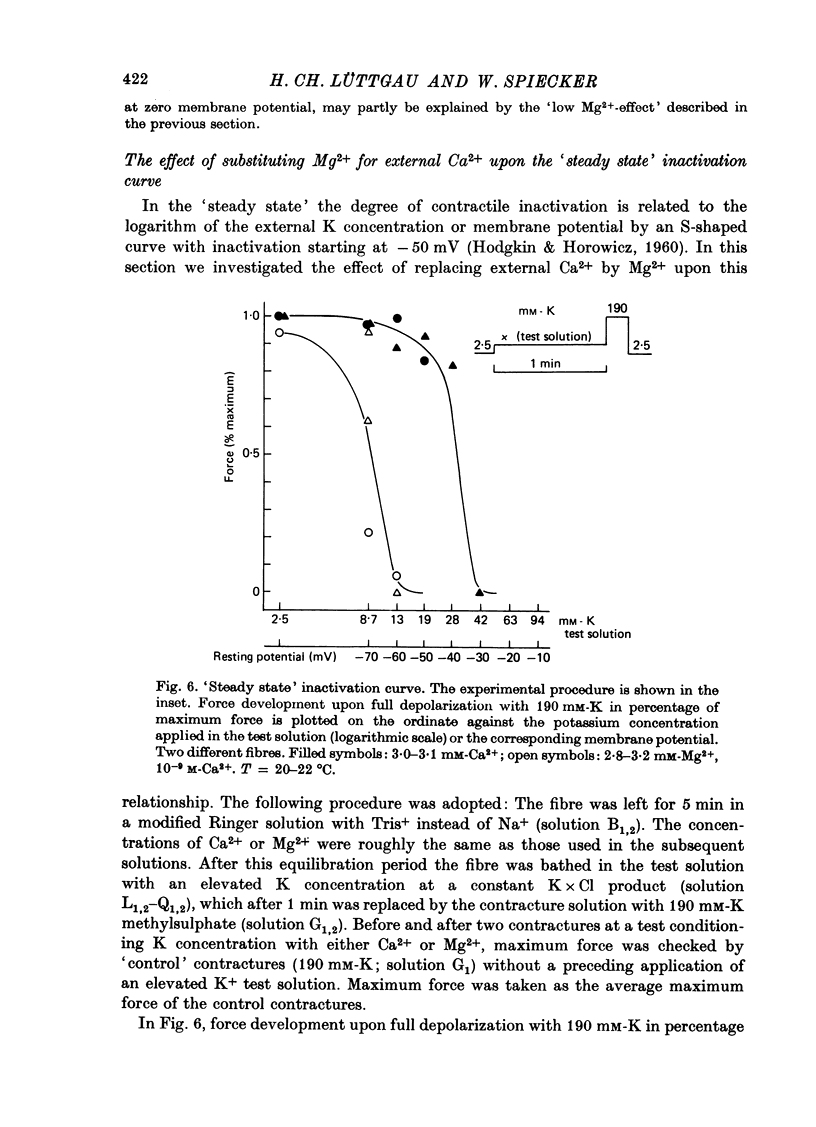

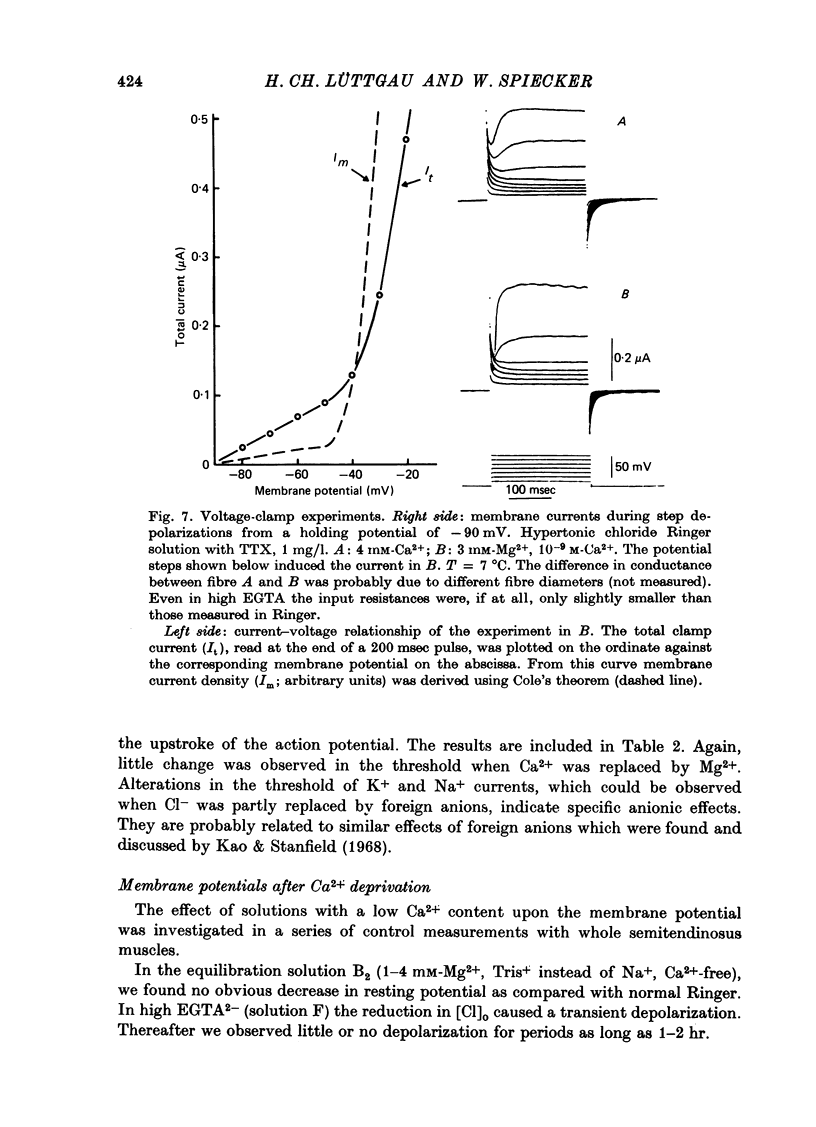





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N., Barrett E. F. Excitation-contraction coupling in skeletal muscle: blockade by high extracellular concentrations of calcium buffers. Science. 1978 Jun 16;200(4347):1270–1272. doi: 10.1126/science.96524. [DOI] [PubMed] [Google Scholar]
- Beaty G. N., Stefani E. Calcium dependent electrical activity in twitch muscle fibres of the frog. Proc R Soc Lond B Biol Sci. 1976 Aug 27;194(1114):141–150. doi: 10.1098/rspb.1976.0070. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Excitation and contraction processes in muscle. Annu Rev Biophys Bioeng. 1978;7:63–83. doi: 10.1146/annurev.bb.07.060178.000431. [DOI] [PubMed] [Google Scholar]
- Caputo C. The effect of low temperature on the excitation-contraction coupling phenomena of frog single muscle fibres. J Physiol. 1972 Jun;223(2):461–482. doi: 10.1113/jphysiol.1972.sp009858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. The time course of potassium contractures of single muscle fibres. J Physiol. 1972 Jun;223(2):483–505. doi: 10.1113/jphysiol.1972.sp009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Biphasic potassium contractures in frog muscle fibers. J Gen Physiol. 1971 Aug;58(2):117–130. doi: 10.1085/jgp.58.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörrscheidt-Käfer M. The action of Ca2+ , Mg2+ and H+ on the contraction threshold of frog skeletal muscle: Evidence for surface charges controlling electro-mechanical coupling. Pflugers Arch. 1976 Mar 11;362(1):33–41. doi: 10.1007/BF00588678. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B., Lännergren J. The effect of calcium on the mechanical response of single twitch muscle fibres of Xenopus laevis. Acta Physiol Scand. 1967 Mar;69(3):242–254. doi: 10.1111/j.1748-1716.1967.tb03518.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut K., Desmedt J. E. Effect of dantrolene sodium on calcium movements in single muscle fibres. Nature. 1974 Dec 20;252(5485):728–730. doi: 10.1038/252728a0. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968 Sep;198(2):291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE EFFECT OF METABOLIC INHIBITORS ON THE FATIGUE OF THE ACTION POTENTIAL IN SINGLE MUSCLE FIBRES. J Physiol. 1965 May;178:45–67. doi: 10.1113/jphysiol.1965.sp007613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- SANDOW A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952 Dec;25(3):176–201. [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Chiarandini D. J. Skeletal muscle: dependence of potassium contractures on extracellular calcium. Pflugers Arch. 1973 Oct 17;343(2):143–150. doi: 10.1007/BF00585709. [DOI] [PubMed] [Google Scholar]
- Takauji M., Nagai T. Effect of dantrolene sodium on the inactivation of excitation-contraction coupling in frog skeletal muscle. Jpn J Physiol. 1977;27(6):743–754. doi: 10.2170/jjphysiol.27.743. [DOI] [PubMed] [Google Scholar]


