Abstract
The BeWo trophoblast cell line does not constitutively express the tryptophan degrading enzyme indolamine 2,3-dioxygenase (IDO), nor can IDO expression be induced by gamma interferon. This correlates with the inability of BeWo cells to control the growth of Chlamydophila abortus, in contrast to effects observed in HeLa cells treated with gamma interferon.
Chlamydophila abortus (previously Chlamydia psittaci immunotype 1) is an obligate intracellular gram-negative bacterium that causes abortion in ruminants and humans (7). Immunological control of this organism is dependent on the ability of the host to mount a cell-mediated response, and protection is strongly associated with gamma interferon (IFN-γ) production (9, 17). This is also true for the related organisms Chlamydia trachomatis and Chlamydophila pneumoniae (24, 25). Control of C. abortus growth by IFN-γ in ovine cells appears to be linked to tryptophan (trp) catabolism, since addition of trp will reverse the effects of IFN-γ and can alter the threshold for IFN-γ-mediated persistence in tissue culture (2). However, the relationship between IFN-γ, trp catabolism, and control of Chlamydia or Chlamydophila spp. is complex and is dependent on both host and pathogen factors, such as sensitivity to IFN-γ, availability of intracellular trp pools, and the presence of functional trp synthase genes in the chlamydial genome (13, 22, 26).
Disease pathogenesis of C. abortus infection follows a distinctive pattern. Abortion in humans appears to be a result of primary gestational infection after direct contact with infected ruminant material, whereas abortion in ruminants can be a result of either primary gestational infection or of a persistent, subclinical infection established prior to pregnancy (7, 12). This link between disease and pregnancy suggests that the organism exploits the immunological and/or physiological status of the pregnant host during this period. By failing to reject the semiallogeneic fetus, the maternal immune system does not react in a manner predicted by the self-nonself model of immune activation, prompting the suggestion that the maternal immune system is suppressed during pregnancy (18). More precisely, it has subsequently been shown that there is immune modulation during pregnancy rather than general maternal immune suppression. This is manifested by a decreased production of IFN-γ and a bias towards Th2-type or regulatory cytokines in the placenta. It appears that production of inflammatory cytokines, particularly at the maternofetal interface, is incompatible with successful pregnancy (5, 23).
Cytokine biasing is not the only mechanism that can explain the maternal acceptance of the semiallogeneic fetus. There is also evidence for induction of maternal T-cell tolerance to the fetal allograft. This is mediated by trp deprivation as a result of constitutive expression of the trp-degrading enzyme indoleamine 2,3-dioxygenase (IDO) in placental trophoblast cells (21). Although this was first shown in mice, IDO expression has subsequently been demonstrated in human placenta trophoblasts (15). This raises an interesting question: why does C. abortus infect trophoblast cells and continue to grow in an environment where trp is readily degraded? It also provokes the following question: what role (if any) does IFN-γ play in IDO expression and control of infection in trophoblast cells?
In an attempt to address these points, an in vitro model of trophoblast infection was established with the BeWo trophoblast cell line. BeWo cells originate from a naturally occurring human choriocarcinoma, and, although transformed, they share several features with normal human trophoblasts (14). They express HLA-G transcripts and they also express functional IFN-γ receptors (6, 8). We therefore reasoned that these cells offer a good system to address our questions. BeWo cells were purchased from the European Collection of Cell Cultures (ECACC; Salisbury, United Kingdom) and were routinely maintained in antibiotic-free Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (FBS; Labtech International, East Sussex, United Kingdom). HeLa cells (ECACC) were used as a control cell line throughout and were cultured under the same conditions as those for BeWo cells.
BeWo (104 cells/well) and HeLa (5 × 103 cells/well) were seeded into 96-well plates (Corning Costar, High Wycombe, United Kingdom) in 100 μl of IMDM supplemented with 5% FBS and were allowed to adhere overnight. The cells were then treated with 100 U of recombinant human IFN-γ (R&D Systems, Abingdon, United Kingdom)/ml and were incubated for a further 24 h. The medium was then removed from the wells, and the cells were infected with C. abortus at a concentration of 5 × 102 inclusion-forming units per well, with a multiplicity of infection of approximately 0.05 to 0.1. The inoculum was removed after 3 h and was replaced with 200 μl of IMDM supplemented with 5% FBS. The cells were cultured for a further 5 days (approximately two cycles of C. abortus growth), and then the supernatants were harvested and analyzed for the presence of chlamydial lipopolysaccharide (LPS) by a specific monoclonal antibody-based capture enzyme-linked immunosorbent assay. This LPS enzyme-linked immunosorbent assay has been shown to correlate well with the number of inclusions in cells and also with the number of infectious elementary bodies in the culture supernatants, thereby giving a rapid quantification of C. abortus growth (2). Each experiment was conducted in quadruplicate and repeated at least twice. Results were analyzed with the Student's t test.
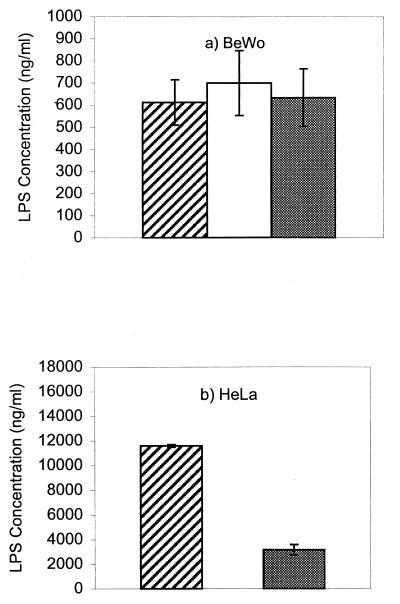
The results of IFN-γ treatment are shown in Fig. 1. Growth of the organism was more rapid in HeLa cells than in BeWo cells, as shown by the amounts of LPS detected. This correlated with lysis of HeLa cells and release of inclusions at this point, whereas there was no lysis in the BeWo cells, despite the presence of multiple inclusions (data not shown). Secondly, treatment of BeWo cells with IFN-γ did not affect the growth of C. abortus, whereas growth was inhibited in HeLa cells (P < 0.001). The failure of BeWo cells to control chlamydial growth in response to IFN-γ treatment is unusual, since this is a common host defense mechanism against this family of organisms. In fact, the failure of IFN-γ to control pathogen growth in BeWo cells was not limited to C. abortus. We also found that neither IFN-γ nor IFN-α could control the replication of Semliki Forest virus in BeWo cells, but both did so in HeLa cells (data not shown). We found no evidence that BeWo cells themselves produce biologically active IFN.
FIG. 1.
Growth of C. abortus in BeWo and HeLa cells cultured in medium alone (hatched bar), medium containing 100 U of IFN-γ/ml (open bar), or medium containing 100 U of IFN-γ/ml and 500 μg of l-tryptophan/ml (filled bar). Error bars represent the standard errors of the means of quadruplicate observations.
Trophoblast cells are a key component of the human hemochorial placenta, forming an interface between the fetus and mother. It is known that there are certain intracellular mechanisms that operate to regulate their response to cytokine exposure. This is important for maintaining the critical balance of invasiveness into maternal decidua while avoiding immunological rejection. For example, there is both in vitro and in vivo evidence that IFN-γ is unable to induce class II expression in trophoblast cells. Detailed studies of JEG-3 cells (a subclone of BeWo) have shown that this is not due to a lack of IFN-γ receptors but rather is due to intracellular regulatory events following receptor ligation, specifically negative regulation or silencing of the class II transactivator. However, IFN-γ can induce transcription of the genes encoding the Janus kinases (Jak1 and Jak2) and also the signal transducer and activator of transcription (STAT1α), demonstrating that the signaling components of the IFN-γ receptor are operational (10, 20). Since C. abortus grows in BeWo cells irrespective of IFN-γ treatment, we were interested to know if these cells were deficient in the constitutive and/or induced expression of IDO. This was done by studying expression of mRNA encoding IDO in BeWo and HeLa cells with PCR following IFN-γ treatment.
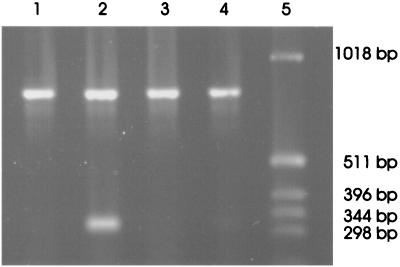
BeWo and HeLa cells were grown to subconfluence in 75-cm2 tissue culture flasks (Corning Costar) and then were treated with 100 U of IFN-γ/ml. Total RNA was isolated from the cells after a further 24 h using the acid phenol/guanidine thiocyanate method (3). RNA (2 μg) was converted to single-stranded cDNA with AMV Reverse Transcriptase (Roche, Lewes, United Kingdom) and oligo(dT)12-15 as the primer. Thirty cycles of the PCR, each of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C, were then performed in order to amplify either the IDO cDNA or the GAPDH cDNA (as an internal control). The primers used for amplifying the IDO cDNA (11) should result in the amplification of a 324-bp fragment of the IDO cDNA, whereas the primers 5′-CCCATGGCAAGTTCCACGGC-3′ and 5′-CACCCTGTTGCTGTAGCCGA-3′ should amplify a 818-bp fragment of the GAPDH cDNA. The results of the PCR amplification are presented in Fig. 2. There is no constitutive expression of mRNA encoding IDO in either cell line. However, expression was induced by IFN-γ in HeLa cells but not in BeWo cells. This pattern fits with the observations shown in Fig. 1, namely that C. abortus grows in both cell lines and that IFN-γ inhibits the growth in HeLa but not in BeWo cells. Based on the assumption that this data reflects trp degradation by IDO, addition of trp to the cells should reverse the antichlamydial effects of IFN-γ.
FIG. 2.
Products from reverse transcription-PCR amplification of IDO and GAPDH mRNAs visualized on a 1% agarose gel. Lanes 1 to 4 correspond to the amplification products obtained from RNA isolated from HeLa cells grown in medium alone, HeLa cells treated with 100 U of IFN-γ/ml, BeWo cells in medium alone, and BeWo cells treated with 100 U of IFN-γ/ml, respectively. Lane 5 contains a 1-kb molecular size marker.
After removal of the inoculum, l-trp (Sigma, Poole, United Kingdom) was added to the culture medium to a final concentration of 500 μg/ml. The results are shown in the final columns in Fig. 1. Addition of trp made no difference to the growth of C. abortus in BeWo cells (P > 0.5) but resulted in growth of the organism in IFN-γ-treated HeLa cells (P < 0.001). The reversal in the HeLa cells was not complete, probably because the antichlamydial effects of IFN-γ are highly dose dependent such that as the concentration of IFN-γ increases, the effect of trp decreases (2).
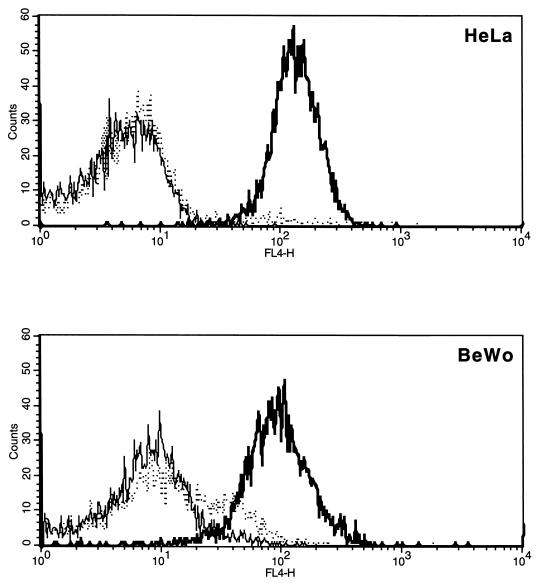
BeWo cells do not express IDO either constitutively or in response to IFN-γ treatment. We have confirmed that the lack of IFN-γ responsiveness is not due to the failure of BeWo cells to express the IFN-γ receptor (CD119). BeWo and HeLa cells were removed from flasks by using nonenzymatic detachment (Accutase; Innovative Cell Technologies, La Jolla, Calif.) and reacted with a mouse monoclonal antibody recognizing human CD119 (Serotec, Oxford, United Kingdom). Cells were then reacted with a biotin conjugated goat anti-mouse immunoglobulin (Dako, Cambridge, United Kingdom) followed by streptavidin-allophycocyanin (Becton Dickinson, San Jose, Calif.). VPM 20, a mouse monoclonal antibody against a ruminant pestivirus nonstructural protein, was used as an isotype-matched (immunoglobulin G2a) control. Data acquisition and analysis was performed with a two-laser four-color FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Mountain View, Calif.). Both BeWo and HeLa cells stained positively for CD119 (Fig. 3). There is some background staining with the control antibody on BeWo cells, most likely as a result of binding via Fc receptors (FcR).
FIG. 3.
Flow cytometry analysis of IFN-γ receptor (CD119) expression on BeWo and HeLa cells. Cells were stained by a three-step procedure involving a primary mouse monoclonal antibody followed by an anti-mouse biotin conjugate and then streptavidin-allophycocyanin. Cells stained with a monoclonal antibody reacting with human CD119 are shown by the heavy solid line. The dotted line shows cells reacted with an isotype-matched control, and the light solid line shows cells stained with the second- and third-step reagents only.
The BeWo cell line, therefore, offers a useful system for dissecting the mechanisms of control of intracellular pathogens, particularly those pathogens that are under control of IFN-γ, where multiple intracellular biosynthetic pathways are triggered. For example, it has been suggested that the persistence, pathogenesis, and tissue tropism of different species and strains of C. trachomatis are linked to IFN-γ responsiveness and trp biosynthesis (26). It is also known that not all gynecologic cancer cells respond to IFN-γ treatment by upregulating IDO expression, compatible with our observations of BeWo cells (16). Interestingly, constitutive IDO expression by tolerizing the reactive T cells via trp starvation may be one method of tumor immune evasion (19). This is not the case with BeWo, despite their placental origin. It would be interesting to study this in other trophoblast lines, but to date many of the established lines have a common origin (19).
These data also support the fact that immunological control of pathogens at the maternofetal interface is different from that in the periphery. The intracellular and extracellular regulatory mechanisms that are in place to prevent maternal allo-rejection render this tissue particularly susceptible to certain invasive intracellular organisms, such as C. abortus and Toxoplasma gondii. It should be noted that constitutive IDO expression is not uniform in human placental trophoblast, as there are areas of low expression (15). This could explain why C. abortus grows in an apparently inhospitable environment.
The mechanisms of infectious abortion vary from organism to organism and reflect a close link between the levels of hormones essential for the maintenance of pregnancy and the cytokine environment (7). The very presence of inflammatory cytokines, such as tumor necrosis factor alpha, interleukin 2, and IFN-γ, can be abortifacient (23). It is therefore possible that infectious abortion represents a form of immunological amputation of the infected fetal unit for the preservation of the mother (4). Although the self-nonself and Danger models of immune activation have different explanations for the role of IDO in the placenta and maternal acceptance of the semiallogeneic fetus, both models predict that placental infection presents a problem for pathogen control without causing fetal damage (1). Therefore, by studying the mechanisms of C. abortus-induced pathology we may also elucidate some of the immunoregulatory processes involved in the maintenance of normal pregnancy.
Acknowledgments
This work was funded by the Scottish Executive Environmental and Rural Affairs Department (SEERAD).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bonney, E. A., and P. Matzinger. 1998. Much IDO about pregnancy. Nat. Med. 4:1128-1129. [DOI] [PubMed] [Google Scholar]
- 2.Brown, J., S. E. M. Howie, and G. Entrican. 2001. A role for tryptophan in immune control of chlamydial abortion in sheep. Vet. Immunol. Immunopathol. 82:107-119. [DOI] [PubMed]
- 3.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidine thiocyanate-phenol-chloroform extraction. Analyt. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 4.Clark, D. A., G. Chaouat, P. C. Arck, H. W. Mittruecker, and G. A. Levy. Cytokine-dependent abortion in CBA × DBA/2 mice is mediated by the procoagulant fgl2 prothombinase. J. Immunol. 160:545-549. [PubMed]
- 5.Dealtry, G. B., M. K. O'Farrell, and N. Fernandez. 2000. The Th2 cytokine environment of the placenta. Int. Arch. Allergy Immunol. 123:107-119. [DOI] [PubMed] [Google Scholar]
- 6.Ellis, S. A., M. S. Palmer, and A. J. McMichael. 1990. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA class I molecule. J. Immunol. 144:731-735. [PubMed] [Google Scholar]
- 7.Entrican, G., D. Buxton, and D. Longbottom. 2001. Chlamydial infection in sheep: immune control versus fetal pathology. J. Royal Soc. Med. 94:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulop, V., M. A. Steller, R. S. Berkowitz, and D. J. Anderson. 1992. Interferon-gamma receptors on human gestational choriocarcinoma cell lines: quantitative and functional studies. Am. J. Obstet. Gynecol. 167:524-530. [DOI] [PubMed] [Google Scholar]
- 9.Graham, S. P., G. E. Jones, M. MacLean, M. Livingstone, and G. Entrican. 1995. Recombinant ovine interferon gamma inhibits the multiplication of Chlamydia psittaci in ovine cells. J. Comp. Pathol. 112:185-195. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson, E., M. Arvola, U. Brunsberg, A. Mattsson, and R. Mattsson. 1997. Lack of detectable major histocompatibility complex class II, a beta-chain messenger ribonucleic acid in placentas of interferon-gamma- and 5-azacytidine-treated mice. Biol. Reprod. 57:715-722. [DOI] [PubMed] [Google Scholar]
- 11.Hissong, B. D., and J. M. Carlin. 1997. Potentiation of interferon-induced indolamine 2,3-dioxygenase mRNA in human mononuclear phagocytes by lipopolysaccharide and interleukin-1. J. Interferon Cytokine Res. 17:387-393. [DOI] [PubMed] [Google Scholar]
- 12.Hyde, S. R., and K. Benirschke. 1997. Gestational psittacosis: case report and literature review. Mod. Pathol. 10:602-607. [PubMed] [Google Scholar]
- 13.Kane, C. D., R. M. Vena, S. P. Ouellette, and G. I. Byrne. 1999. Intracellular tryptophan pool sizes may account for differences in gamma interferon-mediated inhibition and persistence of chlamydial growth in polarized and nonpolarized cells. Infect. Immun. 67:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King, A., L. Thomas, and P. Bischof. 2000. Cell culture models of trophoblast II: trophoblast cell lines-a workshop report. Placenta 21:113-119. [DOI] [PubMed] [Google Scholar]
- 15.Kudo, Y., and C. A. R. Boyd. 2000. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochim. Biophys. Acta 1500:119-124. [DOI] [PubMed] [Google Scholar]
- 16.Leung, B. S., L. E. Stout, E. G. Shaskan, and R. M. Thompson. 1992. Differential induction of indoleamine-2,3-dioxygenase (IDO) by interferon-γ in human gynecologic cancer cells. Cancer Lett. 66:77-81. [DOI] [PubMed] [Google Scholar]
- 17.McCafferty, M. C., S. W. Maley, G. Entrican, and D. Buxton. 1994. The importance of interferon-gamma in an early infection of Chlamydia psittaci in mice. Immunology 81:631-636. [PMC free article] [PubMed] [Google Scholar]
- 18.Medawar, P. B. 1953. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7:320-338. [Google Scholar]
- 19.Mellor, A. L., and D. H. Munn. 1999. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today 20:469-473. [DOI] [PubMed] [Google Scholar]
- 20.Morris, A. C., J. L. Riley, W. H. Fleming, and J. M. Boss. 1998. MHC class II gene silencing in trophoblast cells is caused by inhibition of CIITA expression. Am. J. Reprod. Immunol. 40:385-394. [DOI] [PubMed] [Google Scholar]
- 21.Munn, D. H., M. Zhou, J. T. Attwood, I. Bondarev, S. J. Conway, B. Marshall, C. Brown, and A. L. Mellor. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191-1193. [DOI] [PubMed] [Google Scholar]
- 22.Perry, L. L., H. Su, K. Feilzer, R. Messer, S. Hughes, W. Whitmire, and H. D. Caldwell. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 23.Ragupathy, R. 1997. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 18:478-482. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen, S. J. 1998. Chlamydia immunology. Curr. Opin. Infect. Dis. 11:37-41. [DOI] [PubMed] [Google Scholar]
- 25.Rottenberg, M. E., A. G. Rothfuchs, D. Gigliotti, M. Ceausu, C. Une, V. Levitsky, and H. Wigzell. 2000. Regulation and role of IFN-γ in the innate resistance to infection with Chlamydia pneumoniae. J. Immunol. 164:4812-4818. [DOI] [PubMed] [Google Scholar]
- 26.Shaw, A. C., G. Cristiansen, P. Roepstorff, and S. Birkelund. 2000. Genetic differences in the Chlamydia trachomatis tryptophan synthase α-subunit can explain variations in serovar pathogenesis. Microbes Infect. 2:581-592. [DOI] [PubMed] [Google Scholar]