Abstract
1. Isolated hemisected spinal cords of the frog have been used to investigate the way excitant amino acids depolarize primary afferent fibres and terminals.
2. GABA and excitant amino acids caused depolarization in dorsal roots. But dorsal roots sectioned at the point of exit from the spinal cord responded only to GABA.
3. Prolonged application of kainate or N-methyl-D-aspartate to hemicords caused a depolarization of dorsal roots in association with an increased extra-cellular [K+]. The two effects decayed with similar time courses. The depolarization recorded from ventral roots was maintained in the presence of the excitants.
4. Field potentials, elicited by electrical stimulation of ventral roots and recorded in the ventral horn of Mg blocked preparations, were abolished by prolonged treatment with kainate. Corresponding dorsal horn field potentials elicited by electrical stimulation of dorsal roots were resistant to the presence of kainate.
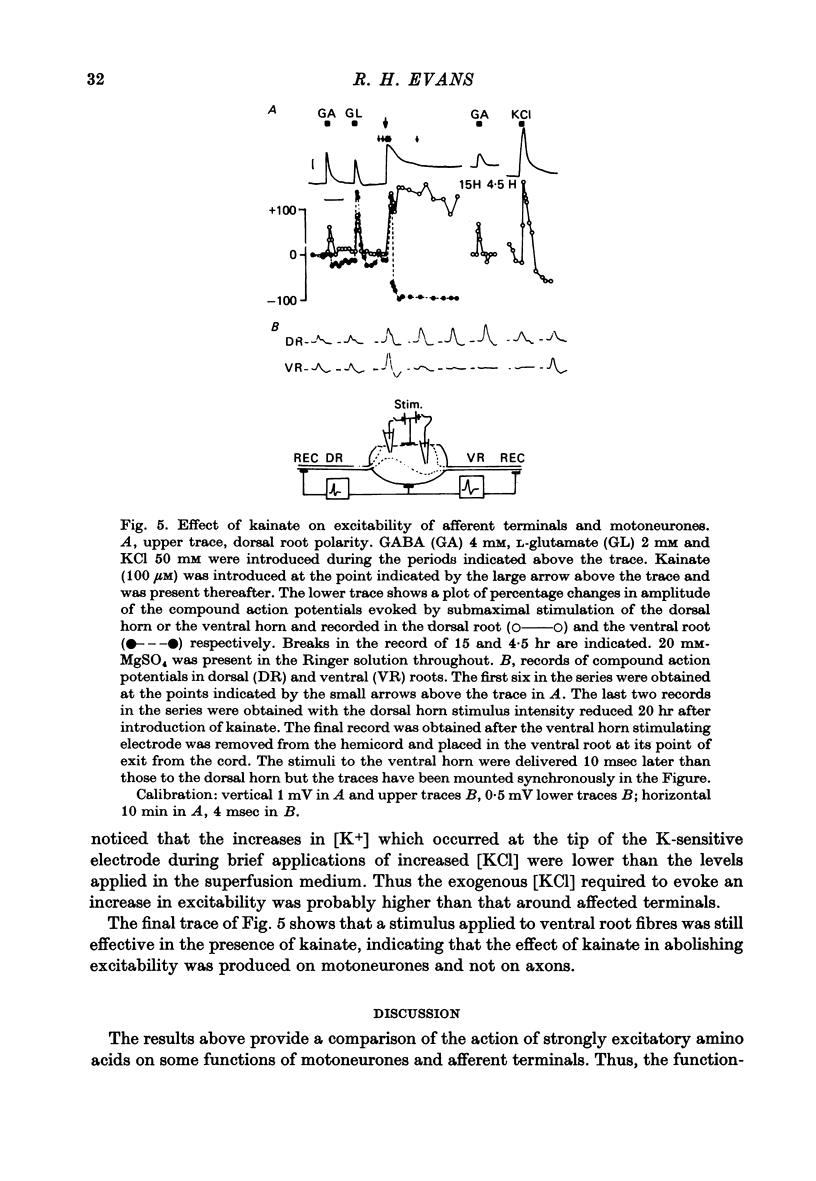
5. Excitability of motoneurones or afferent terminals was measured from the amplitude of action potentials evoked by submaximal cathodal stimulation of ventral or dorsal horns and recorded in ventral or dorsal roots respectively. Prolonged application of kainate to Mg blocked preparations abolished the excitability of motoneurones within 5 min, but the excitability of primary afferent terminals was increased and maintained for several hours.
6. These observations support the hypothesis that primary afferent terminals in the frog do not have receptors for excitatory amino acids and that depolarization of terminals induced by excitatory amino acids is mediated through release of K from other cells within the dorsal horn.
Full text
PDF

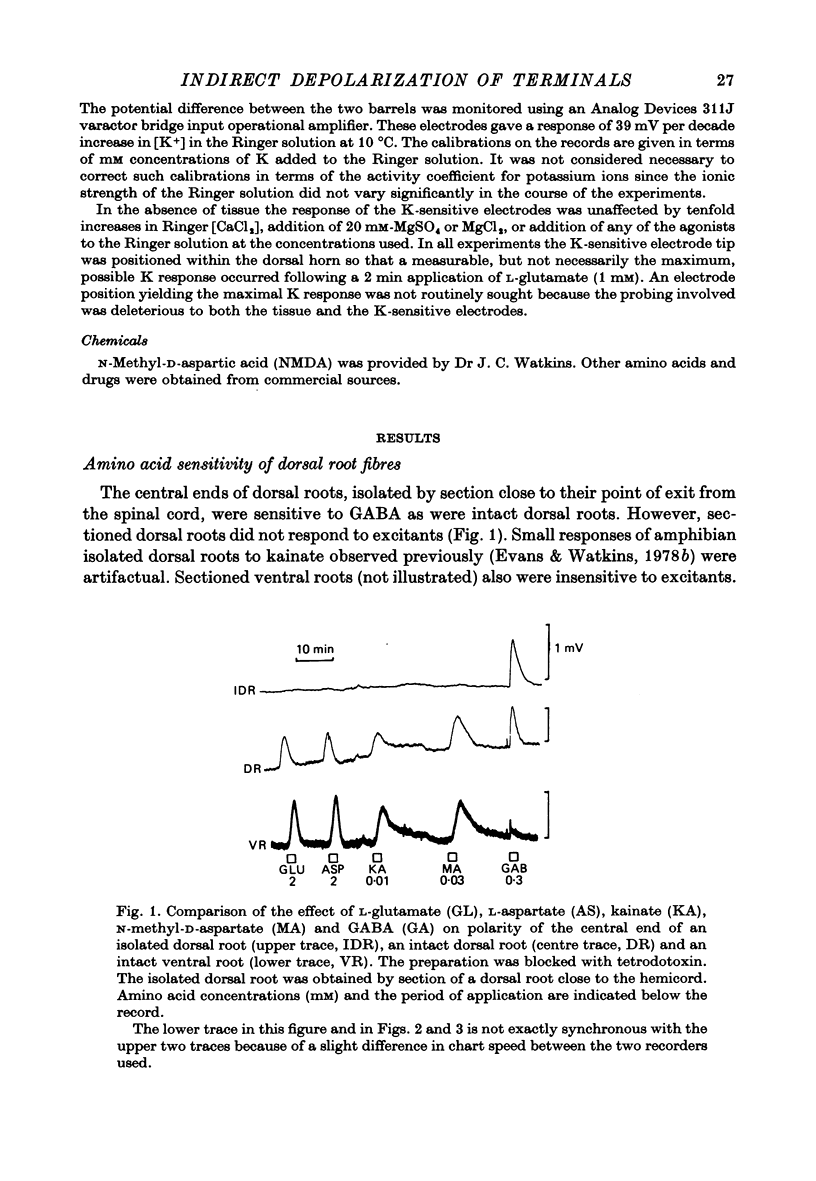
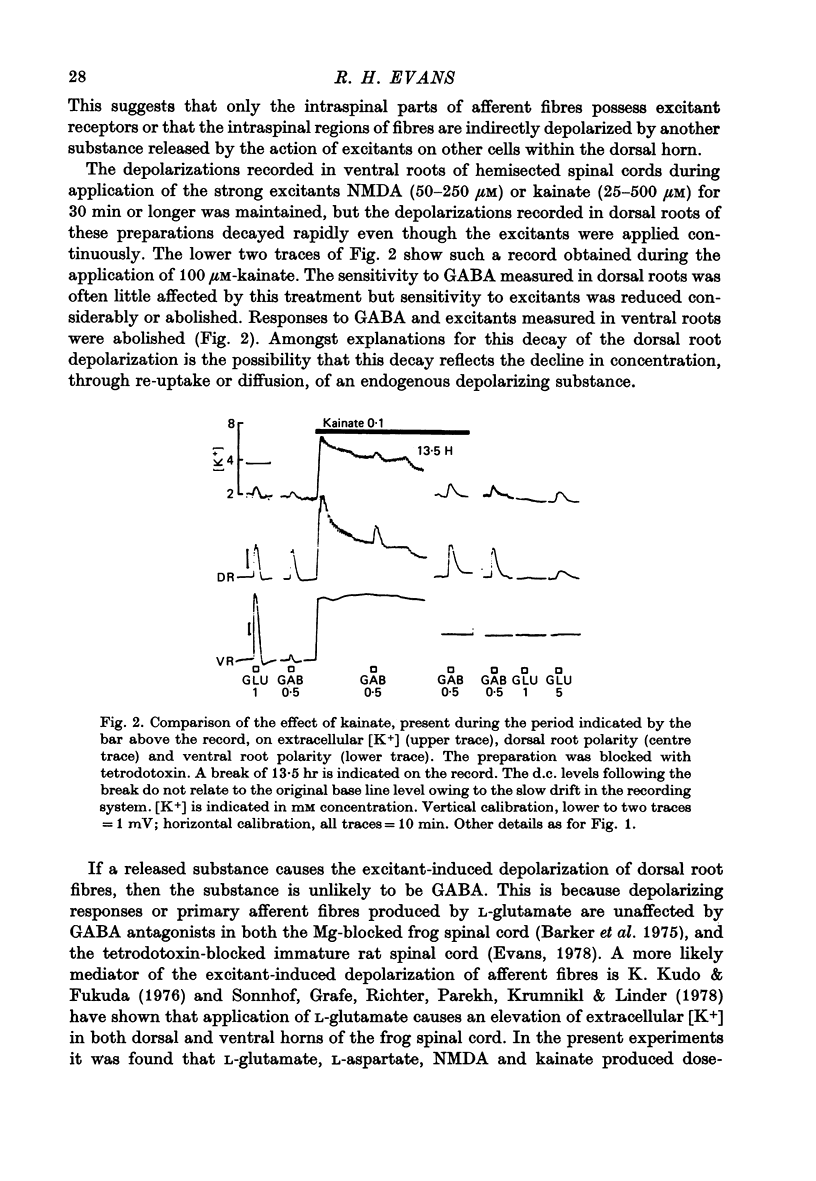
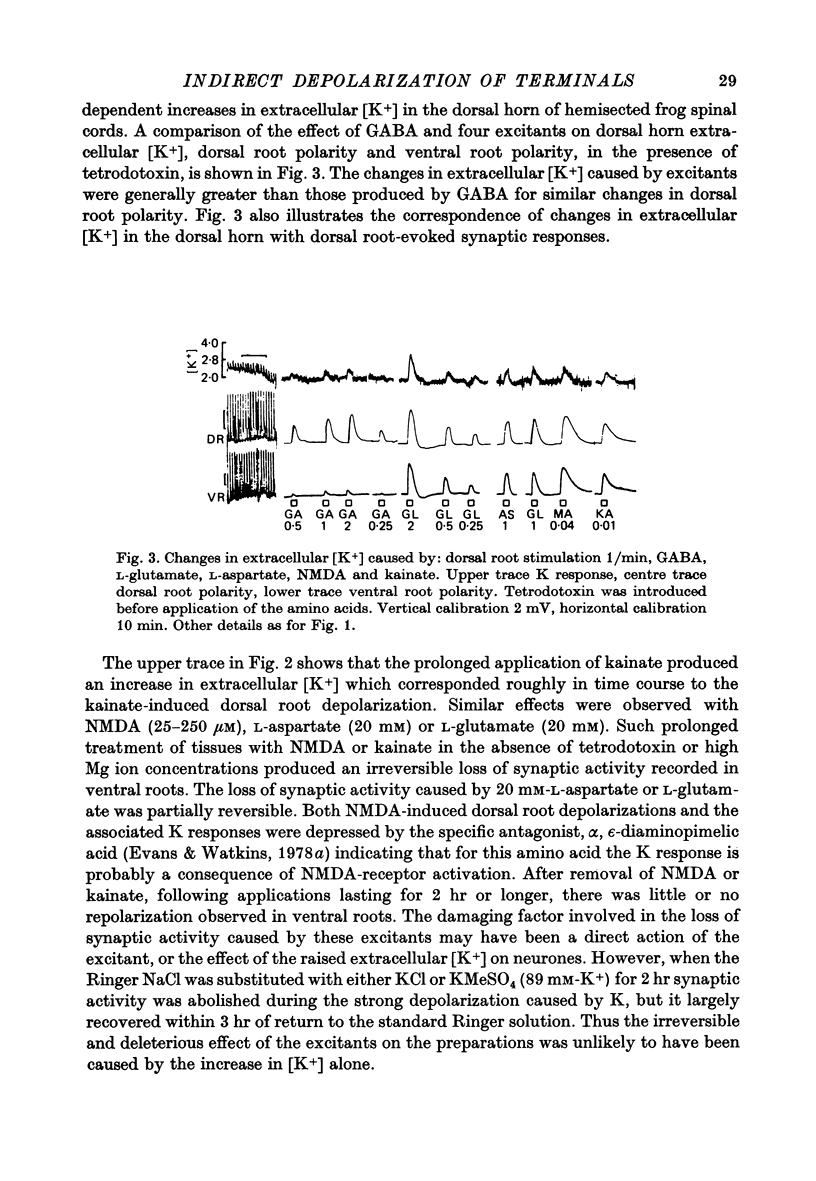

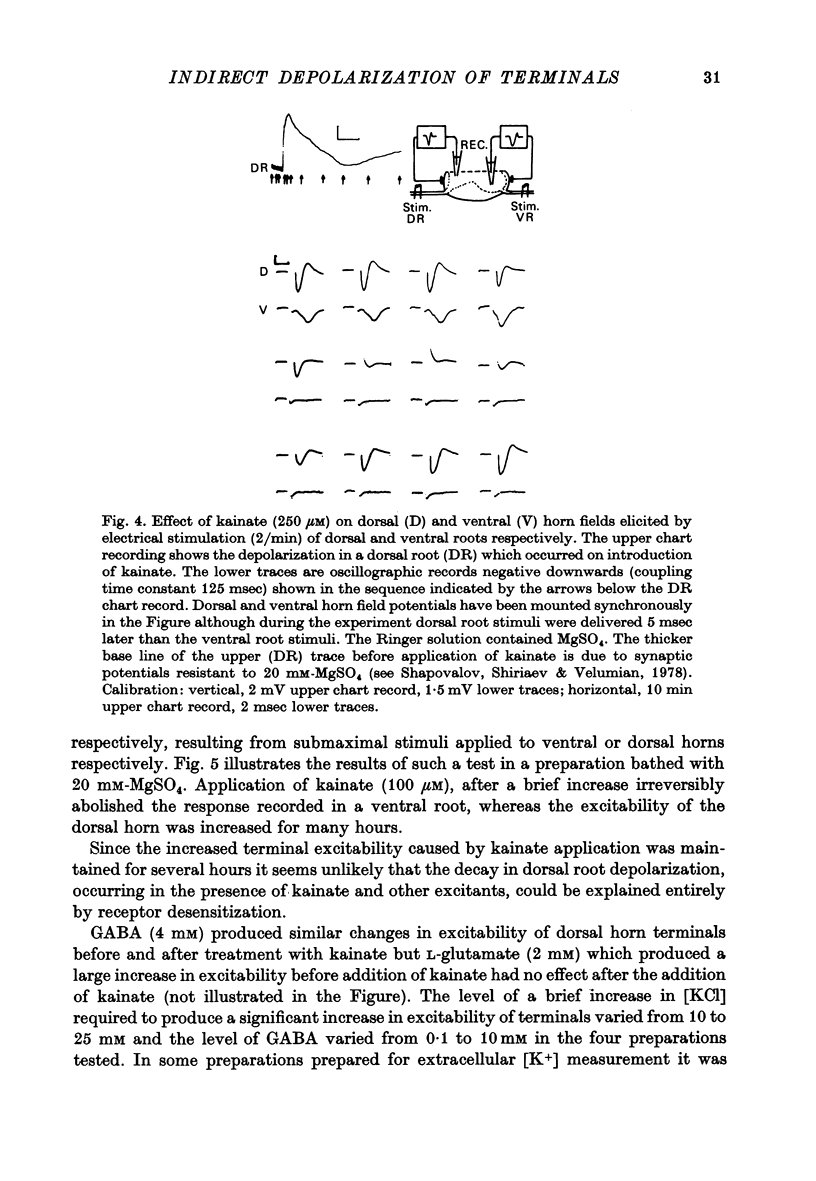



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975 Mar;245(3):521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Headley P. M., Martin M. R., Watkins J. C. Structure-activity relations of excitatory amino acids on frog and rat spinal neurones. Br J Pharmacol. 1976 Nov;58(3):373–382. doi: 10.1111/j.1476-5381.1976.tb07714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960 Mar;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., Teb ecis A. K., Watkins J. C. Excitation of mammalian central neurones by acidic amino acids. Brain Res. 1972 Jun 22;41(2):283–301. doi: 10.1016/0006-8993(72)90503-3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Brand S. J. GABA and spinal afferent terminal excitability in the cat. Brain Res. 1977 Jul 15;130(2):360–363. doi: 10.1016/0006-8993(77)90283-9. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M., Saum W. R. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Sep 15;44(1):273–277. doi: 10.1016/0006-8993(72)90383-6. [DOI] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The action of N-methyl-D-aspartic and kainic acids on motoneurones with emphasis on conductance changes [proceedings]. Br J Pharmacol. 1978 Nov;64(3):384P–385P. [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol. 1979 Mar;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- Evans R. H. Further evidence suggesting the absence of acidic amino acid receptors on primary afferent terminals in the frog [proceedings]. J Physiol. 1979 Aug;293:68P–68P. [PubMed] [Google Scholar]
- Evans R. H. The effects of amino acids and antagonists on the isolated hemisected spinal cord of the immature rat. Br J Pharmacol. 1978 Feb;62(2):171–176. doi: 10.1111/j.1476-5381.1978.tb08442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Watkins J. C. Do primary afferent terminals have acidic amino acid receptors? [proceedings]. Br J Pharmacol. 1978 Nov;64(3):385P–386P. [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Watkins J. C. Specific antagonism of excitant amino acids in the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1978 Jul 15;50(2):123–129. doi: 10.1016/0014-2999(78)90007-9. [DOI] [PubMed] [Google Scholar]
- Feltz P., Rasminsky M. A model for the mode of action of GABA on primary afferent terminals: depolarizing effects of GABA applied iontophoretically to neurones of mammalian dorsal root ganglia. Neuropharmacology. 1974 Jun;13(6):553–563. doi: 10.1016/0028-3908(74)90145-2. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y., Fukuda H. Alteration of extracellular K+-activity induced by amino acids in the frog spinal cord. Jpn J Pharmacol. 1976 Jun;26(3):385–387. doi: 10.1254/jjp.26.385. [DOI] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Sharif N. A. Effects of l-glutamate and related amino acids upon the release of [3H]dopamine from rat striatal slices. Brain Res. 1978 Nov 24;157(2):391–395. doi: 10.1016/0006-8993(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Velumian A. A. Mechanisms of post-synaptic excitation in amphibian motoneurones. J Physiol. 1978 Jun;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]


