Abstract
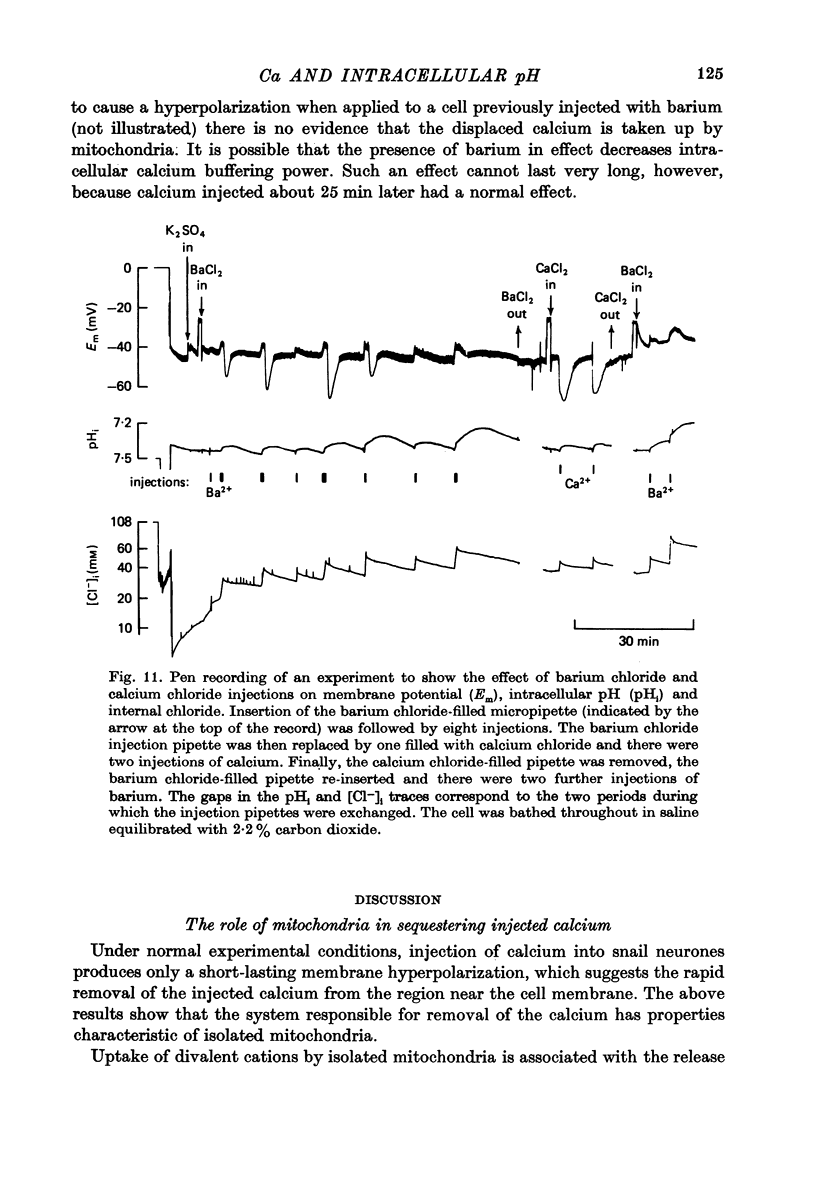
1. Ion-sensitive micro-electrodes were used to measure changes in intracellular pH (pHi) and internal chloride which resulted from the pressure injection of calcium chloride into identified Helix aspersa neurones. The internal chloride measurement allowed the quantity of calcium chloride injected to be estimated. 2. Application of the metabolic inhibitor carbonyl cyanide m-chlorophenyl hydrazone (CCmP) to a calcium-loaded cell caused an increase in the membrane potential comparable to the effect of injecting calcium itself. The effect was not observed in normal cells. 3. When injected in the presence of CCmp, calcium caused a much larger and longer-lasting effect on the membrane potential than that observed in untreated cells. 4. The injection of ruthenium red can increase and/or prolong the hyperpolarization caused by a given quantity of injected calcium. The pHi changes following calcium injection were biphasic and slower than normal. 5. In seven experiments, both hydrogen chloride and calcium chloride were injected into the same cell. The relative changes in pHi corresponded to the production of one hydrogen ion for each calcium ion injected. 6. The relationship between the quantity of calcium injected and the size of the induced hyperpolarization suggested that at least three calcium ions acting cooperatively are required to activate a potassium 'channel'. 7. Injection of barium chloride hyperpolarized the membrane after a delay of about 1 min. After several injections of barium the cell lost this response while retaining its normal response to calcium injection. Injection of barium also caused a slowly developing (biphasic) fall in pHi. 8. We conclude that injected calcium is normally rapidly taken up by mitochondria in exchange for hydrogen ions. If this uptake process is blocked by ruthenium red or CCmP, the calcium is taken up by a second, slower, process which also releases hydrogen ions. When pre-loaded, but not otherwise, the mitochondria will release calcium ions on treatment with CCmP. Injection of barium does not directly affect the membrane conductance, but causes the release of calcium from intracellular binding sites.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLEY W., AMOORE J. E. The effects of manganese on the solute content of rat-liver mitochondria. Biochem J. 1958 Jul;69(3):348–360. doi: 10.1042/bj0690348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Levitan H. Phenols: effects on membrane permeability of molluscan neurons. Brain Res. 1974 Mar 8;67(3):555–561. doi: 10.1016/0006-8993(74)90506-x. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A. Mitochondria and other calcium buffers of squid axon studied in situ. J Gen Physiol. 1978 Jul;72(1):101–127. doi: 10.1085/jgp.72.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- Carvalho A. P. Binding and release of cations by sarcoplasmic reticulum before and after removal of lipid. Eur J Biochem. 1972 Jun 9;27(3):491–502. doi: 10.1111/j.1432-1033.1972.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F. Ion exchange properties of the calcium receptor site of troponin. Biochim Biophys Acta. 1971 Aug 6;245(1):221–229. doi: 10.1016/0005-2728(71)90025-9. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Effects of acetylcholine and parasympathetic nerve stimulation on membrane potential in quiescent guinea-pig atria. J Physiol. 1978 Jun;279:655–668. doi: 10.1113/jphysiol.1978.sp012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela L., Wrobel-Kuhl K. Special characteristics of brain mitochondrial calcium accumulation. Ann N Y Acad Sci. 1978 Apr 28;307:242–245. doi: 10.1111/j.1749-6632.1978.tb41953.x. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Normann T. C., Hall T. A. Calcium and sulphur in neurosecretory granules and calcium in mitochondria as determined by electron microscope x-ray microanalysis. Cell Tissue Res. 1978 Jan 31;186(3):453–463. doi: 10.1007/BF00224934. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Comparison of the mechanisms controlling intracellular pH and sodium in snail neurones. Respir Physiol. 1978 Apr;33(1):63–73. doi: 10.1016/0034-5687(78)90085-3. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Manger J. R., Harris E. J. Cation uptake as the basis for production of proton pulses by mitochondria at the anaerobic-aerobic transition. Eur J Biochem. 1969 Dec;11(3):413–418. doi: 10.1111/j.1432-1033.1969.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASINGTON F. D., MURPHY J. V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962 Aug;237:2670–2677. [PubMed] [Google Scholar]
- Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta. 1972 Jan 21;256(1):43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Brown H. M. Intracellular ionic activity measurements in nerve and muscle. Physiol Rev. 1977 Oct;57(4):729–778. doi: 10.1152/physrev.1977.57.4.729. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]


