Abstract
Cystic fibrosis patients infected with strains from different genomovars of the Burkholderia cepacia complex can experience diverse clinical outcomes. To identify genomovar-specific determinants that might be responsible for these differences, we developed a pulmonary model of infection in BALB/c mice. Mice were rendered leukopenic by administration of cyclophosphamide prior to intranasal challenge with 1.6 × 104 bacteria. Five of six genomovar II strains persisted at stable numbers in the lungs until day 16 with minimal toxicity, whereas zero of seven genomovar III strains persisted but resulted in variable toxicity. We have developed a chronic pulmonary model of B. cepacia infection which reveals differences among genomovars in terms of clinical infection outcome.
Originally described as the cause of soft rot in damaged onion bulbs (2), Burkholderia cepacia has since been identified as a uniquely problematic opportunistic pathogen in patients with cystic fibrosis (CF) (15, 17, 33). B. cepacia is an extremely diverse class of bacteria, and the taxonomy has evolved to accommodate the enhanced understanding of the new genus within which it has been placed (34). At least nine novospecies, termed genomovars, now comprise the B. cepacia complex (BCC) (P. Vandamme, D. Henry, T. Coenye, S. Laevens, J. J. LiPuma, D. P. Speert, J. R. W. Govan, and E. Mahenthiralingam, Sixth Meet. Int. B. cepacia Working Group, abstr. 26, p. 18, 2001).
CF is the most common potentially lethal autosomal recessive disease in North America, affecting approximately 1 in 2,000 live births among Caucasians. CF patients are extremely susceptible to chronic bacterial pulmonary infections, with a characteristic spectrum of pathogens (9). The majority of chronic CF infections are caused by Pseudomonas aeruginosa; however, infection with B. cepacia is also of considerable concern (9). Infection with BCC is associated with a worse prognosis than infection with P. aeruginosa (13). Some strains of BCC are associated with aggressive pneumonia accompanied by bacteremia that is rapidly fatal, in contrast to the generally slowly progressive and noninvasive nature of P. aeruginosa infections (10). Moreover, differential pathogenicity among strains within the complex has been observed in CF patients. Approximately 80% of BCC clinical isolates from Canada are from genomovar III; the most highly transmissible strains also belong to this genomovar, which is also associated with a higher mortality rate than other members of the complex (30). In Canada, Burkholderia multivorans appears to be nontransmissible (except between siblings) and is associated with a relatively benign clinical outcome (30). Although many studies have endeavored to elucidate BCC virulence factors, the basis of this differential pathogenicity among genomovars is unknown.
To better understand the interactions between BCC bacteria and the host, an animal model was required that would sustain a persistent infection and facilitate exploration of the host defenses against this group of organisms. This paper describes a murine model of sustained pulmonary infection that demonstrates differences among the genomovars.
The bacterial strains used in this study are listed in Table 1. They were stored at −70°C in Mueller-Hinton broth with 8.0% dimethyl sulfoxide. All strains were subcultured on blood agar plates (PML Microbiologicals, Richmond, Canada), and viable counts were enumerated on tryptic soy agar (TSA) plates (Becton Dickinson, Cockeysville, Md.). Animal studies were approved by the University of British Columbia Animal Care Committee (UBC-ACC A98-0130). BALB/c mice were purchased from Charles River Laboratories (St. Constant, Quebec, Canada). Females aged 6 to 8 weeks were used in each experiment. The general health of animals was assessed daily on a three-point system based upon weight, food and water consumption, and general appearance. Animals were determined to be unwell when two of the three following observations were made: >10% weight loss, <3 g of water or food consumption daily, or general ill appearance (ruffled coats, huddled position, lack of retreat in handler's presence). For immunosuppression, mice were anesthetized with gaseous methoxyflurane (Janssen, Toronto, Canada). Cyclophosphamide (CPA; 150 mg/kg of body weight; Bristol, Montreal, Canada) was then administered intraperitoneally (i.p.). CPA injections were given on days −1, 4, 9, and 14 of each experiment. On days 0, 2, 4, and 16, mice were anesthetized i.p. with 65 mg of sodium pentobarbitol/kg (MTC Pharmaceuticals, Cambridge, Canada) and exsanguinated by cardiac puncture. Total peripheral leukocyte counts were determined on a Sysmex Toa 9500 hematology system; differential counts were determined microscopically from Giemsa-stained blood smears.
TABLE 1.
BCC strains used in this study
| Strain | Strain typea | Source, locationb | BCESMc | cblAd | Status at day 16 in vivo |
|---|---|---|---|---|---|
| B. multivorans (genomovar II) | |||||
| C5393 | 03 | CF, Canada | − | − | Persistent |
| C3430 | 07 | CF, Canada | − | − | Cleared |
| C1576 | 10 | CF-e, UK | − | − | Persistent |
| C5274 | 12 | CF, Canada | − | − | Persistent |
| FC147 | 12 | CGD, USA | − | − | Persistent |
| C5568 | 19 | CF, Canada | − | − | Persistent |
| B. cepacia (genomovar III) | |||||
| C1257 | 01 | CF-e, USA | + | − | Cleared |
| C5424 | 02 | CF-e, Canada | + | + | Cleared |
| K56-2 | 02 | CF-e, Canada | + | + | Cleared |
| C6433 | 04 | CF-e, Canada | + | − | Cleared |
| Cep511 | 05 | CF-e, Australia | + | − | Cleared |
| C4455 | 06 | CF-e, Canada | + | − | Cleared |
| C1394 | 13 | CF-e, UK | + | − | Cleared |
| B. stabilis (genomovar IV) | |||||
| C7322 | 16 | CF, Canada | − | − | Cleared |
| B. vietnamiensis (genomovar V) | |||||
| FC811 | 08 | CF, USA | − | − | Persistent |
| B. cepacia (genomovar VI) | |||||
| Cep873 | 10 | CF, Canada | − | − | Persistent |
Strain type is the numerical randomly amplified polymorphic DNA type assigned previously to this fingerprint pattern (19).
CF, strain from a CF patient; CF-e, strain that has spread epidemically among patients with CF; CGD, infection of a chronic granulomatous disease patient; UK, United Kingdom; USA, United States.
BCESM, presence of B. cepacia epidemic strain marker (19).
cb1A, presence of cable pilus subunit gene (19).
Bacterial cultures were grown in Luria-Bertani broth for 16 h; cells were harvested by centrifugation (10 min at 30,000 × g) and resuspended in Hanks balanced salt solution with 1% (vol/vol) gelatin (gHBSS). Bacteria were then diluted in gHBSS to 4 × 105 CFU/ml. Mice were challenged intranasally with a dose of 1.6 × 104 CFU in a 40-μL volume as follows: mice were anesthetized with 60 mg of ketamine hydrochloride/kg (MTC Pharmaceuticals) administered i.p., and the infectious dose was given by placing drops on alternate nares. At preselected time points after infection, mice were killed by cervical dislocation. Lungs were excised and weighed, homogenized, diluted in gHBSS, and plated onto TSA plates. Viable bacterial counts were determined after 24 to 48 h of incubation at 37°C.
Table 1 lists the 16 BCC strains initially evaluated for infection kinetics in the mouse model. In five of the six B. multivorans strains tested (C1576, C5274, C5393, C5568, and FC147), immunocompromised mice sustained the infection in the lungs to day 16, whereas healthy cohorts infected with the same organisms cleared the infection by day 16. In contrast, none of the B. cepacia genomovar III strains persisted in either immunocompromised or healthy mice to day 16. Moreover, five of seven genomovar III strains (C1394, C4455, C5424, C6433, and Cep511) were cleared by day 4 of the infection. Genomovar III strains also caused a greater degree of illness in infected animals than did B. multivorans (Fig. 1). While persistent B. multivorans infections caused systemic illness in immunocompromised mice only after several administrations of CPA, genomovar III infections caused a greater degree of systemic illness in a larger number of CPA-treated animals immediately after bacterial challenge. However, the degree of systemic illness experienced by mice infected with genomovar III strains after day 4 was highly variable. The Burkholderia stabilis strain (C7322) was cleared by day 16 in both groups. Immunocompromised mice infected with either Burkholderia vietnamiensis (FC811) or genomovar VI (Cep873) had slight increases in pulmonary bacterial load by days 10 and 14 that were above the initial dose delivered. In light of these observations and, particularly, the apparent differential virulence between B. multivorans and genomovar III strains within the model, further studies were conducted with five strains: B. multivorans C1576 and C5568, genomovar III strains C1394 and C6433, and B. vietnamiensis FC811.
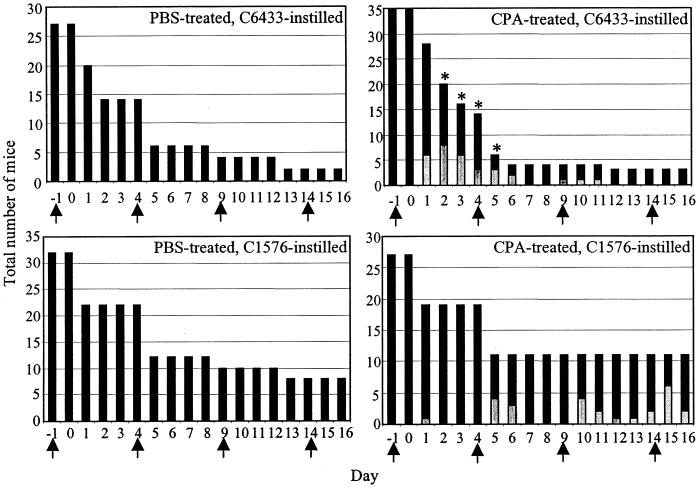
FIG. 1.
Effect of CPA treatment and bacterial infection on animal health over time. PBS-treated and CPA-treated mice were challenged with ≈1.6 × 104 B. cepacia genomovar III strain C6433 or B. multivorans strain C1576. Animals were monitored daily for the duration of the experiment and assessed on a three-point system for ill health. Unhealthy animals (light bars) and healthy animals (dark bars) are shown for each day. The total number of animals studied decreased over time as animals were removed for analysis. Asterisks denote days on which animals were euthanatized due to extreme systemic illness. Arrows denote administration of either CPA or PBS.
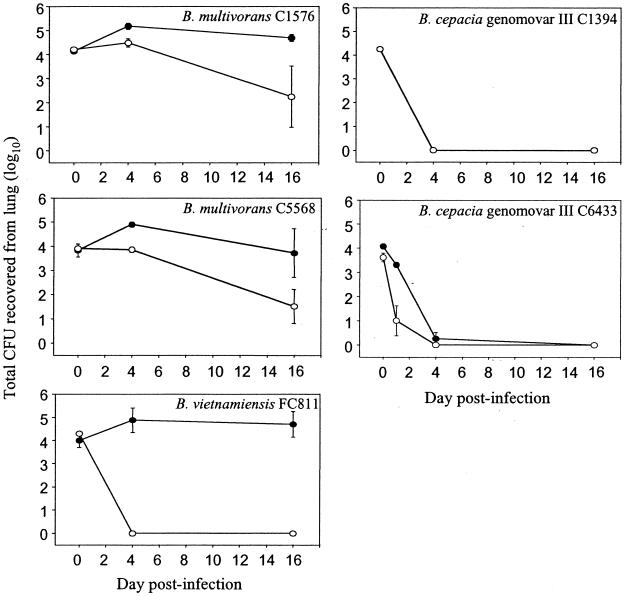
Figure 2 displays the kinetics of infection with five BCC strains in both healthy and immunocompromised mice. Animals were injected i.p. with either sterile phosphate-buffered saline (PBS) or CPA (150 mg/kg) prior to and every fifth day during the course of infection. Mice were then challenged with ≈1.6 × 104 CFU intranasally. Viable counts were obtained from diluted lung homogenates on days 0, 4, and 16 of the infection. Immunocompromised mice infected with either of the two B. multivorans strains (C1576 and C5568) or B. vietnamiensis strain FC811 sustained pulmonary bacterial loads at high titers for up to 16 days. Healthy control mice infected with strains C1576 and C5568 demonstrated slow clearance of the infection from their lungs, while healthy controls infected with strain FC811 displayed rapid clearance. In contrast, both genomovar III strains (C1394 and C6433) were cleared by day 4 from healthy and immunocompromised animals. CPA treatment therefore impeded pulmonary clearance of the B. multivorans and B. vietnamiensis strains but not the genomovar III strains.
FIG. 2.
Effect of CPA treatment on pulmonary bacterial load after infection with five BCC strains. CPA-treated (closed symbols) and PBS-treated (open symbols) BALB/c mice were intranasally challenged with ≈1.6 × 104 CFU. Quantitative bacteriology of the lung was assessed at 0 (3 h), 4, and 16 days. Values for the C1394-infected CPA group were identical to those for the PBS-treated group. Data are the mean and standard error of the mean from six animals at each time point.
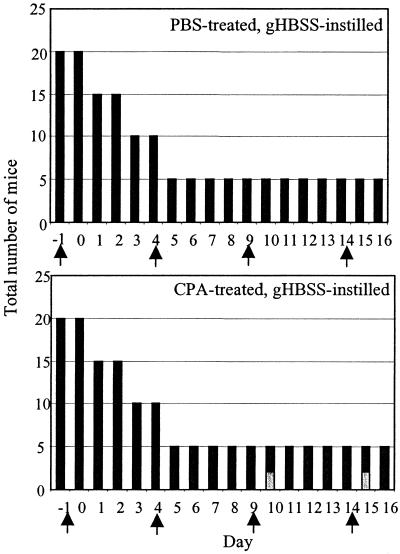
The efficacy of CPA as an inducer of leukopenia was also evaluated. Animals were monitored daily for general health, and signs of illness were recorded. Figure 3 demonstrates that CPA treatment prior to intranasal introduction of a gHBSS bolus caused minimal systemic illness in BALB/c mice. Systemic ill health was only observed 1 day after the third and fourth CPA injections, and animals appeared to recover from this state of ill health within 24 h. These observations suggest that CPA treatment alone rendered BALB/c mice mildly ill, but recovery was swift. Total peripheral leukocyte counts were measured in the CPA-treated and PBS-treated mice on days 0, 2, 4, and 16. CPA effected a minimal decrease in the total peripheral leukocyte count; none of the measured counts was significantly different from the control except at day 16 (3,823 ± 620 cells/mm3 in PBS-treated controls, compared to 1,255 ± 134 cells/mm3 in CPA-treated mice; P = 0.02). Differential counts of blood smears demonstrated that CPA elicited mild panleukopenia rather than neutropenia: mononuclear and polymorphonuclear populations in the peripheral blood were equally decreased by drug administration, and the percentages of each group in the peripheral blood did not change (data not shown).
FIG. 3.
Effect of CPA treatment on animal health over time. PBS-treated and CPA-treated mice were challenged with gHBSS only. Animals were monitored daily for the duration of the experiment and assessed on a three-point system for ill health. Unhealthy animals (light bars) and healthy animals (dark bars) are shown at each day. The total number of animals studied decreased over time as animals were removed for analysis. Arrows denote administration of either CPA or PBS.
The BCC is an important and diverse group of opportunistic pathogens demonstrating differential virulence among genomovars, but the relationship between bacterium and host is poorly understood. The purpose of these studies was to develop a physiologically relevant animal model that would permit investigation of bacterial virulence determinants or host responses resulting in the persistent infections that are characteristic of B. cepacia colonization of immunocompromised individuals. Several animal models have already been adapted to, and developed for, the study of B. cepacia infection. Multiple mouse models of CF have been described (7), two of which have been extensively examined in the context of lung disease during B. cepacia infection (8, 24). Wild-type mouse models have also been used in which agar beads were employed to enhance persistence of the bacterial inoculum (28). These different model systems have been used to characterize infection with clinical BCC strains and to evaluate possible virulence factors (3, 5, 12, 14, 16, 20-23, 25, 26, 28). Previous models utilized high and/or repetitive inocula to establish sustained or acute infections (8, 24, 28). However, none of the models yielded chronic infection of 2 weeks duration after a single low dose, as is reported herein. While previously reported models have proven useful for studying acute infection with B. cepacia in the lung, they do not serve the goal of our studies, which is to clarify the mechanisms of genomovar-specific differences in a chronic lung infection.
We evaluated several candidate model systems in an effort to create a suitable infection scenario; these included the use of neutrophil elastase to alter local host defenses, encapsulation of bacteria in agar beads, and infection via intubation and intratracheal instillation (5). None of these strategies resulted in chronic infection (data not shown), and so we adopted a system of pulmonary infection following intranasal instillation in CPA-immunocompromised BALB/c mice, similar to that described by Cryz et al. (6). Administration of CPA resulted in mild leukopenia in our studies, rather than acute neutropenia as previously reported (1, 4, 11, 27). A relatively low bacterial challenge dose was chosen in the interest of mimicking a physiologically relevant level of exposure. The noninvasive, mildly immunosuppressive nature of the model allowed sustained infection without causing extreme ill health in the animals. Infection dynamics were also highly reproducible in this model, enabling us to discern differences between genomovars II and III during chronic infection.
A panel representing five genomovars, with heavy representation of clinically relevant B. multivorans and genomovar III strains, was examined in an effort to establish genomovar-specific differences which may elucidate factors controlling the disparate clinical outcomes associated with B. multivorans versus genomovar III infection. While genomovar III strains are popular CF prototypes, their overrepresentation in mouse studies may be misleading, since mice readily clear these strains (8, 24, 28). Furthermore, B. multivorans, genomovar IV, and B. vietnamiensis strains have also been regularly isolated from CF patients (30). Indeed, we showed B. multivorans persistence in the mouse, albeit in a benign fashion. This persistence mimics infection in the CF lung, in which prolonged carriage of BCC strains occurs in most BCC-infected CF patients and only about 20% of patients experience septicemia and fulminant pulmonary decompensation (the cepacia syndrome) (10).
This model was initially developed with B. multivorans strain C1576 because previous studies in an i.p. infection model showed that B. multivorans is able to establish splenic persistence, while strains representing other genomovars are often cleared (31). The observation that B. multivorans strains from the panel persisted while genomovar III strains were rapidly cleared not only demonstrated similar kinetics to the i.p. model but also showed a clear difference between B. multivorans and genomovar III. The more effective clearance of genomovar III in our model may be viewed as contrary to the pattern of disease seen in CF in Canada, where the majority of aggressive pathogens come from this genomovar. However, both genomovars persist in the human disease and any differences seen in their clearance from the murine lung may be due to host response. Genomovar III infections were cleared more rapidly than those with B. multivorans, but clearance was accompanied by a variable degree of systemic illness in the mice, whereas B. multivorans infections caused minimal systemic illness. We speculate that genomovar III strains provoked higher levels of toxicity, thereby inducing a more effective immune response and clearance. These effects may be more evident with a higher challenge dose in studies currently under development.
We report that this model is able to support persistent infections in the lung in a physiologically relevant, noninvasive manner and shows differential persistence among the genomovars. We believe this system has the potential to yield valuable insights into host responses to BCC infections and the basis of the differential virulence among genomovars.
Acknowledgments
This work was supported by an operating grant (to D.P.S.) and studentships (to K.K.C. and J.W.C.) from the Canadian CF Foundation and by the British Columbia Lung Association (D.P.S.). D.J.D. is a Wellcome Trust (United Kingdom) fellow.
We thank Daniel W. Doxsee for excellent technical assistance in the animal work.
Editor: V. J. DiRita
REFERENCES
- 1.Amura, C. R., P. A. Fontan, S. Norberto, and D. O. Sordelli. 1994. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin. Immunol. Immunopathol. 73:261-266. [DOI] [PubMed] [Google Scholar]
- 2.Burkholder, W. H. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115-117. [Google Scholar]
- 3.Burns, J. L., M. Jonas, E. Y. Chi, D. A. Clark, A. Berger, and A. Griffith. 1996. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect. Immun. 64:4054-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calame, W., A. E. Douwes-Idema, M. T. van den Barselaar, R. van Furth, and H. Mattie. 1994. Influence of cytostatic agents on the pulmonary defence of mice infected with Klebsiella pneumoniae and on the efficacy of treatment with ceftriaxone. J. Infect. 29:53-66. [DOI] [PubMed] [Google Scholar]
- 5.Chiu, C. H., A. Ostry, and D. P. Speert. 2001. Invasion of murine respiratory epithelial cells in vivo by Burkholderia cepacia. J. Med. Microbiol. 50:594-601. [DOI] [PubMed] [Google Scholar]
- 6.Cryz, S. J., E. Furer, and R. Germanier. 1983. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect. Immun. 39:1067-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, D. J., and M. Rolfe. 2001. Mouse models of cystic fibrosis. Trends Genet. 17:S29-S37. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, D. J., J. R. Dorin, G. McLachlan, V. Ranaldi, D. Lamb, C. Doherty, J. Govan, and D. J. Porteous. 1995. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat. Genet. 9:351-357. [DOI] [PubMed] [Google Scholar]
- 9.Gilligan, P. H. 1991. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 4:35-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govan, J. R. W., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Hirakata, Y., N. Furuya, K. Tateda, and K. Yamaguchi. 1995. The influence of immunosuppressive agents on the immune system of mice. Immun. Infect. Dis. 5:88-93. [Google Scholar]
- 12.Hughes, J. E., J. Stewart, G. R. Barclay, and J. R. W. Govan. 1997. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect. Immun. 65:4281-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchison, M. L., and J. R. Govan. 1999. Pathogenicity of microbes associated with cystic fibrosis. Microbes Infect. 1:1005-1014. [DOI] [PubMed] [Google Scholar]
- 14.Hutchison, M. L., I. R. Poxton, and J. R. Govan. 1998. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect. Immun. 66:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 16.Lefebre, M. D., and M. A. Valvano. 2001. In vitro resistance of Burkholderia cepacia complex isolates to reactive oxygen species in relation to catalase and superoxide dismutase production. Microbiology 147:97-109. [DOI] [PubMed] [Google Scholar]
- 17.Lewin, L. O., P. J. Byard, and P. B. Davis. 1990. Effect of Pseudomonas cepacia colonization on survival and pulmonary function of cystic fibrosis patients. J. Clin. Epidemiol. 43:125-131. [DOI] [PubMed] [Google Scholar]
- 18.LiPuma, J. J. 1998. Burkholderia cepacia: management issues and new insights. Clin. Chest Med. 19:473-486. [DOI] [PubMed] [Google Scholar]
- 19.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. W. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from t he Burkholderia cepacia complex. J. Clin. Microbiol. 38:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnikov, A., O. Zaborina, N. Dhiman, B. S. Prabhakar, A. M. Chakrabarty, and W. Hendrickson. 2000. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol. Microbiol. 36:1481-1493. [DOI] [PubMed] [Google Scholar]
- 21.Palfreyman, R. W., M. L. Watson, C. Eden, and A. W. Smith. 1997. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect. Immun. 65:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini, L. S., S. B. Galsworthy, M. A. John, and M. A. Valvano. 1999. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology 145:3465-3475. [DOI] [PubMed] [Google Scholar]
- 23.Sajjan, U. S., F. A. Sylvester, and J. F. Forstner. 2000. Cable-piliated Burkholderia cepacia binds to cytokeratin 13 of epithelial cells. Infect. Immun. 68:1787-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sajjan, U., G. Thanassoulis, V. Cherapanov, A. Lu, C. Sjolin, B. Steer, Y. J. Wu, O. D. Rotstein, G. Kent, C. McKerlie, J. Forstner, and G. P. Downey. 2001. Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in Cftr−/− mice. Infect. Immun. 69:5138-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sajjan, U., Y. Wu, G. Kent, and J. Forstner. 2000. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J. Med. Microbiol. 49:875-885. [DOI] [PubMed] [Google Scholar]
- 26.Shaw, D., I. R. Poxton, and J. R. W. Govan. 1995. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. FEMS Immunol. Med. Microbiol. 11:99-106. [DOI] [PubMed] [Google Scholar]
- 27.Shirai, R., J. Kadota, K. Tomono, K. Ogawa, K. Iida, K. Kawakami, and S. Kohno. 1997. Protective effect of granulocyte colony-stimulating factor (G-CSF) in a granulocytopenic mouse model of Pseudomonas aeruginosa lung infection through enhanced phagocytosis and killing by alveolar macrophages through priming tumour necrosis factor-alpha (TNF-alpha) production. Clin. Exp. Immunol. 109:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokol, P. A., P. Darling, D. E. Woods, E. Mahenthiralingam, and C. Kooi. 1999. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: characterization of pvdA, the gene encoding l-ornithine N5-oxygenase. Infect. Immun. 67:4443-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speert, D. P. 2001. Understanding Burkholderia cepacia: epidemiology, genomovars, and virulence. Infect. Med. 18:49-56. [Google Scholar]
- 30.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speert, D. P., B. Steen, K. Halsey, and E. Kwan. 1999. A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen. Infect. Immun. 67:4027-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stotland, P. K., D. Radzioch, D., and M. M. Stevenson. 2000. Mouse models of chronic lung infections with Pseudomonas aeruginosa: models for the study of cystic fibrosis. Pediatr. Pulmonol. 30:413-424. [DOI] [PubMed] [Google Scholar]
- 33.Tablan, O. C., W. J. Martone, C. F. Doershuk, R. C. Stern, M. J. Thomassen, J. D. Klinger, J. W. White, L. A. Caron, and W. R. Jarvis. 1987. Colonization of the respiratory tract with Pseudomonas cepacia in cystic fibrosis: risk factors and outcomes. Chest 91:527-532. [DOI] [PubMed] [Google Scholar]
- 34.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36:1251-1275. [DOI] [PubMed] [Google Scholar]