Abstract
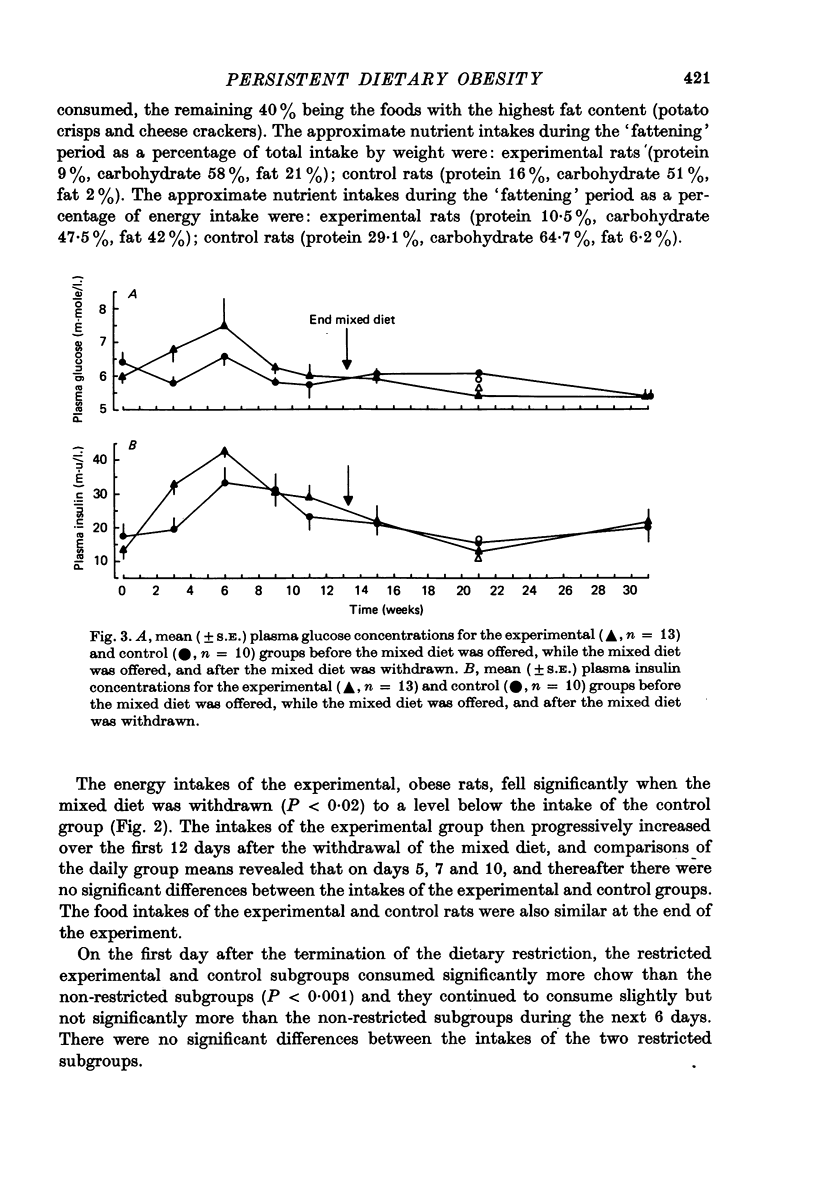
1. Adult male hooded rats which were offered a mixed, high energy diet for 90 days were hyperphagic and became significantly obese compared to chow-fed control rats. Fasting plasma insulin and glucose levels were initially elevated in the experimental rats, but later in the 90 day period were similar to control levels. 2. When the high energy foods were withdrawn after 90 days and just chow was available, the obese rats maintained the elevated body weights. The obese rats were initially hypophagic, but chow intakes rapidly reached control levels. Plasma insulin and glucose levels were similar in both groups, suggesting that the persisting obesity may not be associated with altered insulin resistance. 3. Five weeks after withdrawal of the 'fattening' diet, half of the experimental rats were offered restricted access to chow for 27 days to reduce their weights to control levels. When the rats were again given free access to chow, they returned to the previously elevated weight. 4. Eighteen weeks after withdrawal of the 'fattening' diet, the experimental rats had significantly elevated body weights and fat stores. The elevated body weight was not simply due to increased growth because, although the experimental rats had slightly more lean body mass than the control rats, the increase in fat was not related to body size.
Full text
PDF


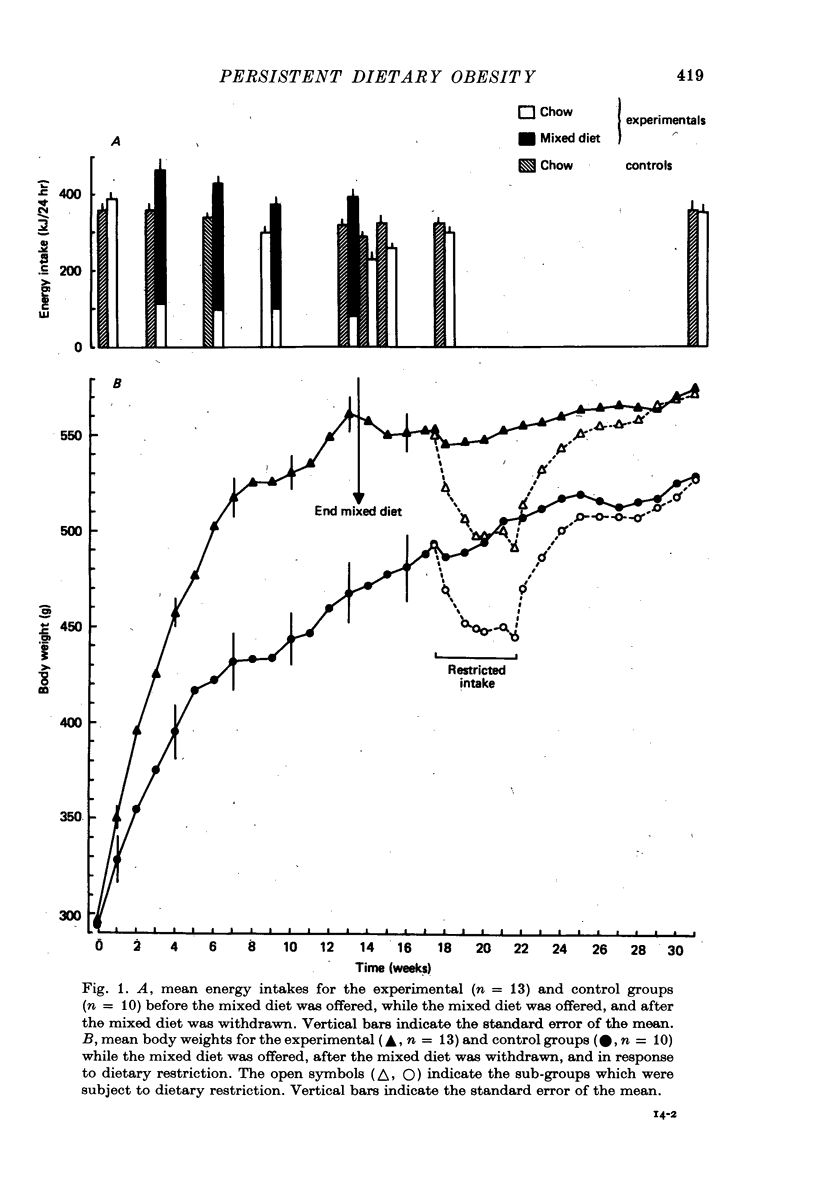
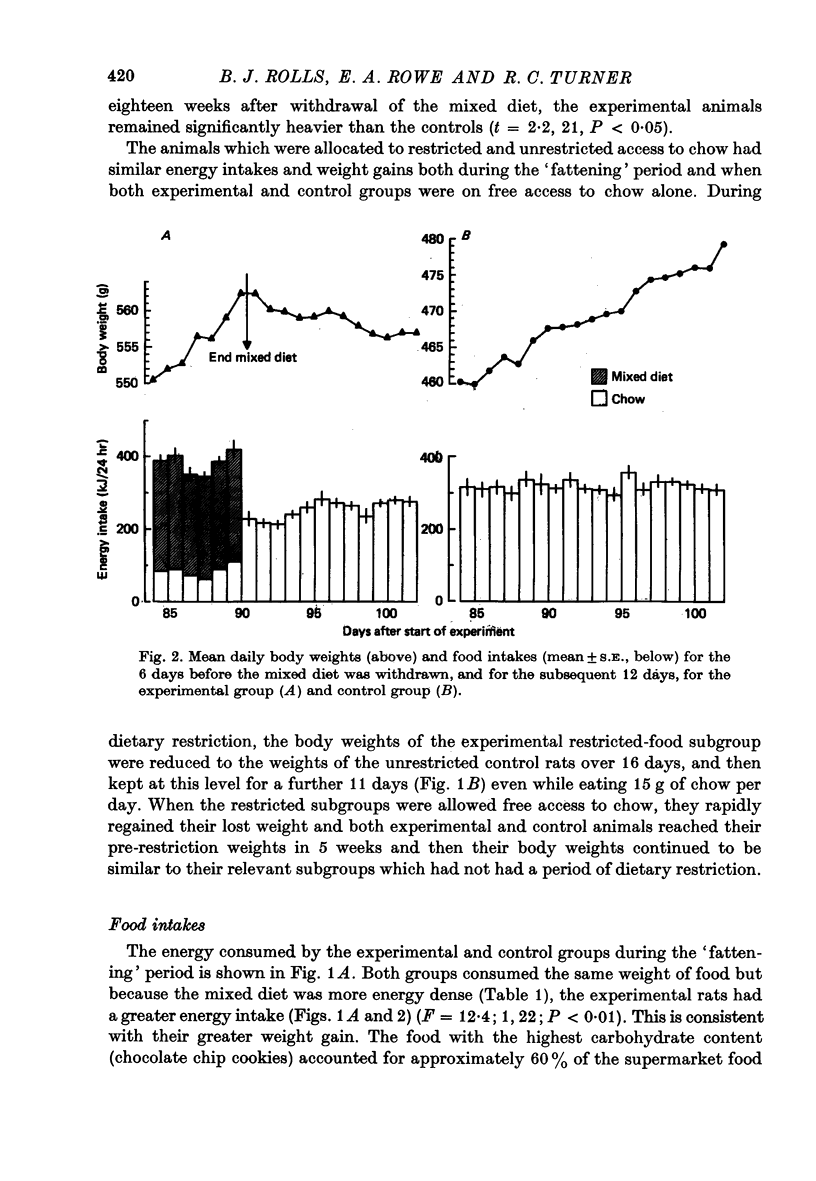





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- COHN C., JOSEPH D. Influence of body weight and body fat on appetite of "normal" lean and obese rats. Yale J Biol Med. 1962 Jun;34:598–607. [PMC free article] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Friedman M. I., Stricker E. M. The physiological psychology of hunger: a physiological perspective. Psychol Rev. 1976 Nov;83(6):409–431. [PubMed] [Google Scholar]
- Grey N., Kipnis D. M. Effect of diet composition on the hyperinsulinemia of obesity. N Engl J Med. 1971 Oct 7;285(15):827–831. doi: 10.1056/NEJM197110072851504. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Kirtland J. Adipose tissue cellularity: a review. I. Techniques for studying cellularity. Int J Obes. 1978;2(4):401–427. [PubMed] [Google Scholar]
- Hughes P. C., Tanner J. M. A longitudinal study of the growth of the black-hooded rat: methods of measurement and rates of growth for skull, limbs, pelvis, nose-rump and tail lengths. J Anat. 1970 Mar;106(Pt 2):349–370. [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Bray G. A., Mullen Y. S. Effect of transplantation of pancreas on development of hypothalamic obesity. Nature. 1977 Apr 21;266(5604):742–744. doi: 10.1038/266742a0. [DOI] [PubMed] [Google Scholar]
- Inoue S., Campfield L. A., Bray G. A. Comparison of metabolic alterations in hypothalamic and high fat diet-induced obesity. Am J Physiol. 1977 Sep;233(3):R162–R168. doi: 10.1152/ajpregu.1977.233.3.R162. [DOI] [PubMed] [Google Scholar]
- Kanarek R. B., Hirsch E. Dietary-induced overeating in experimental animals. Fed Proc. 1977 Feb;36(2):154–158. [PubMed] [Google Scholar]
- Kirtland J., Gurr M. I. Adipose tissue cellularity: a review. 2. The relationship between cellularity and obesity. Int J Obes. 1979;3(1):15–55. [PubMed] [Google Scholar]
- MICKELSEN O., TAKAHASHI S., CRAIG C. Experimental obesity. I. Production of obesity in rats by feeding high-fat diets. J Nutr. 1955 Dec 10;57(4):541–554. doi: 10.1093/jn/57.4.541. [DOI] [PubMed] [Google Scholar]
- Morrison S. D. Differences between rat strains in metabolic activity and in control systems. Am J Physiol. 1973 Jun;224(6):1305–1308. doi: 10.1152/ajplegacy.1973.224.6.1305. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding in diabetes. Relationships with plasma insulin levels and insulin sensitivity. Diabetes. 1977 Jul;26(7):680–688. doi: 10.2337/diab.26.7.680. [DOI] [PubMed] [Google Scholar]
- PECKHAM S. C., ENTENMAN C., CARROLL H. W. The influence of a hypercalric diet on gross body and adipose tissue composition in the rat. J Nutr. 1962 Jun;77:187–197. doi: 10.1093/jn/77.2.187. [DOI] [PubMed] [Google Scholar]
- Rolls B. J., Rowe E. A. Dietary obesity: permanent changes in body weight [proceedings]. J Physiol. 1977 Oct;272(1):2P–2P. [PubMed] [Google Scholar]
- Schemmel R., Mickelsen O., Gill J. L. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. J Nutr. 1970 Sep;100(9):1041–1048. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- Schemmel R., Mickelsen O., Tolgay Z. Dietary obesity in rats: influence of diet, weight, age, and sex on body composition. Am J Physiol. 1969 Feb;216(2):373–379. doi: 10.1152/ajplegacy.1969.216.2.373. [DOI] [PubMed] [Google Scholar]
- Sclafani A., Gorman A. N. Effects of age, sex, and prior body weight on the development of dietary obesity in adult rats. Physiol Behav. 1977 Jun;18(6):1021–1026. doi: 10.1016/0031-9384(77)90006-3. [DOI] [PubMed] [Google Scholar]
- Sclafani A., Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976 Sep;17(3):461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Snowdon C. T. Motivation, regulation, and the control of meal parameters with oral and intragastric feeding. J Comp Physiol Psychol. 1969 Sep;69(1):91–100. doi: 10.1037/h0027941. [DOI] [PubMed] [Google Scholar]


