Abstract
Haemophilus ducreyi, the etiologic agent of the sexually transmitted disease chancroid, produces a cytolethal distending toxin (HdCDT) that inhibits mammalian cell proliferation. We investigated the effects of HdCDT on normal human endothelial cells and on tubule formation in an in vitro model of angiogenesis. Endothelial cells were arrested in the G2 phase of the cell cycle, and tubule formation was inhibited in a dose-dependent manner. The antiproliferative activities of HdCDT on endothelial cells might contribute to the characteristic slow healing and persistence of chancroid ulcers.
Cytolethal distending toxins (CDTs) are produced by a number of gram-negative bacteria (5, 22, 24, 25, 29, 36, 42), including Haemophilus ducreyi (6). CDTs are three-component toxins, consisting of CdtA, CdtB, and CdtC. The H. ducreyi CDT (HdCDT) has been shown to affect both transformed cells, i.e., HEp-2, HeLa, THP-1, and HaCaT lineages, and normal human cells, such as T and B cells, fibroblasts, and keratinocytes (7, 14, 27, 37). CDTs have been shown to induce cell cycle arrest in the G2 phase and the accumulation of the tyrosine phosphorylated (inactive) form of cyclin-dependent kinase 1 (cdk1 or cdc2) (26). Cell cycle arrest in the G1 phase of fibroblasts (8) and apoptosis of T cells (14, 32) caused by CDTs have also been described. The cellular response to HdCDT resembles the response to double-strand breaks caused by insults such as ionizing radiation (8). This notion is supported by recent studies showing that the B components of several CDTs, including HdCDT (13), have homology with DNase I and express DNase activity in vitro and that the toxin is inactivated through mutation of DNase I-homologous residues (11, 17). Most investigators find that all three components are required for toxicity (11, 13, 18, 19, 26, 36) and that CdtB alone is toxic only when microinjected or endogenously produced (11, 17). The three components have been shown to form a complex and can be copurified through binding one component (10, 18, 28). There are also, however, reports describing that extracellularly administered, recombinantly (individually) produced Actinobacillus actinomycetemcomitans CdtB alone (31) or in combination with CdtA (28) or with CdtC (2) are enough for cytotoxicity. H. ducreyi CdtB together with CdtC was shown to have low toxic activity, which was greatly enhanced by addition of CdtA (10). There are recent data of a sequence from Salmonella enterica serovar Typhi which encodes a CdtB homologue without genes for A or C present (23), but it has not yet been assayed for CDT activity.
H. ducreyi causes chancroid (soft chancre, ulcus molle), a sexually transmitted disease that is characterized by mucocutaneous lesions on the external genitals, which if left untreated heal very slowly (40). HdCDT is produced by more than 80% of H. ducreyi strains (1) and is expressed in infected human hosts (39). The role of HdCDT in pathogenesis is unclear; HdCDT mutants defective in CdtC expression were noncytopathic in vitro but were as virulent as the wild type in both the rabbit model and human model of early H. ducreyi infection (35, 41).
Endothelial cells (ECs) play an important role in the early stages of inflammation, since they regulate the recruitment of inflammatory cells via the expression of surface receptors, the production of chemokines, and altered permeability. The endothelial leukocyte adhesion molecule 1 (E-selectin, CD62E) and the intercellular adhesion molecule 1 (ICAM-1) are both early markers of EC activation (4, 34). ECs normally have a very slow turnover, but rapid cell division is necessary during wound healing for the formation of new blood vessels (9). Although EC proliferation is necessary for angiogenesis, vascular sprouting can still occur even if proliferation is inhibited (33).
Histological examination of chancroid ulcers shows blood vessels that are degenerated and infiltrated with polymorphonuclear leukocytes or thrombosed in the superficial zone. Below this zone, ECs are the dominating cell type and small, dilated blood vessels are oriented in a vertical arrangement (12, 15, 30). This observation and the fact that angiogenesis is important in wound healing suggest that ECs may be important targets in chancroid pathogenesis.
The aim of this study was to investigate the impact of HdCDT on ECs and on tubule formation in vitro.
ECs are sensitive to HdCDT.
The effect of HdCDT on two normal human EC types was investigated. Normal human microvascular endothelial cells from adult dermal tissue (HMVEC-d; BioWhittaker, Walkersville, Md.) were cultured in microvascular endothelial growth medium (EGM-2MW; BioWhittaker) supplemented according to the manufacturer's instructions. Human umbilical vein ECs (HUVEC; Cascade Biologics Inc., Portland, Oreg.) were cultured in medium M200 (Cascade Biologics Inc.) without antibiotics.
The HdCDT preparation used was partially purified from the culture medium of Escherichia coli DH5α carrying cdtABC as described previously (37). All three CDT proteins are detected in this preparation by immunoblotting (13). The total amount of protein was 200 μg/ml, and the toxic titer, determined as previously described (27), was 107 cytopathic units (CPU)/ml (corresponding to 20 pg of protein/CPU/ml). Briefly, 1 CPU/ml was determined as the concentration required to reduce the number of HEp-2 cells by 50% compared to untreated control cultures.
ECs were seeded onto plates at a density of 5 × 103 cells/well, incubated overnight, and treated with 10 to 103 CPU/ml of the HdCDT preparation. A colorimetric assay based on the cleavage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma) by dehydrogenase enzymes in living cells was used to measure metabolic activity (20). Briefly, 50 μg of MTT was added to cells cultured for 1, 2, 3, or 4 days in 100 μl of medium, and the plates were incubated for a further 5 h at 37°C in 6% CO2. Formed crystals were dissolved in acid isopropanol, and the absorbance was measured by a Titertek Multiscan reader. The results are expressed as the differences between the absorbance at 570 and 630 nm with background subtraction (i.e., medium without cells).
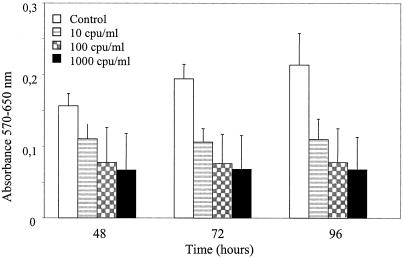
The metabolic activities of both EC types showed similar dose-dependent inhibition by HdCDT. Figure 1 shows the impact of HdCDT on HMVEC-d. A concentration of 10 CPU/ml was sufficient for metabolic inhibition to about 50% after 72 h, which is 10 to 100 times the amount required to affect the epithelial cell line HEp-2. One CPU/ml reduced the number of HEp-2 cells to 50% after 48 h, and metabolic activity was reduced to about 50% after 72 h with as little as 0.1 CPU/ml (results not shown). This perhaps reflects a difference between continuous cell lines, which proliferate homogeneously, and normal cell lines (such as HMVEC-d and HUVEC), which consist of a more heterogeneous population of cells. The difference in sensitivity could also simply reflect a difference between the two types of cells.
FIG. 1.
The influence of HdCDT on the metabolic activity of HMVEC-d cells was measured by using MTT. White bars represent controls (PBS) and shaded bars represent cells treated with 10, 102, or 103 CPU of HdCDT per ml. The data are shown as the means ± standard deviations of two representative and independent experiments performed in triplicate.
HdCDT arrests HMVEC-d in the G2 phase of the cell cycle.
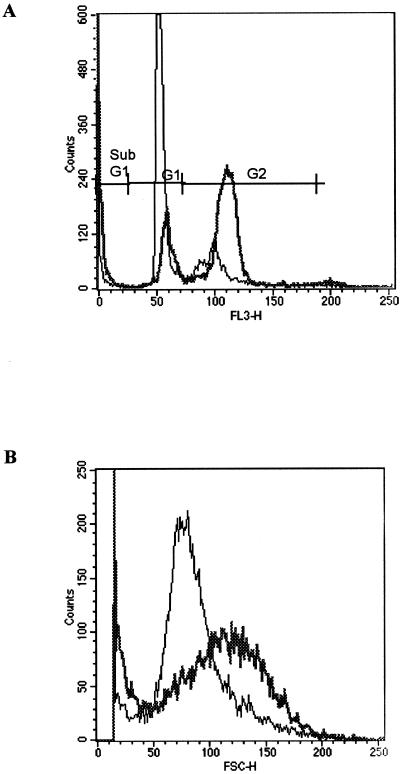
To investigate the effect of HdCDT on cell cycling, HMVEC-d cells (2.5 × 104 cells/well in 24-well plates) were treated with the toxin at concentrations ranging from 10 to 104 CPU/ml. To include all cells, culture supernatants (containing detached cells) were collected and the remaining, adherent cells were trypsinized. The cells were pelleted together, washed once with phosphate-buffered saline (PBS) and then fixed in 70% ethanol on ice for at least 15 min. The cells were resuspended in a propidium iodide solution and reincubated for 1 h at 4°C. The cell cycle inhibitors nocodazole (100 nM; Sigma) and hydroxyurea (2.5 mM; Sigma) were used as controls for G2 and G1 arrest, respectively. The cells were analyzed in a FACSCalibur (Becton Dickinson, San Jose, Calif.). The data from 104 cells were collected and analyzed by using CellQuest (Becton Dickinson) as previously described (7). After 72 h of incubation, 13 to 19% of the control cells were in the G2 phase compared with 51 to 59% of the cells incubated with 100 CPU of HdCDT per ml. The results from one of four representative experiments are shown in Fig. 2A. Not all cells were arrested in the G2 phase; even after 96 h, a significant percentage of cells remained in the G1 phase. However, nocodazole treatment resulted in a similar distribution (51% of cells in G2 phase), suggesting that HMVEC-d cells have a finite life span and that some cells do not divide or divide slowly. Cells that stained with an intensity lower than that of the G1 peak (sub-G1) were considered apoptotic cells (8). This population increased with time and was larger in the HdCDT-treated samples than in untreated controls (Fig. 2A). After 96 h of toxin treatment, this population was threefold larger in HdCDT-treated cells than in the untreated control. This increase was concomitant with a decrease in the G2 peak, probably due to the death of G2-arrested cells. Furthermore, HdCDT treatment increased the cell size, as determined by increased forward scatter signal (Fig. 2B), thus reflecting the distending properties of CDT. The increase in size was also obvious when cells were still attached to plastic surfaces (data not shown).
FIG. 2.
(A) Influence of HdCDT on cell cycle progression. The percentage of cells in the G2 phase of the cell cycle was increased. (B) Cells treated with HdCDT were increased in size compared to the control (PBS-treated) cells. The thin line represents the control. The thick line represents HdCDT. Data are from one of four representative experiments.
The expressions of cyclin-dependent kinase 1 (cdc-2) and p53 phosphorylated on serine 15 (p53-ser 15), which are involved in cell cycle regulation, were determined by Western blotting. Cells were cultured in 25-cm2 flasks (105 cells/flask) and incubated with HdCDT for 2 to 24 h. Cells were then trypsinized, washed with PBS, disrupted in electrophoresis sample buffer (16), and boiled for 10 min. The total protein concentration was determined by the Bio-Rad (Hercules, Calif.) DC protein assay. Equal amounts (30 to 50 μg) of total cellular protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Criterion gel; Bio-Rad) and blotted onto polyvinylidene difluoride transfer membranes (Hybond-P; Pharmacia Biotech, Uppsala, Sweden). The blots were probed with an anti-cdc-2 antibody (Transduction Laboratories, Lexington, Ky.) and anti-phospho-p53 (ser 15) (Cell Signaling Technology, Beverly, Mass.), followed by an appropriate secondary antibody conjugated to horseradish peroxidase, and developed with an Enhanced Chemiluminescence Kit (Amersham Pharmacia Biotech).
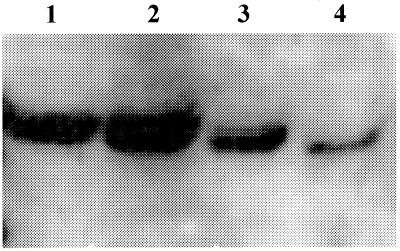
After 2 h in the presence of HdCDT, the cells expressed two bands that reacted with the anti-cdc-2 antibody, whereas only one reactive band could be seen in control cells (Fig. 3). The band with the higher molecular weight has previously been shown to be the phosphorylated (and inactive) cdc2 (7), and the lower-molecular-weight band has been shown to be the active and dephosphorylated variant. Dephosphorylation of cdc2 on Thr-14 and Tyr-15 is the final requirement before the cell can leave G2 and start mitosis. The two cdc-2-specific bands persisted for at least 12 h of toxin treatment. This finding is similar to that observed with other cell types, e.g., HEp-2 cells. Fibroblasts were previously shown to arrest in both the G2 and G1 phases and also expressed increased amounts of p53-ser 15 (8). p53 is involved mainly in regulation of G1 checkpoint through transcriptional induction of p21, which in turn can inhibit G1 cyclin-dependent kinases (21). p53 can also be involved in the G2 checkpoint (38). We were unable to detect any bands of p53-ser 15 in either control or HdCDT-treated cells during the 24 h assayed. It is possible that the cells express phosphorylated p53 at later time points, but this was not tested.
FIG. 3.
HdCDT induces the phosphorylation of cyclin-dependent kinase 1 at early time points in HMVEC-d cells. Cells were treated with HdCDT (103 CPU/ml) for 2 h (lane 1), 6 h (lane 2), 9 h (lane 3), and control at 9 h (lane 4).
HdCDT does not affect the expression of early adhesion molecules.
HMVEC-d cells were incubated with extracts of sonicated bacteria in order to test whether HdCDT had any impact on the expression of early adhesion molecules, such as endothelial leukocyte adhesion molecule 1 (E-selectin) and ICAM-1. Briefly, E. coli strain XL1-Blue (Stratagene, San Diego, Calif.) carrying the gene for HdCDT (13) and its parent strain with vector alone were grown in Luria-Bertani broth at 37°C with shaking. The two strains were adjusted to the same optical density, and equal volumes were centrifuged. The pellet was resuspended in PBS and sonicated on ice and then filtered through a 0.2-μm-pore-size filter. The toxic titer for the strain expressing HdCDT was 105 CPU/ml (measured as described above). The parent strain was cytotoxic at a dilution of 1:10. The sonicates were added to HMVEC-d cells in dilutions ranging from 1:102 to 1:105. After 6 h the cells were trypsinized, washed once with fluorescence-activated cell sorting buffer (1% bovine serum albumin in PBS), stained with 5 μg of anti-human ICAM-1 (CD54) or anti-human E-selectin (CD62E; DAKO A/S, Glostrup, Denmark) per ml for 30 min, centrifuged, and incubated in the dark for 30 min with fluorescein isothiocyanate-labeled F(ab′)2 rabbit anti-mouse immunoglobulin (DAKO) and then fixed with Cellfix (Becton Dickinson). The cells were analyzed using FACSCalibur and CellQuest, as described above.
Similar dose-dependent increases in ICAM-1 and E-selectin expression occurred in the presence of bacterial sonicates at dilutions from 1:102 to 1:105. The HdCDT-positive sonicates induced ICAM-1 expression in 76 and 88% of cells, and the control sonicates induced ICAM-1 in 75 and 91% of cells at concentrations of 100 and 1,000 CPU/ml, respectively. E-selectin expression was also induced to the same extent by the two sonicates but to a lower magnitude, i.e., 48 and 59% with HdCDT-positive sonicates and 41 and 56% with control sonicates, at concentrations of 100 and 1,000 CPU/ml, respectively (results not shown).
HdCDT inhibits the formation of tubuli.
We used an in vitro angiogenesis model comprising human ECs cocultured with other human, fibroblast-like cells in a specially designed medium (Angiokit; TCS Cellworks, Buckinghamshire, United Kingdom). The ECs initially form small islands within the culture matrix. They subsequently proliferate and then migrate, forming tubule structures. Tubule networks emerge after about 2 weeks in culture.
The partially purified HdCDT described above was added to cultured cells, in concentrations ranging from 102 to 105 CPU/ml, on day 1. On day 10 the cultures were fixed and stained for PECAM-1 (CD31; Tubule Staining Kit; TCS Cellworks) to allow the visualization of tubuli.
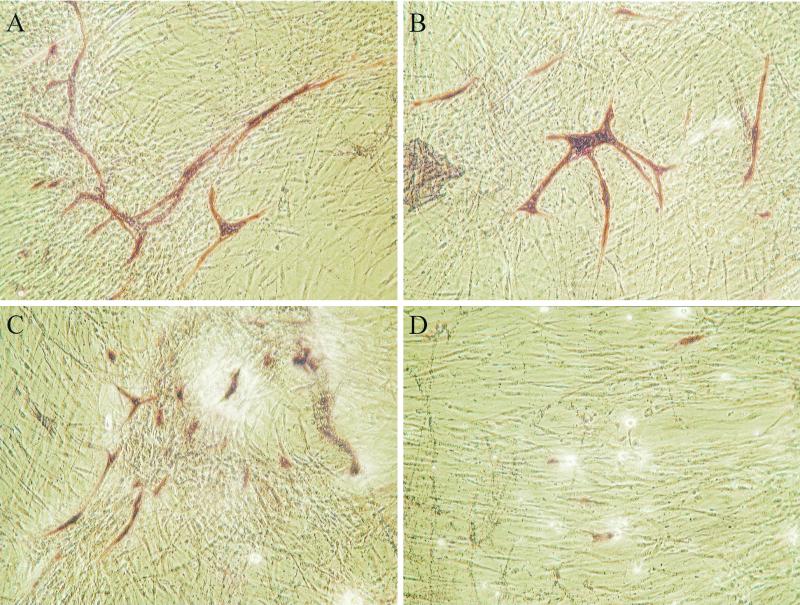
We found that HdCDT added at high concentrations (105 CPU/ml) abolished the formation of tubuli (Fig. 4D), while at lower concentrations (102 to 104 CPU/ml) it reduced tubular length and branching of tubuli (Fig. 4B and C). A relatively high concentration of HdCDT was needed to inhibit tubulus formation. A possible reason for this is the presence of a high number of other cell types making up the matrix in which small islands of ECs are found. The fibroblast-like cells were also affected by the high concentrations of HdCDT, seen as a reduced cellular density (Fig. 4D). This is in agreement with previous studies showing that human fibroblasts are sensitive to HdCDT (8, 35).
FIG. 4.
Impact of HdCDT on an in vitro model of angiogenesis. ECs and tubuli were visualized by staining for PECAM (CD31) (shown in red). In control (PBS-treated) wells long tubuli are formed (A). With 102 CPU (B) and 104 CPU (C) of HdCDT per ml the tubuli are shorter and less branched. Increasing the concentration further to 105 CPU HdCDT per ml (D) totally inhibits the formation of tubuli. The cell density of the fibroblast-like cells is decreased at high concentrations of HdCDT (D) compared to control (A).
This is the first study showing that HdCDT arrests normal human ECs in the G2 phase of the cell cycle. The response of ECs to HdCDT is similar to that of many other cells to this toxin. In this experimental setup, the toxin did not influence the expression of E-selectin or ICAM-1, indicating that the toxin does not influence leukocyte adhesion per se. The impact, shown, of HdCDT on new blood vessel formation is probably largely due to antiproliferative effects of the toxin on ECs. This effect of the toxin may influence the outcome of the complex process of angiogenesis. In vivo, a large number of other factors with pro- or antiangiogenic properties are present, and further studies regarding HdCDT's involvement in this process are required. There is at present no relevant in vivo model that accurately reflects the whole disease process of chancroid. The human model of H. ducreyi infection (3) does not allow the study of later phases of chancroid, e.g., wound healing. None of the animal models accurately models human H. ducreyi infection, i.e., a very large inoculum is required to induce a lesion and/or the lesion does not mimic the disease process in humans. In both the human and the rabbit models, HdCDT mutants (nontoxic on cell cultures) were shown to be as virulent as the wild type (35, 41).
HdCDT affects keratinocytes, fibroblasts, lymphocytes, and, as we have shown, ECs. All these cell types are important in the wound-healing process, and it therefore seems likely that HdCDT plays an important role in the characteristically slow healing of chancroid.
Acknowledgments
This work was supported by the Swedish Agency for Research Cooperation with Developing Countries (SIDA/SAREC) and Swedish Medical Research Council (grants 12630 and 05969). L. A. Svensson is supported by grants from the Infection and Vaccinology program, Swedish Foundation for Strategic Research.
We thank Teresa Frisan, MTC, Karolinska Institute, Stockholm, Sweden, and Leif Lindholm, Got-a-Gene AB, Göteborg, Sweden, for valuable discussions, and Vincent Collins, Dept. of Rheumatology, Göteborg University, for revising the manuscript and for helpful discussions.
Editor: A. D. O'Brien
REFERENCES
- 1.Ahmed, H. J., L. A. Svensson, L. D. Cope, J. L. Latimer, E. J. Hansen, K. Ahlman, J. Bayat-Turk, D. Klamer, and T. Lagergard. 2001. Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J. Med. Microbiol. 50:860-864. [DOI] [PubMed] [Google Scholar]
- 2.Akifusa, S., S. Poole, J. Lewthwaite, B. Henderson, and S. P. Nair. 2001. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect. Immun. 69:5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 4.Bevilacqua, M. P., S. Stengelin, M. A. Gimbrone, Jr., and B. Seed. 1989. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science 243:1160-1165. [DOI] [PubMed] [Google Scholar]
- 5.Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49:525-534. [DOI] [PubMed] [Google Scholar]
- 6.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes-Bratti, X., E. Chaves-Olarte, T. Lagergard, and M. Thelestam. 1999. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J. Clin. Investig. 103:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 276:5296-5302. [DOI] [PubMed] [Google Scholar]
- 9.Denekamp, J. 1984. Vascular endothelium as the vulnerable element in tumours. Acta Radiol. Oncol. 23:217-225. [DOI] [PubMed] [Google Scholar]
- 10.Deng, K., J. L. Latimer, D. A. Lewis, and E. J. Hansen. 2001. Investigation of the interaction among the components of the cytolethal distending toxin of Haemophilus ducreyi. Biochem. Biophys. Res. Commun. 285:609-615. [DOI] [PubMed] [Google Scholar]
- 11.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37:952-963. [DOI] [PubMed] [Google Scholar]
- 12.Freinkel, A. L. 1987. Histological aspects of sexually transmitted genital lesions. Histopathology 11:819-831. [DOI] [PubMed] [Google Scholar]
- 13.Frisk, A., M. Lebens, C. Johansson, H. Ahmed, L. Svensson, K. Ahlman, and T. Lagergard. 2001. The role of different protein components from the Haemophilus ducreyi cytolethal distending toxin in the generation of cell toxicity. Microb. Pathog. 30:313-324. [DOI] [PubMed] [Google Scholar]
- 14.Gelfanova, V., E. J. Hansen, and S. M. Spinola. 1999. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect. Immun. 67:6394-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King, R., S. H. Choudhri, J. Nasio, J. Gough, N. J. Nagelkerke, F. A. Plummer, J. O. Ndinya-Achola, and A. R. Ronald. 1998. Clinical and in situ cellular responses to Haemophilus ducreyi in the presence or absence of HIV infection. Int. J. STD AIDS 9:531-536. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 18.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, D. A., M. K. Stevens, J. L. Latimer, C. K. Ward, K. Deng, R. Blick, S. R. Lumbley, C. A. Ison, and E. J. Hansen. 2001. Characterization of Haemophilus ducreyi CdtA, CdtB, and CdtC mutants in in vitro and in vivo systems. Infect. Immun. 69:5626-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 21.Norbury, C. J., and I. D. Hickson. 2001. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 41:367-401. [DOI] [PubMed] [Google Scholar]
- 22.Okuda, J., H. Kurazono, and Y. Takeda. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 18:167-172. [DOI] [PubMed] [Google Scholar]
- 23.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 24.Pickett, C. L., D. L. Cottle, E. C. Pesci, and G. Bikah. 1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickett, C. L., E. C. Pesci, D. L. Cottle, G. Russell, A. N. Erdem, and H. Zeytin. 1996. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect. Immun. 64:2070-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292-297. [DOI] [PubMed] [Google Scholar]
- 27.Purven, M., and T. Lagergard. 1992. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect. Immun. 60:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiki, K., K. Konishi, T. Gomi, T. Nishihara, and M. Yoshikawa. 2001. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol. Immunol. 45:497-506. [DOI] [PubMed] [Google Scholar]
- 29.Scott, D. A., and J. B. Kaper. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheldon, W. H., and A. Heyman. 1946. Studies on chancroid: observations on the histology with an evaluation of biopsy as diagnostic procedure. Am. J. Pathol. 22:415-425. [PubMed] [Google Scholar]
- 31.Shenker, B. J., R. H. Hoffmaster, T. L. McKay, and D. R. Demuth. 2000. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J. Immunol. 165:2612-2618. [DOI] [PubMed] [Google Scholar]
- 32.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. R. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167:435-441. [DOI] [PubMed] [Google Scholar]
- 33.Sholley, M. M., G. P. Ferguson, H. R. Seibel, J. L. Montour, and J. D. Wilson. 1984. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab. Investig. 51:624-634. [PubMed] [Google Scholar]
- 34.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 35.Stevens, M. K., J. L. Latimer, S. R. Lumbley, C. K. Ward, L. D. Cope, T. Lagergard, and E. J. Hansen. 1999. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect. Immun. 67:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugai, M., T. Kawamoto, S. Y. Peres, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 66:5008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson, L. A., A. Tarkowski, M. Thelestam, and T. Lagergard. 2001. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in immune response. Microb. Pathog. 30:157-166. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, W. R., and G. R. Stark. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803-1815. [DOI] [PubMed] [Google Scholar]
- 39.Throm, R. E., and S. M. Spinola. 2001. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect. Immun. 69:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young, R. S., K. R. Fortney, V. Gelfanova, C. L. Phillips, B. P. Katz, A. F. Hood, J. L. Latimer, R. S. Munson, Jr., E. J. Hansen, and S. M. Spinola. 2001. Expression of cytolethal distending toxin and hemolysin is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 69:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, V. B., K. A. Knox, and D. B. Schauer. 2000. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect. Immun. 68:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]