Abstract
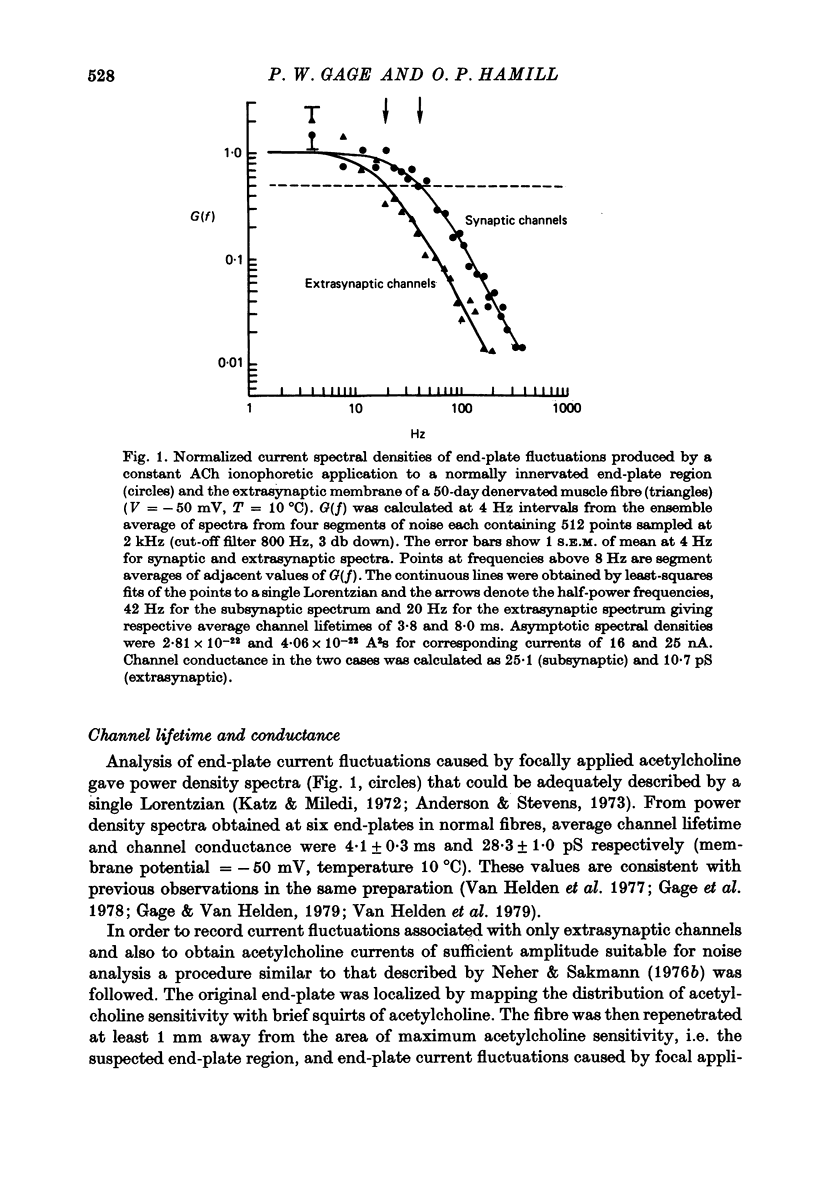
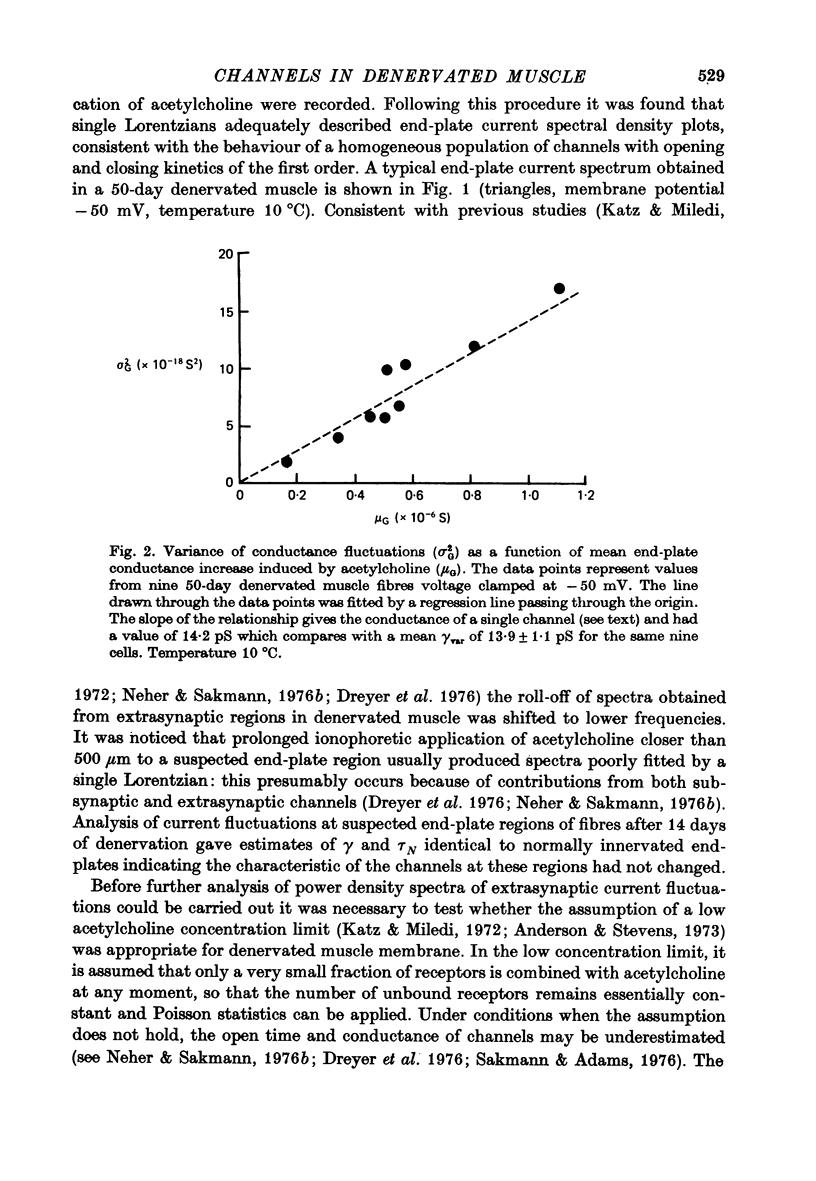
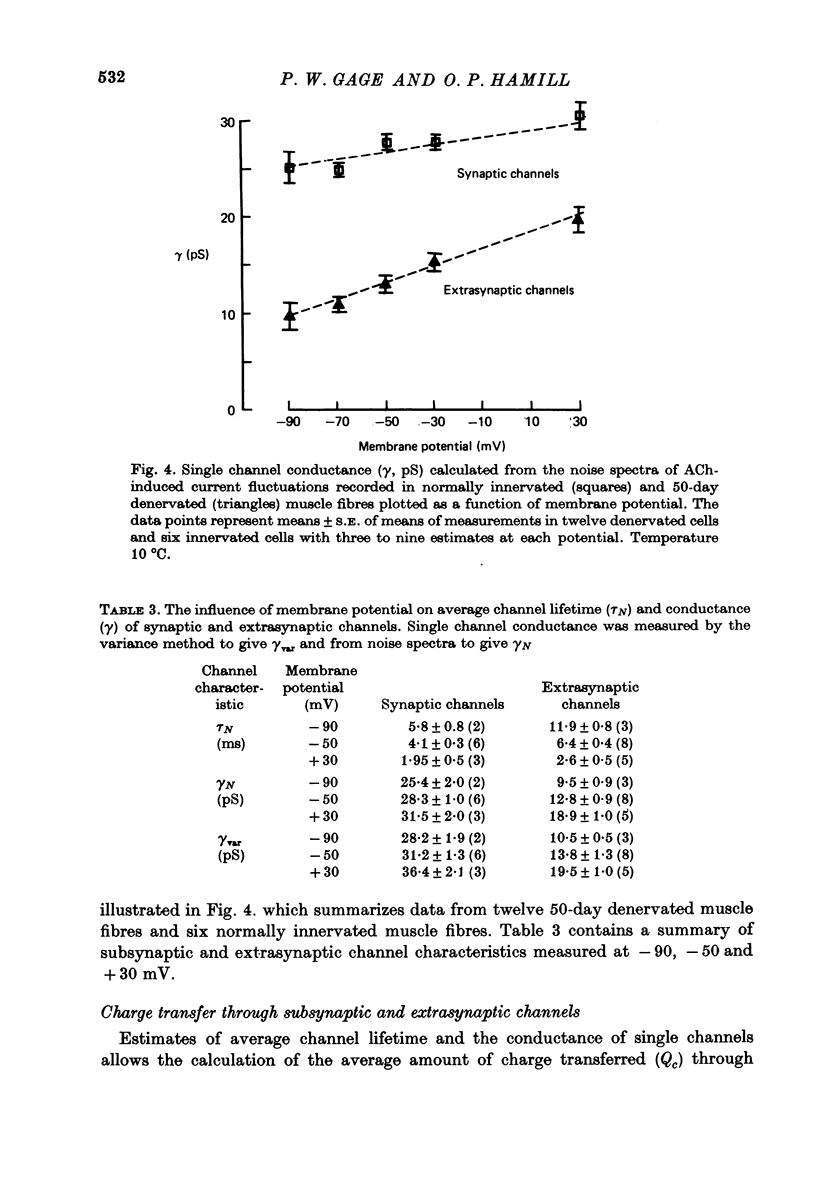
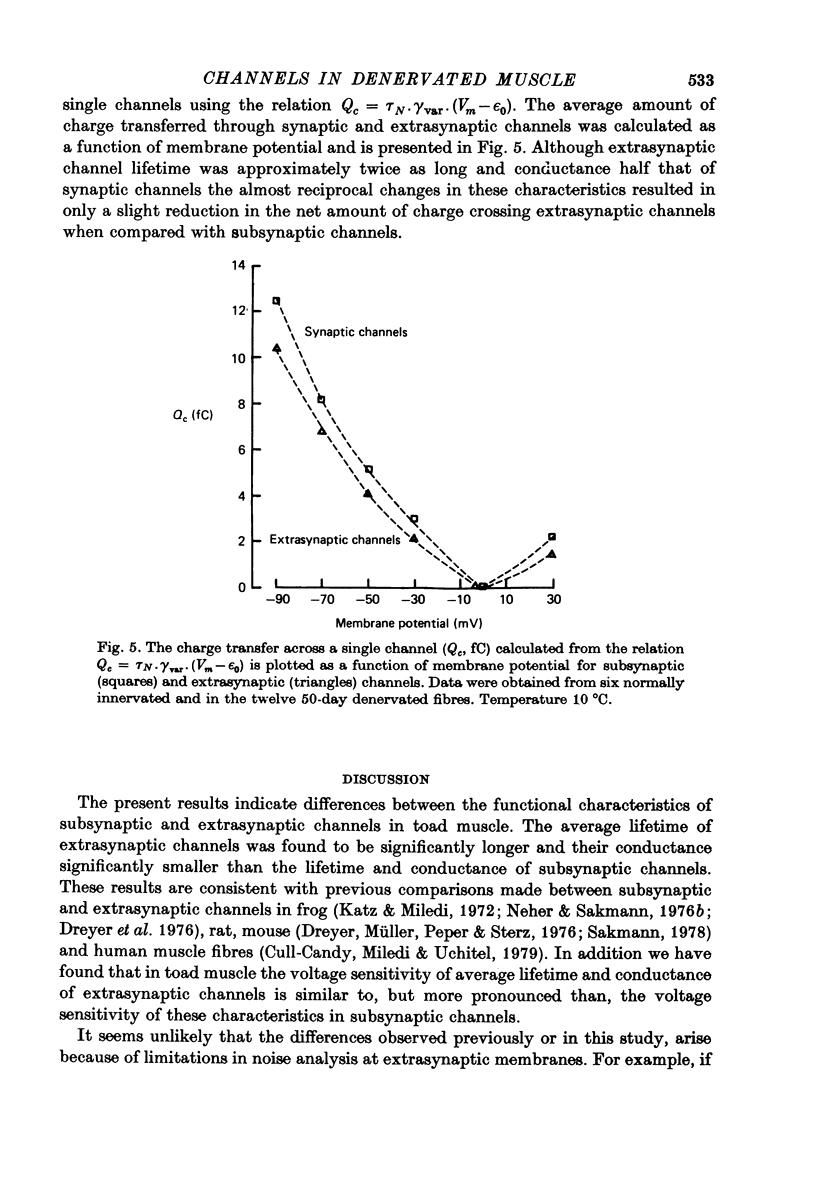
1. The average lifetime and conductance of acetylcholine-activated channels were measured in normal and denervated, voltage-clamped toad sartorius muscle fibres at 10 degrees C. 2. The null potential was -4 +/- 1 mV for subsynaptic channels in normal fibres and -6 +/- 3 mV for extrasynaptic channels in denervated fibres. 3. There was a linear relationship between variance of conductance fluctuations and mean conductance for acetylcholine-induced currents up to 50 nA, in denervated fibres clamped at -50 mV. The ratio gave a channel conductance of 14 pS. 4. At the same membrane potential, the average lifetime of extrasynaptic channels in denervated fibres was approximately double, whereas channel conductance was approximately half, that of subsynaptic channels in normal fibres: there was little difference in net charge transfer through the two types of channel under similar conditions. 5. Single channel conductance increased, whereas average channel lifetime decreased, as the membrane potential became more positive (depolarized). The effect of potential on channel lifetime and conductance was more pronounced in denervated than in normal fibres.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. A comparison of current-voltage relations for full and partial agonists. J Physiol. 1978 Oct;283:621–644. doi: 10.1113/jphysiol.1978.sp012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Temperature-dependent properties of gramicidin A channels. Biochim Biophys Acta. 1974 Oct 29;367(2):127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Barry P. H., Gage P. W., Van Helden D. F. Cation permeation of the amphibian motor end-plate. J Membr Biol. 1979 Apr 9;45(3-4):245–276. doi: 10.1007/BF01869288. [DOI] [PubMed] [Google Scholar]
- Brenner H. R., Sakmann B. Gating properties of acetycholine receptor in newly formed neuromuscular synapses. Nature. 1978 Jan 26;271(5643):366–368. doi: 10.1038/271366a0. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Synthesis of acetylcholine receptor by denervated rat diaphragm muscle. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1368–1372. doi: 10.1073/pnas.72.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chuang S. T., Huang M. C. Effects of chronic treatment with various neuromuscular blocking agents on the number and distribution of acetylcholine receptors in the rat diaphragm. J Physiol. 1975 Aug;250(1):161–173. doi: 10.1113/jphysiol.1975.sp011047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Uchitel O. D. Acetylcholine receptors in organ-cultured human muscle fibres. Nature. 1979 Jan 18;277(5693):236–238. doi: 10.1038/277236a0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. The spread of acetylcholine sensitivity after denervation of frog skeletal muscle fibers. Pflugers Arch. 1974 May 6;348(4):287–292. doi: 10.1007/BF00589218. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P. Effects of several inhalation anaesthetics on the kinetics of postsynaptic conductance changes in mouse diaphragm. Br J Pharmacol. 1976 Jun;57(2):263–272. doi: 10.1111/j.1476-5381.1976.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Van Helden D. Octanol reduces end-plate channel lifetime. J Physiol. 1978 Jan;274:279–298. doi: 10.1113/jphysiol.1978.sp012147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Bamberg E. Influence of membrane thickness and ion concentration on the properties of the gramicidin a channel. Autocorrelation, spectral power density, relaxation and single-channel studies. Biochim Biophys Acta. 1977 Jan 4;464(1):127–141. doi: 10.1016/0005-2736(77)90376-5. [DOI] [PubMed] [Google Scholar]
- Letter: Lenses and the compression of black lipid membranes by an electric field. Biophys J. 1975 Jan;15(1):77–81. doi: 10.1016/S0006-3495(75)85793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Szabo G., Urry D. W. N-acetyl gramicidin: single-channel properties and implications for channel structure. Science. 1979 Jan 5;203(4375):55–57. doi: 10.1126/science.83000. [DOI] [PubMed] [Google Scholar]
- Van Helden D., Hamill O. P., Gage P. W. Permeant cations alter endplate channel characteristics. Nature. 1977 Oct 20;269(5630):711–713. doi: 10.1038/269711a0. [DOI] [PubMed] [Google Scholar]
- van Helden D. F., Gage P. W., Hamill O. P. Conductance of end-plate channels is voltage dependent. Neurosci Lett. 1979 Feb;11(2):227–232. doi: 10.1016/0304-3940(79)90133-2. [DOI] [PubMed] [Google Scholar]


