Abstract
Recent evidence suggests that immune-mediated gastric epithelial cell apoptosis through Fas-Fas ligand interactions participates in Helicobacter pylori disease pathogenesis. To define the role of Fas signaling in vivo, H. pylori strain SS1 infection in C57BL/6 mice was compared to that in mice deficient in the Fas ligand (gld). gld mice had a degree of gastritis similar to that of C57BL/6 mice after 6 weeks (gastritis score, 5.2 ± 0.6 [mean ± standard error] versus 3.5 ± 0.8) and 12 weeks (4.0 ± 0.7 versus 3.4 ± 0.5) of infection. Bacterial colonization was comparable in each group of mice at 12 weeks of infection (2.1 ± 0.3 versus 1.6 ± 0.3 for gld and C57BL/6, respectively; the difference is not significant). Sixty-seven percent of H. pylori-infected gld mice displayed atrophic changes in the gastric mucosa, compared with 37% of infected C57BL/6 mice, at 12 weeks. In addition, atrophic changes were more severe in H. pylori-infected gld mice (P < 0.05). Splenocytes isolated from H. pylori-infected C57BL/6 mice had a twofold increase in production of the Th1 cytokine gamma interferon (IFN-γ) in response to H. pylori antigens at both 6 and 12 weeks compared to controls (143 ± 65 versus 69 ±26 pg/ml and 336 ± 73 versus 172 ± 60, respectively). In contrast, there was a lack of detectable IFN-γ in gld mice infected with the bacterium. H. pylori-infected C57BL/6 mice had increased epithelial cell apoptosis compared with sham-infected C57BL/6 mice (35.0 ± 8.9 versus 12.3 ± 6.9; P < 0.05). Epithelial cell apoptosis did not differ between H. pylori-infected and control gld mice (5.2 ± 1.6 versus 6.5 ± 2.9 [not significant]). These data demonstrate that mice with mutations in the Fas ligand develop more severe premalignant mucosal changes in response to infection with H. pylori in association with both an impaired gastric epithelial cell apoptotic response and IFN-γ production. The Fas death pathway modulates disease pathophysiology following murine infection with H. pylori. Deregulation of the Fas pathway could be involved in the transition from gastritis to gastric cancers during H. pylori infection.
Chronic infection with the gastric pathogen Helicobacter pylori is associated with the development of peptic ulcer disease and gastric cancers (4). The mechanisms involved in disease pathogenesis remain unclear. Alterations in the gastric epithelial cell cycle, including both enhanced proliferation (3) and increased apoptosis of gastric cells, are identified during infection with the bacterium (34). These changes in cell turnover are present in both H. pylori-infected children (16) and adults (21, 24). Current evidence indicates that alterations in cell turnover are likely to play a central role in H. pylori-mediated disease pathogenesis. An increase in epithelial cell apoptosis is detected in tissue obtained from subjects with active duodenal ulceration compared to those with healing ulcers (18). In contrast, an increase in cell proliferation in the absence of enhanced apoptosis is detected in gastric tissues obtained from patients with the premalignant changes of intestinal metaplasia (33). Similarly, during H. pylori infection in gerbils, which has been reported to lead to gastric adenocarcinomas, a transient increase in apoptosis is detected, followed by a persistent increase in proliferation (30).
Recent studies indicate that both host determinants and bacterial factors are involved in mediating such changes in epithelial cell turnover. Peek and colleagues (29) have demonstrated that infection with cagA+ H. pylori strains is associated with enhanced proliferation without a corresponding increase in apoptosis. Mice deficient in secretary phospholipase A have altered apoptosis in response to infection with the related bacterium Helicobacter felis (39). In vitro studies indicate that the bacterium is capable of directly inducing apoptosis of gastric cells (8, 17, 37). Fan and colleagues (7) provided evidence that binding of urease to class II major histocompatibility complex molecules mediates apoptosis in vitro. In addition, H. pylori infection increases the sensitivity of gastric epithelial cells to Fas-triggered apoptosis by enhancing Fas receptor expression (17). Inflammatory cytokines present during H. pylori infection, such as gamma interferon (IFN-γ), also enhance activation of the Fas signaling pathway in vitro (13). Furthermore, among H. pylori-infected children, gastric epithelial cell apoptosis returns to baseline levels only following both eradication of bacterial colonization and resolution of the accompanying inflammatory cell infiltrate (16). These findings suggest that immune-mediated cell death through the Fas pathway could contribute to the apoptosis that is observed during infection in vivo.
To determine if Fas signaling is involved in H. pylori disease pathogenesis in vivo, H. pylori infection in C57BL/6 mice was compared with infection of an inbred gld (for generalized lymphoproliferative disease) strain with alterations in the Fas signaling pathway due to a point mutation in the Fas ligand (25). Following infection, colonization, mucosal inflammatory changes, gastric cell apoptosis, and cytokine responses were measured.
MATERIALS AND METHODS
Bacteria and growth conditions.
The mouse-adapted H. pylori strain SS1, originally described by Lee et al. (19) and kindly received from Ken Croitoru (McMaster University, Hamilton, Ontario, Canada), was employed in these studies. Bacteria were grown under microaerophilic conditions on Columbia blood agar plates for 72 h at 37°C, harvested, and resuspended in brucella broth (Difco, Detroit, Mich.) supplemented with 10% heat-inactivated fetal calf serum (Gibco BRL, Grand Island, N.Y.), 10 mg of vancomycin per liter, and 5 mg of trimethoprim per liter (17). Bacteria were grown overnight at 37°C in an Erlenmeyer flask with shaking at 120 rpm, as described previously (17). Cells were then pelleted and resuspended in sterile phosphate-buffered saline (PBS) at a concentration of 109 CFU/ml. Before use, the suspension was assessed for motility of organisms under bright-field microscopy and cultured on blood agar plates under microaerophilic conditions to exclude contamination.
Animals.
Specific-pathogen-free female mice (6 to 8 weeks old), strains C57BL/6 and B6Smn.C3H-Faslgld (gld), were purchased from Jackson Laboratories (Bar Harbor, Maine). Animals were housed in microisolater cages under barrier conditions with free access to commercially available chow and water for the duration of the experiments. All animal experimentation was performed according to institutional guidelines and with approval of the local institutional review board.
Infection of mice with H. pylori strain SS1.
Mice were inoculated with 108 bacteria in 0.1 ml of PBS by orogastric lavage three times within a 5-day interval as described previously (19). A control group for each strain of mice was given 0.1 ml of PBS alone by orogastric lavage under the same conditions.
Groups of 10 to 12 mice from each strain were either sham inoculated or challenged with H. pylori strain SS1 on two different occasions. At 6 or 12 weeks following infection, mice were killed by an intraperitoneal injection of chloral hydrate and then necropsied. The stomach was removed en bloc, opened, and rinsed with PBS before assessment of visual evidence of macroscopic changes. Gastric tissue was then cut into strips from the squamocolumnar junction to the duodenum, fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned. In a subset of mice from each group, the spleen was also removed for isolation of splenic mononuclear cells, as described below.
Following 8 weeks of infection, another group of H. pylori SS1-infected gld mice (n = 7) received eradication therapy consisting of 6.15 mg of bismuth per ml, 50 mg of tetracycline per ml, and 22.5 mg of metronidazole per ml administered by orogastric lavage daily for 7 days (19). Mice were then sacrificed at 21 (n = 4) or 42 (n = 3) days after infection.
Bacterial colonization.
The degree of gastric colonization with H. pylori was assessed as described previously (19), with minor modifications. Briefly, Giemsa-stained gastric tissue sections (5 μm) were examined microscopically under oil immersion and assigned semiquantitative scores for colonization by a blinded observer, as follows: 0, no bacteria detected; 1, mild level of colonization, with bacteria not detected in every gastric crypt; 2, mild level of colonization, with bacteria detected in the majority of crypts; 3, moderate to heavy colonization in all crypts; and 4, severe colonization, with all crypts densely packed with bacteria.
Determination of apoptotic cells.
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed on sections of paraffin-embedded tissue from a subset of mice from each group with a commercially available kit (ApopTag; Intergen, Purchase, N.Y.) according to the manufacturer's directions with minor modifications. Briefly, sections were deparaffinized in xylene and rehydrated through graded concentrations of ethanol at room temperature. Triton-X (0.5%) was applied directly onto slides and incubated for 15 min at 25°C. After blocking of endogenous peroxidase by incubation in hydrogen peroxide (1.2%) in methanol for 25 min at 25°C, the slides were washed and an equilibration buffer was applied for a minimum of 10 s. The terminal deoxynucleotidyltransferase enzyme diluted in reaction buffer (1:5) was then applied to the slides and incubated for 1 h in a humidified chamber at 37°C. The stop buffer was then applied for 10 min at 25°C. Peroxidase conjugate was then applied to the sections and incubated for 30 min at 25°C. Detection of peroxidase conjugate was achieved by using a commercially available kit (Histostain Plus; Zymed Laboratories, San Francisco, Calif.). Sections were counterstained with hematoxylin, washed, and mounted with a coverslip.
Apoptotic cells were quantitated by enumerating the number of TUNEL-stained cells in 40 well-oriented gastric glands in antral tissue. Enumeration was performed in a blinded fashion. The apoptotic index represents the mean number of apoptotic cells counted in 40 well-oriented antral glands.
Gastritis score.
Giemsa-stained sections of gastric tissue were viewed under bright-field microscopy and graded for the severity of antral gastritis, as described previously (16). Briefly, the presence of an increased number of mononuclear cells, the presence and severity of mucus depletion, and the presence of polymorphonuclear leukocytes within the mucosa were each assessed separately and graded from 0 to 3+ by a pathologist in a blinded fashion with coded slides. Submucosal inflammation was not included in the antral gastritis score.
The presence of atrophic changes, as defined by atrophy of glandular cells and hyperplasia of mucus cells, was determined in a blinded fashion and scored based on the percentage of altered gastric mucosa, as described previously (10): 0, no mucosal alterations; 1, less than 5%; 2, 10 to 25%; 3, 25 to 50%; and 4, 50 to 75%.
Production of IFN-γ and IL-5 by splenic mononuclear cells.
Spleens harvested at necropsy were mashed through sterile filter screens into RPMI culture medium to obtain single-cell suspensions. Cell suspensions were incubated in red blood cell lysis buffer for 2 min to lyse erythrocytes. The cells were then washed three times and resuspended in RPMI medium containing 10% fetal calf serum. Viable cells (106) were then incubated with sterile H. pylori strain SS1 whole-cell sonicate (1 μg/ml) for 72 h at 37°C in 96-well flat-bottom microtiter plates in triplicate. Supernatants were collected and stored at −70°C until used for cytokine assessment. Production of IFN-γ and interleukin-5 (IL-5) by splenic mononuclear cells was measured in collected supernatants by using commercially available immunoassays (Biosource, Camarillo, Calif.).
Statistics.
Results are expressed as means ± standard errors (SE). Comparison of results between multiple groups was performed using analysis of variance (ANOVA) followed by post-hoc comparisons with the Newman-Keuls or Duncan test. For comparisons between two groups, an unpaired Student t test was performed.
RESULTS
Effect of Fas ligand deficiency on bacterial colonization.
All mice that were challenged with H. pylori strain SS1 were colonized when assessed at both 6 and 12 weeks following inoculation. All sham-infected mice remained free of H. pylori infection. The majority of C57BL/6 and gld mice infected with H. pylori displayed mild to moderate bacterial colonization with bacteria detected in most crypts (2.1 ± 0.3 versus 1.6 ± 0.3; the difference was not significant [P = NS]).
Increased gastric mucosal atrophy in mice deficient in the Fas ligand.
At both 6 and 12 weeks following infection, no macroscopic evidence of mucosal ulceration was detected in stomachs obtained from either the C57BL/6 or gld mice. However, one of the infected gld mice had a thickened gastric wall that corresponded histopathologically to an increase in inflammatory infiltrate predominantly within the submucosa of the corpus region.
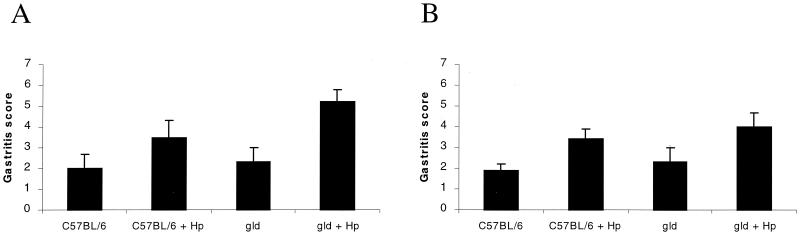
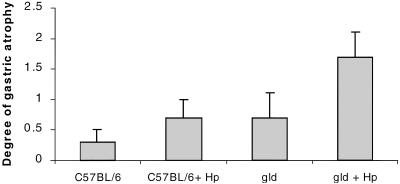
Following 6 weeks of infection, when the degree of gastritis was quantitated, gld mice demonstrated mucosal inflammation comparable to that in the stomachs of H. pylori strain SS1-infected C57BL/6 mice (gastritis score at 6 weeks, 5.2 ± 0.6 versus 3.5 ± 0.8; P = NS) (Fig. 1). In addition, the degree of gastric atrophic changes were similar in infected wild-type and gld mice (P = NS). Following 12 weeks of infection, there was no detectable difference in the degree of gastritis between SS1-infected C57BL/6 and gld mice (3.4 ± 0.5 versus 4 ± 0.7; P = NS) (Fig. 1). However, as shown in Fig. 2, atrophic changes, including mucus cell hyperplasia and parietal cell loss, were detected more frequently in the corpus regions of gld mice following 12 weeks of infection than in the infected control group of mice (67% of gld mice versus 37% of C57BL/6 mice). When the degree of atrophic changes was quantitated, both groups of mice displayed an approximately twofold increase in atrophic changes compared to uninfected mice. However, H. pylori-infected gld mice displayed more severe mucosal alterations (Fig. 3) (P < 0.05, ANOVA).
FIG. 1.
Quantitation of the degree of gastritis in sham-infected and H. pylori (Hp) strain SS1-infected C57BL/6 mice and gld mice following 6 weeks (A) and 12 weeks (B) of infection. The degree of gastritis did not differ between groups of mice at 6 and 12 weeks postinfection (P = NS). Error bars represent the SE.
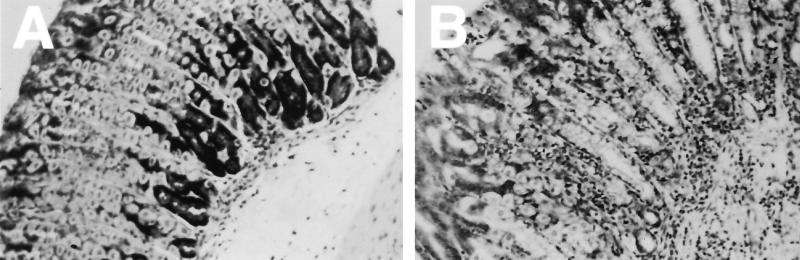
FIG. 2.
Photomicrographs of Giemsa-stained gastric tissue sections demonstrating mucosal alterations in response to H. pylori strain SS1 infection of C57BL/6 (A) and gld (B) mice. Tissues from infected C57BL/6 mice showed a mild inflammatory infiltrate in the lamina propria. In contrast, panel B shows an increase in infiltrating inflammatory cells within the lamina propria extending through the muscularis mucosa in gld mice with associated atrophic changes, including loss of parietal cells and mucous cell hyperplasia. Original approximate magnifications, ×40.
FIG. 3.
Determination of severity of atrophic changes in gastric mucosa of sham-infected and H. pylori (Hp)-infected C57BL/6 and gld mice following 12 weeks of infection. gld mice displayed more severe atrophic changes following infection (P < 0.05, ANOVA). Error bars represent the SE.
In the gld mice that received eradication therapy 8 weeks after H. pylori infection, bacteria were not detected in gastric tissue by using the modified Steiner stain. In addition, in mice that received bacterial eradication therapy, the degree of mucosal inflammation was comparable to that in uninfected mice (gastritis score, 2.0 ± 0.8 versus 2.2 ± 1.1; P = NS).
Fas signaling mediates an apoptotic response in epithelial cells following infection with H. pylori.
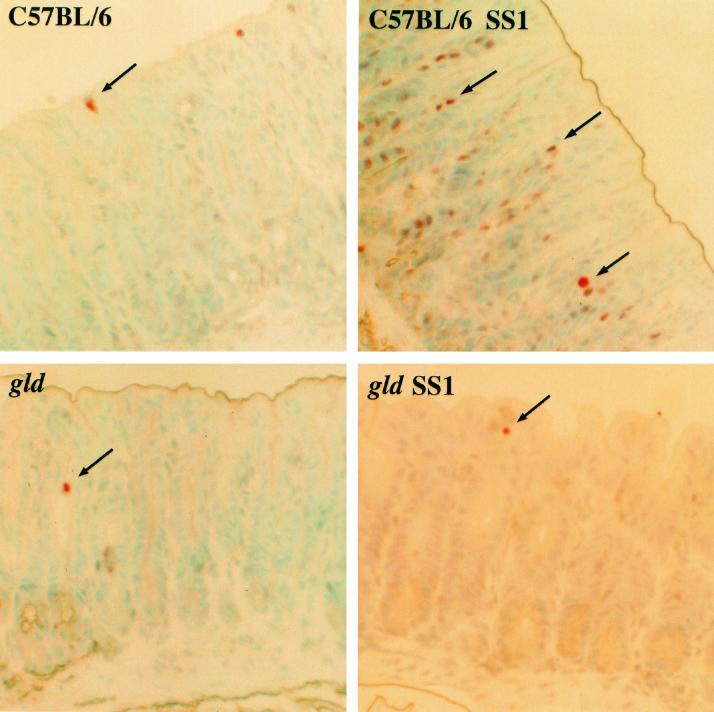
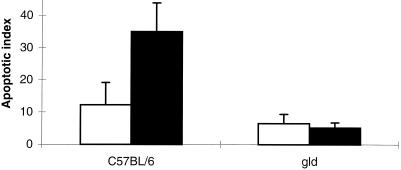
As shown in Fig. 4, apoptotic epithelial cells were detected in superficial epithelium confined to the upper one-third of gastric crypts by the TUNEL assay in tissues obtained from each of the four groups of mice. Compared to uninfected controls, H. pylori strain SS1-infected C57BL/6 mice had increased apoptosis of gastric epithelial cells extending into the neck region (35.0 ± 8.9 versus 12.3 ± 6.9; P < 0.05) (Fig. 5). In contrast, an increase in the number of apoptotic gastric epithelial cells was not detected in tissues obtained from gld mice infected with H. pylori. The degrees of apoptosis were comparable in H. pylori strain SS1-infected and sham-infected gld mice (5.2 ± 1.6 versus 6.5 ± 2.9; P = NS).
FIG. 4.
Photomicrograph demonstrating apoptotic cells (arrows) as assessed by the TUNEL assay in gastric antral tissue from sham-infected and H. pylori strain SS1-infected C57BL/6 and gld mice. Compared with sham-infected C57BL/6 mice, H. pylori SS1-infected C57BL/6 mice had an increase in the number of gastric cells undergoing apoptosis. In contrast, an increase in apoptosis was not detected in infected gld mice. Original approximate magnification, ×40.
FIG. 5.
Quantitation of apoptosis in gastric tissue obtained from sham-infected (open bars) and H. pylori (Hp) SS1-infected (solid bars) C57BL/6 and gld mice following 12 weeks of infection. C57BL/6 mice had an increase in apoptosis of gastric cells compared to both groups of gld mice. C57BL/6 mice infected with the bacteria demonstrated an enhanced degree of apoptosis (P < 0.05, ANOVA). Results are expressed as the mean apoptotic index. Error bars represent the SE.
Lack of detectable Th1 inflammatory cytokine response during H. pylori infection in gld mice.
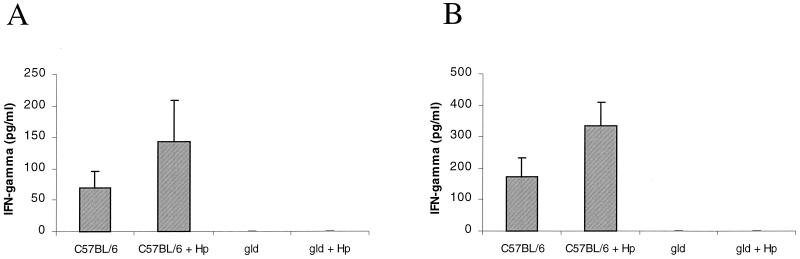
C57BL/6 mice displayed an approximately twofold increase in production of the Th1 cytokine IFN-γ following 6 and 12 weeks of infection (143 ± 65 versus 69 ± 26 pg/ml and 337 ± 73 versus 172 ± 60 pg/ml, respectively; P = NS) (Fig. 6). In contrast, production of IFN-γ was undetectable in both sham- and H. pylori-infected gld mice.
FIG. 6.
Th1 (IFN-γ) cytokine profiles in isolated splenocytes stimulated with H. pylori (Hp) antigens obtained from sham-infected and H. pylori strain SS1-infected C57BL/6 and gld mice at 6 weeks (A) and 12 weeks (B) of infection. H. pylori-infected C57BL/6 mice displayed a predominant Th1 response. In contrast, gld mice lacked an IFN-γ response during infection. Error bars represent the SE.
Th2 response to H. pylori infection in gld mice.
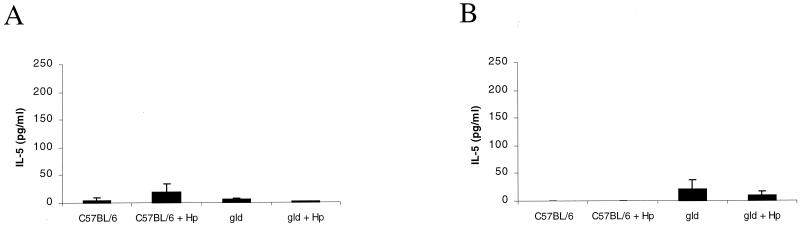
Production of the Th2 cytokine IL-5 was minimal in sham-infected C57BL/6 and gld mice at both 6 (5.2 ± 2.8 and 4.5 ± 4.5 pg/ml) and 12 (0 and 20.8 ± 16.7 pg/ml) weeks. Levels of IL-5 did not change in response to infection with H. pylori in either group of mice (Fig. 7).
FIG. 7.
Th2 (IL-5) cytokine profiles in isolated splenocytes stimulated with H. pylori (Hp) antigens obtained from sham-infected and H. pylori strain SS1-infected C57BL/6 and gld mice at 6 weeks (A) and 12 weeks (B) of infection. Production of IL-5 was minimal in each group of mice. Error bars represent the SE.
DISCUSSION
Fas, or CD95, is a member of the tumor necrosis factor receptor family which when bound by its natural ligand, Fas ligand, stimulates an apoptotic signal through activation of the caspase cascade (1). Expression of the Fas ligand is found predominantly on activated T lymphocytes (20). The Fas death signal regulates immune responses by deleting autoreactive T cells, triggering activation-induced cell death, and mediating tolerance (20). Expression of the Fas ligand in specific tissues, such as the testes, is implicated in immune privilege of this site (9).
In the gastrointestinal tract, Fas receptor normally is constitutively expressed in epithelial cells. By contrast, there is little or no detectable Fas ligand expression, except on the surface of Paneth cells (23). Current evidence indicates that disruption of Fas signaling is involved in the pathogenesis of intestinal diseases. For example, Fas ligand is highly expressed on infiltrating T lymphocytes in the colons of patients with ulcerative colitis (35). In a murine model of autoimmune gastritis, enhanced Fas expression is detected on gastric parietal cells in association with the induction of apoptosis (26).
Apoptosis is implicated in the regulation of inflammation in response to bacterial pathogens. The gastric pathogen H. pylori induces apoptosis of gastric epithelial cells both in vivo and in vitro (34). In addition, infection with H. pylori enhances expression of the Fas receptor on gastric epithelial cells and increases sensitivity to Fas-triggered cell death in vitro (17). An increase in infiltrating Fas ligand-expressing lymphocytes is detected in gastric tissues obtained from H. pylori-infected individuals (31). In addition, H. pylori infection of T-cell lines in vitro induces apoptotic cell death mediated by the Fas pathway (38). However, the relative contribution of the Fas death pathway in mediating disease during H. pylori infection is not clear. This study is the first to directly investigate the importance of Fas signaling during H. pylori infection in vivo using a murine model of infection.
Consistent with enhanced programmed cell death observed in humans infected with H. pylori (16, 21, 24), C57BL/6 mice demonstrated an increase in apoptosis of gastric epithelial cells during infection. However, in gastric tissues obtained from mice deficient in the Fas ligand, enhanced gastric epithelial cell apoptosis was not detected. Thus, gastric epithelial cells from mice deficient in the Fas ligand are resistant to H. pylori-mediated cell death. Therefore, although H. pylori can directly induce apoptosis of gastric epithelial cells in vitro (8, 17), the Fas signaling cascade is the predominant form of cell death which occurs during infection in vivo. Indeed, the Fas pathway may be the preferential cell death pathway utilized by helicobacters. In support of this contention, Houghton et al. (14) recently demonstrated that mice with a mutation in the Fas receptor (lpr) show a lack of an apoptotic response during infection with a related gastric helicobacter, H. felis.
We have demonstrated that in gld mice deficient in the Fas ligand, H. pylori infection results in more severe disease than that in infected C57BL/6 mice. Although there was a trend towards increased mucosal inflammation in the infected wild-type and gld mice compared with the sham-infected controls, this was not statistically significant. This finding is in agreement with the original study characterizing murine infection with the Sydney strain. Lee et al. (19) described a significant difference in the degree of antral inflammation between control and SS1-infected mice only following 8 months of infection. Atrophic changes characterized by a loss of parietal cell mass and replacement with metaplastic mucus cells are considered premalignant lesions (10). In our study, gastric atrophy was detected more frequently in gld mice than in C57BL/6 mice in response to infection. The exact mechanisms underlying the transition from gastritis to atrophic changes during gastric Helicobacter infection are not known.
An increase in bacterial colonization due to insufficient Fas-mediated elimination of infected cells is one possible explanation for the enhanced pathological changes observed in gld mice. The importance of Fas-triggered cell death in modulating disease severity during infection was recently demonstrated during Pseudomonas aeruginosa infection in mice (11). In comparison with wild-type mice, mice deficient in either the Fas receptor or Fas ligand rapidly developed sepsis with increased splenic colonization in association with a reduction in lung epithelial cell apoptosis and markedly decreased survival. Similarly, studies with both Mycobacterium tuberculosis (28) and Listeria monocytogenes (15) indicate that Fas-mediated cell death is involved in host defense. Fas-triggered apoptosis reduces the viability of M. tuberculosis within infected macrophages in vitro (28). Compared to wild-type mice, Fas-deficient mice have elevated bacterial titers in the liver during infection with L. monocytogenes (15). However, in our study levels of bacterial colonization were similar in both groups of mice, suggesting that the enhanced degree of mucosal injury was not a result of increased bacterial load.
Previous studies with mice indicate that Helicobacter infection is associated with a predominant Th1 cytokine profile (10). In addition, murine studies suggest that levels of Th1 cytokines, in particular IFN-γ, contribute to the degree of gastric injury during Helicobacter infection (10). For example, mice deficient in IFN-γ or administered anti-IFNγ develop less severe inflammation than controls (32). Similarly, Fox et al. (10) recently reported that coinfection with the Th2-promoting helminth Heligmosmoides polygyrus during H. felis infection in C57BL/6 mice causes a reduction in atrophic changes in association with an enhanced Th2 profile and a reduced Th1 cytokine profile.
To determine if the increased gastric atrophy observed in H. pylori-infected gld mice was associated with altered Th1 or Th2 responses, we measured the levels of the Th1 cytokine IFN-γ and the Th2 cytokine IL-5 in response to infection in isolated splenocytes. In preliminary experiments we were not able to reliably measure IL-4 production from isolated splenocytes. Therefore, IL-5 was measured as a representative Th2 cytokine. Previous studies have demonstrated that cytokine responses in splenocytes correlate with gastric cytokine responses during Helicobacter infection in mice (22). Both groups of mice displayed similar levels of IL-5 before and after infection. However, splenocytes from mice deficient in Fas signaling showed a lack of IFN-γ production in response to infection compared to control mice. This finding suggests that multiple factors, in addition to IFN-γ, are involved in mediating the transition to gastric atrophy during infection in gld mice.
Conflicting data exist regarding the stimulation of IFN-γ from unfractionated spleen cells obtained from gld mice. For example, Davignon et al. (6) presented evidence that concanavalin A-stimulated unfractionated splenocytes from gld mice produce less IFN-γ than stimulated control cells. In contrast, Davidson et al. (5) reported that unfractionated lymphocytes from gld mice do not spontaneously produce IFN-γ but can produce IFN-γ after concanavalin A stimulation. The majority of lymphoid cells that accumulate in the lymph nodes and spleens of gld mice are a unique subset of double-negative T cells (5). When purified, these T cells proliferate weakly and fail to produce cytokines, including IFN-γ, following stimulation with a variety of agents (6). Further studies are required to determine if infection with H. pylori enhances the proportion of unresponsive, double-negative T-cell subsets in the spleens of gld mice.
Whereas Fas-triggered cell death likely serves as a host defense mechanism, dysregulation of the pathway could result in complications associated with infection. For example, excessive apoptosis would result in disruption of the epithelial barrier and thereby cause the development of mucosal ulceration. Indeed, H. pylori-infected subjects with duodenal ulcers have an increase in apoptosis of gastric epithelial cells compared to subjects with healing peptic ulcer disease (18). By contrast, a resistance to apoptosis could promote the development of gastric malignancies (33). In support of this contention, enhanced proliferation in the absence of increased cell death is detected in tissues obtained from patients with the precancerous lesion intestinal metaplasia compared to tissues from subjects with H. pylori-mediated gastritis alone (33). In our study the degree of apoptosis did not correlate with the degree of proliferation as assessed by proliferating cell nuclear antigen immunohistochemistry in serial sections (data not shown). Our findings in mice showing that programmed cell death is triggered through Fas and that the absence of Fas signaling enhances premalignant changes suggest that disruption of this signaling pathway could promote carcinogenesis.
Deregulation of the Fas pathway has been implicated in the development of a variety of human cancers, including gastric malignancies (27). Differential expression of the Fas receptor is detected both in cell lines derived from human gastric cancers (12) and in gastric carcinoma tissue specimens (36). Bennett et al. (2) detected enhanced Fas ligand expression in 30 gastric carcinomas and suggested that expression of Fas ligand allows gastric cancers to escape immune surveillance by inducing cell death in infiltrating lymphocytes.
In summary, this study demonstrates the importance of the Fas death pathway in disease pathophysiology following murine infection with H. pylori. Determination of whether chronic infection with H. pylori-induced dysregulation of the Fas signaling pathway ultimately participates in the multistep pathway leading to gastric carcinogenesis requires further study.
Acknowledgments
N. L. Jones is the recipient of an American Digestive Health Foundation Research Scholar Award and a Canadian Institute of Health Research Operating Grant. A. S. Day was the recipient of a Research Initiative Award from the Canadian Association of Gastroenterology/Astra Zeneca Pharmaceuticals/Medical Research Council of Canada. H. A. Jennings was the recipient of a Summer Studentship Award from the Canadian Association of Gastroenterology. P. M. Sherman is the recipient of a Canada Research Chair in Gastrointestinal Disease.
Editor: J. D. Clements
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, M. W., J. O'Connell, G. C. O'Sullivan, D. Roche, C. Brady, J. Kelly, J. K. Collins, and F. Shanahan. 1999. Expression of Fas ligand by human gastric adenocarcinomas: a potential mechanism of immune escape in stomach cancer. Gut 44:156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenes, F., B. Ruiz, P. Correa, F. Hunter, T. Rhamakrishnan, E. Fontham, and T. Y. Shi. 1993. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre- and post-eradication indices of proliferating cell nuclear antigen. Am. J. Gastroenterol. 88:1870-1875. [PubMed] [Google Scholar]
- 4.Covacci, A., J. L. Telford, G. Del Guidice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 5.Davidson, W. F., C. Calkins, A. Hugins, T. Giese, and K. L. Holmes. 1991. Cytokine secretion by C3H-lpr and -gld T cells. J. Immunol. 146:4138-4148. [PubMed] [Google Scholar]
- 6.Davignon, J. L., R. C. Budd, R. Ceredig, P. F. Piguet, H. R. MacDonald, J. C. Cerottini, P. Vasalli, and S. Izui. 1985. Functional analysis of T cell subsets from mice bearing the lpr gene. J. Immunol. 135:2423-2428. [PubMed] [Google Scholar]
- 7.Fan, X., H. Gunasena, Z. Cheng, R. Espejo, S. E. Crowe, P. B. Ernst, and V. E. Reyes. 2000. Helicobacter urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J. Immunol. 165:1918-1924. [DOI] [PubMed] [Google Scholar]
- 8.Fan, X. J., S. E. Crowe, S. Behar, H. Gunasena, G. Ye, H. Haeberle, N. Van Houten, W. K. Gourley, P. B. Ernst, and V. E. Reyes. 1998. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J. Exp. Med. 187:1659-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson, T. A., and T. S. Griffith. 1997. A vision of cell death: insights into immune privilege. Immunol. Rev. 156:167-184. [DOI] [PubMed] [Google Scholar]
- 10.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 11.Grassme, H., S. Kirschnek, J. Reithmueller, A. Riehle, G. von Kurthy, F. Lang, M. Weller, and E. Gulbins. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527-530. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, H., S. Tatebe, M. Osaki, A. Goto, Y. Suzuki, and H. Ito. 1997. Expression of Fas antigen and its mediation of apoptosis in human gastric cancer cell lines. Jpn. J. Cancer Res. 88:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houghton, J., R. M. Korah, M. R. Condon, and K. H. Kim. 1999. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig. Dis. Sci. 44:465-478. [DOI] [PubMed] [Google Scholar]
- 14.Houghton, J. M., L. M. Bloch, M. Goldstein, S. Von Hagen, and R. M. Korah. 2000. In vivo disruption of the fas pathway abrogates gastric growth alterations secondary to Helicobacter infection. J. Infect. Dis. 182:856-864. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, E. R., A. A. Glass, W. R. Clark, E. J. Wing, E. F. Miller, and S. H. Gregory. 1998. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect. Immun. 66:4143-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, N. L., P. T. Shannon, E. Cutz, H. Yeger, and P. M. Sherman. 1997. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 151:1695-1703. [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, N. L., A. S. Day, H. Jennings, and P. M. Sherman. 1999. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 67:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohda, K., K. Tanaka, Y. Aiba, M. Yasuda, T. Miwa, and Y. Koga. 1999. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut 44:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, A., J. O'Rourke, M. C. de Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 20.Lenardo, M. J. 1996. Fas and the art of lymphocyte maintenance. J. Exp. Med. 183:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannick, E. E., L. E. Bravo, G. Zarama, J. L. Realpe, X. J. Zhang, B. Ruiz, E. T. Fontham, R. Mera, M. J. Miller, and P. Correa. 1996. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 56:3238-3243. [PubMed] [Google Scholar]
- 22.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 23.Moller, P., H. Walczak, S. Reidl, J. Strater, and P. H. Krammer. 1996. Paneth cells express high levels of CD95 ligand transcripts: a unique property among gastrointestinal epithelia. Am. J. Pathol. 149:9-13. [PMC free article] [PubMed] [Google Scholar]
- 24.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. R. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata, S., and T. Suda. 1995. Fas and Fas ligand: lpr and gld mutations. Immunol. Today 16:39-43. [DOI] [PubMed] [Google Scholar]
- 26.Nishio, A., T. Katakai, C. Oshima, S. Kasakura, M. Sakai, S. Yonehara, T. Suda, S. Nagata, and T. Masuda. 1996. A possible involvement of Fas-Fas ligand signaling in the pathogenesis of murine autoimmune gastritis. Gastroenterology 111:959-967. [DOI] [PubMed] [Google Scholar]
- 27.O'Connell, J., M. W. Bennett, G. C. O'Sullivan, J. K. Collins, and F. Shanahan. 1999. Fas counter-attack—the best form of tumor defense? Nat. Med. 5:267-268. [DOI] [PubMed] [Google Scholar]
- 28.Oddo, M., T. Renno, A. Attinger, T. Bakker, H. R. MacDonald, and P. R. Meylan. 1998. Fas ligand-induced apoptosis of infected macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J. Immunol. 160:5448-5454. [PubMed] [Google Scholar]
- 29.Peek, R. M., Jr., S. F. Moss, K. T. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89:863-868. [DOI] [PubMed] [Google Scholar]
- 30.Peek, R. M., H.-P. Wirth, S. F. Moss, M. Yang, A. M. Abdalla, K. T. Tham, T. Zhang, L. H. Tang, I. M. Modlin, and M. J. Blaser. 2000. Helicobacter pylori alters gastric epithelial cell events and gastrin secretion in Mongolian gerbils. Gastroenterology 118:48-59. [DOI] [PubMed] [Google Scholar]
- 31.Rudi, J., D. Kuck, S. Strand, A. von Herbay, S. M. Mariani, P. H. Krammer, P. R. Galle, and W. Stremmel. 1998. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Investig. 102:1506-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scotiniotis, I. A., T. Rokkas, E. E. Furth, B. Rigas, and S. J. Shiff. 2000. Altered gastric epithelial cell kinetics in Helicobacter pylori-associated intestinal metaplasia: implications for gastric carcinogenesis. Int. J. Cancer 85:192-200. [PubMed] [Google Scholar]
- 34.Shirin, H., and S. F. Moss. 1998. Helicobacter pylori induced apoptosis. Gut 43:592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueyama, H., T. Kiyohara, N. Sawada, K. Isozaki, S. Kitamura, S. Kondo, J. Miyagawa, S. Kanayama, Y. Shinomura, H. Ishikawa, T. Ohtani, R. Nezu., S. Nagata, and Y. Matsuzawa. 1998. High Fas ligand expression on lymphocytes in lesions of ulcerative colitis. Gut 43:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vollmers, H. P., J. Dammrich, F. Hensel, H. Ribbert, A. Meyer-Bahlburg, T. Ufken-Gaul, M. von Korff, and H. K. Muller-Hermelink. 1997. Differential expression of apoptosis receptors on diffuse and intestinal type stomach carcinoma. Cancer 79:433-440. [DOI] [PubMed] [Google Scholar]
- 37.Wagner, S., W. Beil, J. Westermann, R. P. H. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836-1847. [DOI] [PubMed] [Google Scholar]
- 38.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 39.Wang, T. C., J. R. Goldenring, C. Dangler, S. Ito, A. Mueller, W. K. Jeon, T. J. Koh, and J. G. Fox. 1998. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114:675-689. [DOI] [PubMed] [Google Scholar]