Abstract
Disruption of the barrier properties of the enterocyte tight junction is believed to be important in the pathogenesis of diarrhea caused by enteropathogenic Escherichia coli (EPEC). This phenotype can be measured in vitro as the ability of EPEC to reduce transepithelial resistance (TER) across enterocyte monolayers and requires the products of the locus of enterocyte effacement (LEE) and, in particular, the type III secreted effector protein EspF. We report a second LEE-encoded gene that is also necessary for EPEC to fully reduce TER. rorf10 is not necessary for EPEC adherence, EspADB secretion, or formation of attaching and effacing lesions. However, rorf10 mutants have a diminished TER phenotype, reduced intracellular levels of EspF, and a reduced ability to translocate EspF into epithelial cells. The product of rorf10 is a 14-kDa intracellular protein rich in α-helices that specifically interacts with EspF but not with Tir or other EPEC secreted proteins. These properties are consistent with the hypothesis that rorf10 encodes a type III secretion chaperone for EspF, and we rename this protein CesF, the chaperone for EPEC secreted protein F.
Diarrhea remains one of the leading causes of childhood mortality in developing nations, and enteropathogenic Escherichia coli (EPEC) is a leading cause of bacterial diarrhea in infants (28). However, the pathogenesis of diarrhea due to EPEC is incompletely understood and may involve production of an enterotoxin (25), the formation of attaching and effacing lesions on host epithelial cells (28), or alterations in tight-junction permeability (33).
Epithelial tight junctions may be studied in vitro by using polarized T84 epithelial cell monolayers. When T84 monolayers are infected with EPEC, an increase in myosin light chain phosphorylation and redistribution of the tight-junction-associated protein occludin are seen and correlate with disruption of the tight-junction barrier (32, 37). This is measured as a dramatic reduction in transepithelial resistance (TER) across the monolayer and an increase in solute flux across the paracellular space (33). The reduction in barrier function may be an important factor in EPEC diarrhea.
To reduce TER, EPEC requires proteins encoded by the locus of enterocyte effacement (LEE) pathogenicity island (21, 32). The LEE contains genes encoding an outer membrane protein (intimin), a regulator of LEE gene expression (Ler) (9, 24), a type III secretion system (Esc, Sep, Ces), and several type III secreted proteins, including Map (15) and Tir (14) as well as EspA, EspB, EspD, EspF, and EspG (5, 8, 12, 22). Type III secretion by EPEC is believed to involve a bacterial membrane complex of Esc/Sep proteins upon which is assembled an extracellular filament of polymerized EspA (17). EspB and EspD proteins are proposed to form a pore in the host membrane at the distal end of the EspA filament (11, 35). Together, these components function to translocate effector proteins directly from the bacterial cytoplasm into the host via the EspA filament. Translocation of EspF in particular has been demonstrated to be absolutely essential for the EPEC-induced drop in TER (23).
The process of translocation of type III secreted proteins may also require cytoplasmic chaperones, which typically perform multiple roles, including preventing premature and inappropriate protein-protein interactions, assisting protein folding, stabilizing the protein, and otherwise enabling protein secretion (reviewed in references 4 and 36). Chaperones involved in type III secretion are not highly similar at the amino acid level but in general are small (15- to 20-kDa), cytoplasmic, acidic proteins with a putative amphipathic α-helix in the C-terminal portion, and they are usually specific for a given secreted protein. Two LEE-encoded EPEC chaperones have been described. CesD, the chaperone for E. coli secretion of EspD (34), directly interacts with EspD, is necessary for EspD stability in the cytoplasm, and is required for secretion of both EspD and EspB but not EspA. CesT, the chaperone for Tir (1, 6), binds the N terminus of Tir and is necessary for Tir translocation and stability in the cytoplasm. CesT is not absolutely required for Tir secretion, although secretion is markedly reduced in a cesT mutant.
Here we report that the LEE gene rorf10 encodes a protein with properties consistent with those of an EspF-specific chaperone. Following previous nomenclature for the LEE, we rename this protein CesF, the chaperone for EPEC secreted protein F.
MATERIALS AND METHODS
Construction, expression, and purification of His6CesF fusion.
A plasmid expressing CesF fused to an N-terminal MRGSHis6 tag (pQE30::His6CesF) (Table 1) was constructed by amplification of a 360-bp fragment with primers K1362 (5′-ACGCGGATCCAATGAACAATTTGCAAAGATCTT-3′) and K1363 (5′-GCAAATAAATCAAAGTGAAAGTAGTT-3′), digestion with BamHI, and cloning into BamHI/SmaI sites of pQE30. The template used for this and other PCR amplifications was wild-type EPEC strain E2348/69 (18). The fidelity of the construct was checked by sequencing. Clones of DH5α containing the pQE30::His6CesF plasmid were unstable unless they were grown on Luria-Bertani (LB) agar supplemented with 100 mM glucose at 30°C. To examine expression, overnight cultures were grown in LB medium containing 100 mM glucose at 30°C, and 1 ml was inoculated into 100 ml of LB medium and grown at 37°C for 2 h. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a concentration of 2 mM, and then the culture was allowed to grow for an additional 3 h. The bacterial pellet was resuspended in buffer containing 300 mM NaCl and 50 mM NaH2PO4 (pH 8.0) (buffer) supplemented with 10 mM imidazole and lysed with a French pressure cell. The cleared bacterial cell lysate was incubated at 4°C for 1 h with 1 ml of Ni2+-nitrilotriacetic acid (NTA) beads (Qiagen) with gentle mixing. The suspension was then poured into a column, and the flowthrough was collected and passed over the packed beads two more times. The column was washed three times with 4 ml of buffer containing 20 mM imidazole, and bound proteins were eluted with 0.5-ml aliquots of buffer containing 250 mM imidazole. Proteins were examined in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels stained with Coomassie blue or in Western blots by utilizing antibodies directed against the His6 tag (Pierce) at a dilution of 1:5,000.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Reference |
|---|---|---|
| Strains | ||
| E2348/69 | Wild-type E. coli O127::H7 EPEC | 18 |
| E2348/69 cesF | E. coli E2348/69 cesF::pSE887 | This study |
| E2348/69 escN | Type III secretion-deficient mutant | 12 |
| E2348/69 ΔespF | espF deletion | 22 |
| Plasmids | ||
| pCesF-F | pBluescript KS::PlacZ-cesF | This study |
| pCesF-R | pBluescript KS::cesF-PlacZ | This study |
| pQE30::His6CesF | N-terminal MRGSHis6 fusion to CesF | This study |
| pJP5603 | oriR6K mobRP4 lacZ::MCS kan | 29 |
| pBPM37 | EspF-CyaA fusion | 23 |
| pSE887 | pJP5603::cesF internal fragment | This study |
Construction of CesF mutant and clones.
A fragment containing cesF and the cesF promoter was amplified as an 815-bp fragment using primers K168 (5′-GCAAATAAATCAAAGTGAAAGTAG-3′) and K184 (5′-GGGTAGTCAGTTTGCCATGATTAA-3′) and ligated into pGEM-T. The insert orientation was determined by PCR and restriction digestion. Plasmids containing cesF in the same orientation as lac (pCesF-F) and containing cesF transcribed in the orientation opposite that of lac (pCesF-R) were isolated.
Insertional inactivation of cesF was achieved by using previously described protocols (7). cesF was amplified as described above and was digested with BglII and EcoRI, resulting in a 275-bp fragment that was internal to cesF. This fragment was cloned into the EcoRI/BamHI site of the suicide plasmid pJP5603, and the resultant plasmid was introduced into EPEC strain E2348/69 (Nalr) via conjugation. Kanr Nalr transconjugants were examined for loss of the suicide plasmid and insertion into cesF using plasmid extraction, PCR, and Southern blotting.
TER assay.
T84 cells were grown to confluence on 0.33-cm2 collagen-coated permeable supports and infected with bacteria for 6 h as described previously (23). A simplified apparatus for measuring electrophysiological parameters described by Madara et al. (20) was used. TER was determined by passing 25 μA of current, measuring voltage deflection, and applying Ohm's law (V = IR, where V is voltage, I is current, and R is resistance) to calculate resistance.
Analysis of EspF-CyaA translocation into HeLa cells.
The adenylate cyclase reporter assay was performed as previously described (22). Briefly, overnight cultures of bacterial strains were diluted 1:100 in Dulbecco modified Eagle medium (Invitrogen) and grown for 4.5 h at 37°C with aeration. Then 0.5 ml of a bacterial cell culture was added to HeLa cells to obtain a final multiplicity of infection of 100 and incubated for 1.5 h. The infected cells were then washed, harvested, and lysed, and each aliquot was adjusted so that equivalent protein concentrations were obtained. cAMP levels were determined in extracts by using the Biotrak cAMP enzyme immunoassay system (Amersham Pharmacia Biotech).
Assays for virulence-associated phenotypes.
Bacterial adherence to HEp-2 cells was examined after 3 h by the modified method of Scaletsky et al. (31), as previously described (7). In the fluorescent actin stain (FAS) test (16) fluorescein isothiocyanate-phalloidin is utilized to visualize the accumulation of actin beneath and around bacteria attached to HEp-2 cells, and this test was used as a marker for the ability of bacteria to cause attaching and effacing lesions after a 3-h infection.
Expression of bacterial proteins was examined in supernatants and bacterial fractions which were prepared as outlined previously (6, 13). Following separation through SDS-PAGE gels, proteins were either stained with Coomassie blue or blotted onto polyvinylidene difluoride membranes and Western blotted with murine monoclonal antibodies against the His6 epitope or rabbit polyclonal antibodies against EspF, Tir, intimin, and all EPEC secreted proteins as previously described (6, 13).
Column binding assay.
The ability of column-immobilized His6CesF to interact with EspF was determined by previously described methods (6). Briefly, His6CesF was bound to a Ni2+-NTA column and washed with buffer (see above) containing 20 mM imidazole, as described above for the purification of His6CesF. Secreted proteins from E2348/69 were concentrated from 100 ml of a minimal essential medium-grown culture, resuspended in buffer containing 20 mM imidazole, and passed through the column three times. The column was then washed three times with 4 ml of buffer containing 20 mM imidazole. CesF was eluted in 0.5-ml aliquots with buffer containing 250 mM imidazole. In a control experiment secreted protein preparations were passed through a column that had not been previously incubated with His6CesF. The resulting samples were examined by Western blotting. All samples of His6CesT and secreted proteins used in the protocol described above were prepared fresh and were never frozen.
Molecular techniques.
When cloning required PCR amplification, the proofreading polymerase Pwo (Boeringher Mannheim) was used, and the resultant clones were examined for fidelity by sequencing. All other PCR were performed using Taq polymerase (Life Technologies). Automated sequencing was performed at the University of Maryland Biopolymer Core Facility. All other molecular techniques were performed according to standard protocols. DNA analysis was performed with DNAsis v5 (Hitachi) and with the suite of programs provided by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Homology searches were performed by using PSI-BLAST (http://www.ncbi.nlm.gov/blast/psiblast.cgi) with the filter off and the gap function activated. Protein localization was predicted by using the PSORT algorithm (http://www.psort.nibb.ac.jp/). Secondary structure was predicted by using the Jpred program, which gives the consensus of multiple algorithms and is found at the European Bioinformatics Institute website (http://jura.ebi.ac.uk).
RESULTS
CesF is a small intracellular protein with features consistent with those of a chaperone.
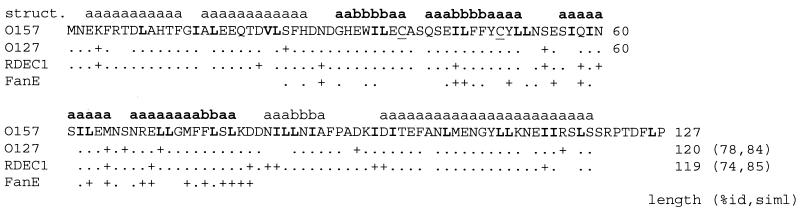
The EPEC cesF gene (previously named rorf10) is 360 bp long and is predicted to encode a 120-amino-acid 14-kDa protein. However, the length of CesF may vary in different isolates, from 119 amino acids in RDEC-1 to 120 amino acids in an EPEC O127:H7 isolate and 127 amino acids in an enterohemorrhagic E. coli (EHEC) O157:H7 isolate. Interestingly, the relative lengths of CesF in the isolates reflect differences in the length of EspF in the strains, which ranges from 160 amino acids in RDEC-1 to 206 amino acids in EPEC and 248 amino acids in EHEC. A comparison also demonstrated that CesF may diverge in isolates by up to 26% (Fig. 1), which is greater than the divergence observed in most LEE-encoded proteins that constitute the type III secretion machinery; this level of divergence is more typical of secreted proteins, such as EspA (20% divergence) (5, 30, 38). PSI-BLAST homology searches also demonstrated that CesF was weakly similar to the K99 pilus chaperone FanE (27). Unlike FanE, CesF does not possess an obvious signal sequence, and computer analysis (http://www.psort.nibb.ac.jp) predicted that CesF was located in the bacterial cytoplasm, supporting the experimental observations. Secondary structural analyses predicted that CesF is rich in α-helices containing leucine, isoleucine, and valine residues, a feature that is often associated with protein-protein interactions.
FIG. 1.
Alignment of CesF amino acid sequence from EHEC O157:H7 (O157) with the 120-amino-acid CesF sequence of EPEC E2348/69 (O127:H6) and the 119-amino-acid CesF sequence of RDEC1. The region of similarity of the chaperone FanE with CesF is also shown. Identical amino acids are indicated by periods, similar amino acids are indicated by plus signs, and dissimilar amino acids are indicated by spaces. The percentages of identity (%id) and similarity (siml) relative to the O157:H7 sequence are indicated at the ends of the aligned sequences. Structural details (struct.) were predicted by Jpred and are indicated above the CesF amino acid sequence as follows: a, α-helix; b, β sheet; boldface type, potential amphipathic regions. Also indicated in the CesF amino acid sequence are paired cysteine residues (underlined) and the large number of L, I, and V residues (boldface type) that occur, especially in the α-helices.
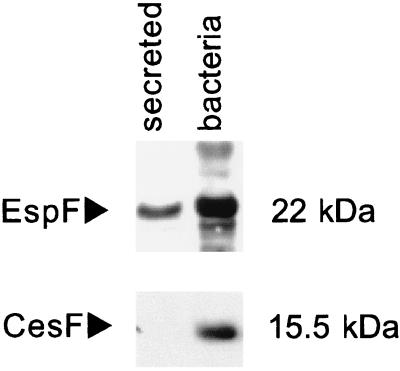
To examine the properties of CesF, the cesF gene was cloned into vector pQE30, generating pQE30::His6CesF, which expressed an N-terminal His6-tagged fusion protein (His6CesF) in DH5α. Upon induction, large amounts of a 15.5-kb protein were observed in Coomassie blue-stained SDS-PAGE gels and in Western blots when monoclonal antibodies directed against the His6 tag were used (data not shown). These data are in agreement with the predicted molecular mass of the fusion protein, 15.2 kDa. E2348/69 was transformed with pQE30::His6CesF and grown in minimal essential medium with 2 mM IPTG to induce His6CesF production. Unlike EspF, which was present in both fractions, a 15-kDa protein was observed in the whole-cell preparations but not in the supernatant (Fig. 2). These results indicate that CesF is not secreted into the supernatant and are consistent with earlier predictions.
FIG. 2.
Western blots of secreted proteins and whole bacteria probed with antiserum against EspF and the His6 tag, which recognized His6CesF. CesF is found in whole bacteria but is not secreted into the supernatant, unlike the type III secreted protein EspF.
cesF mutants show diminished ability to disrupt intestinal epithelial barrier function.
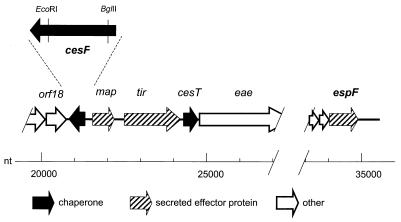
cesF is monocistronic and transcriptionally divergent from its flanking genes, orf19/map and orf18 (Fig. 3). We therefore mutated the cesF gene by cloning an internal fragment of cesF into the suicide vector pJP5603 and recombining the entire plasmid into the homologous site in E2348/69.
FIG. 3.
Region of the E2348/69 LEE containing cesF. cesF, encoding the chaperone for the type III secreted effector protein EspF, is located near the map and tir genes, which encode other type III secreted effector proteins, and also near cesT, which encodes the chaperone for tir. However, cesF is distant from and transcriptionally isolated from the LEE4 operon that contains espF. cesF is also transcribed divergently from the adjacent map gene and from orf18, which is part of the LEE3 operon that encodes components of the type III secretion system. An expanded view of cesF shows the EcoRI and BglII sites that were used to construct the cesF mutant strain. The nucleotide (nt) scale is from the LEE sequence described by Elliott et al. (5).
To provide cesF in trans and complement the chromosomal mutation, an 815-bp fragment containing the cesF structural gene and the cesF promoter was cloned into pBluescript KS in forward and reverse orientations with respect to lacZ, generating pCesF-F and pCesF-R, respectively. Both plasmids were stable in DH5α and E2348/69 cesF, unlike pQE30::His6CesF, which was unstable in both hosts (data not shown).
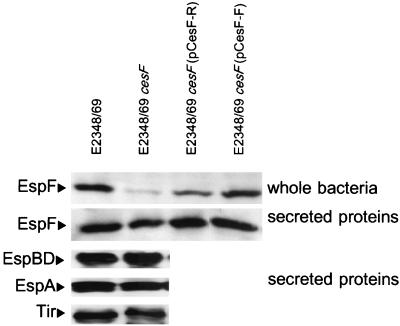
To examine the role of CesF in EPEC virulence phenotypes, we compared wild-type E2348/69, E2348/69 cesF, and E2348/69 cesF transformed with either pCesF-F or pCesF-R in several in vitro assays for EPEC virulence phenotypes. These four strains were indistinguishable in terms of their ability to form microcolonies on HEp-2 cells in the modified localized adherence assay and in the 3-h FAS test for formation of attaching and effacing lesions (data not shown). The presence or absence of CesF also did not affect the ability of EPEC to secrete proteins EspABD or Tir (Fig. 4).
FIG. 4.
Western blots of secreted proteins and whole bacteria probed with antiserum against EspF. Mutation of cesF does not affect secretion of EspABD or Tir, and the secretion of EspF by the cesF mutant is only slightly less than that by the wild type. In contrast, the EspF levels in whole bacteria are substantially lower in a cesF mutant. EspF levels are restored by complementation with cloned CesF.
In contrast, expression of CesF affected the ability of EPEC to alter the TER of T84 monolayers. Uninfected polarized T84 cell monolayers showed increased resistance over time, while polarized T84 cell monolayers infected with wild-type EPEC strain E2348/69 showed a significant decrease in TER (37) (Table 2). In contrast, E2348/69 ΔespF did not induce a significant drop in TER (23) (Table 2). When polarized T84 monolayers were infected with E2348/69 cesF, an attenuated decrease in the TER response was observed that was 38% of the wild-type decrease. Infection with E2348/69 cesF strains complemented with multicopy plasmids encoding cesF restored and even enhanced the TER response compared to the response seen with the wild type (Table 2). E2348/69 cesF containing pCesF-F with cesF in the same orientation as the lac promoter showed an 80% decrease in the TER, while E2348/69 cesF containing pCesF-R with cesF transcribed against the lac promoter decreased the TER by 60%. These values were slightly greater than the 52% decrease in TER seen with wild-type strain E2348/69. As shown in Fig. 4, higher levels of EspF were observed in cells containing CesF-F than in cells containing pCesF-R, suggesting that the TER response parallels the amount of CesF produced.
TABLE 2.
Change in TER across polarized T84 monolayers after 6 h of infection with bacteria
| Strain | Change in TERa | % EPECb | P < 0.05c |
|---|---|---|---|
| E2348/69 | −52 (3) | 100 | |
| E. coli K-12 | 4 (2) | −8 | + |
| No bacteria | −13 (3) | 25 | + |
| EPEC escN | 4 (4) | −8 | + |
| EPEC espF | −4 (2) | 8 | + |
| EPEC cesF | −20 (4) | 38 | + |
| EPEC cesF (pUC18) | −20 (3) | 38 | + |
| EPEC cesF (pCesF-R) | −60 (3) | 115 | + |
| EPEC cesF (pCesF-F) | −80 (5) | 154 | + |
Mean change in TER between zero time and 6 h, expressed as a percentage of the TER at zero time. The values in parentheses are standard errors of the means.
Change in TER expressed as a percentage of the E2348/69 change in TER.
+, significantly different from E2348/59 at P values of <0.05, as determined by a single-tailed t test.
CesF affects intracellular levels of EspF and EspF translocation into epithelial cells.
E2348/69 cesF exhibited a marked reduction in the amount of EspF in the bacterial cell compared with the wild type, and complementation of the cesF mutation with cesF on a multicopy plasmid restored expression of intracellular EspF to wild-type levels (Fig. 4). However, the presence or absence of CesF did not appear to have a significant effect upon the levels of EspF secreted into the supernatant, as the cesF mutant exhibited only slightly less EspF secretion into the supernatant than the wild type (Fig. 4). The slightly decreased levels of secreted EspF observed may have been due to a reduced intracellular pool of EspF to be secreted, or, less likely, EspF may have an accessory function in secretion.
To measure the levels of EspF translocated into epithelial cells, bacteria were transformed with pBPM37, which expresses an EspF-CyaA fusion protein. By measuring the resulting intracellular cAMP levels due to the translocation of calmodulin-dependent adenylate cyclase (CyaA), the efficiency of EspF translocation in the absence of CesF was estimated. In this assay, mutation of the escN gene encoding the predicted ATPase of the type III secretion system completely abolished translocation of EspF (Table 3). Mutation of cesF greatly reduced but did not abolish translocation of EspF [55% reduction of EspF translocation compared to the wild type; P = 0.001 for E2348/69(pBPM37) versus E2348/69 cesF(pBPM37) (Table 3)]. These data parallel those from the TER assay (Table 2). In the absence of CesF the amount of EspF delivered to host cells was reduced, as was the TER.
TABLE 3.
Translocation of EspF-CyaA into HEp-2 cells
| Strain | Genotype | Plasmid added | cAMP total protein (pmol/mg)a | P value vs E2348/69(pBPM37)b | P value vs CVD452(pBPM37)b |
|---|---|---|---|---|---|
| E2348/69 | Wild type | None | 7.5 ± 4.5 | <0.001 | 0.007 |
| E2348/69 | Wild type | pBPM37 (EspF-Cya) | 1,697.5 ± 222.4 | <0.001 | |
| CVD452 | ΔescN | pBPM37 (EspF-Cya) | 14.1 ± 4.3 | <0.001 | |
| E2348/69 cesF | ΔcesF | pBPM37 (EspF-Cya) | 792.2 ± 126.5 | 0.001 | <0.001 |
Mean ± standard error based on triplicate samples from three independent experiments.
P values were calculated by using a t test.
CesF specifically interacts with EspF.
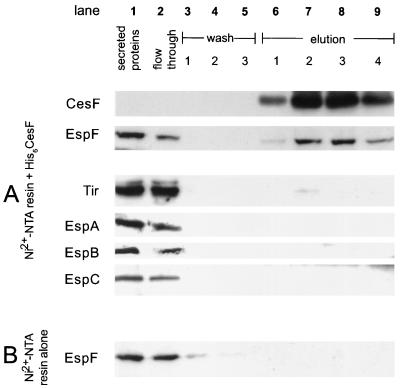
To determine if a specific interaction occurred between EspF and CesF, we examined the ability of His6CesF immobilized on an Ni2+-NTA column to bind EspF compared to its ability to bind other secreted EPEC proteins (Fig. 5A). When a secreted protein preparation containing all EPEC secreted proteins (Fig. 5A, lane 1) was applied to a column containing immobilized CesF, EspF was retained on the column and could be eluted from the column with CesF (lanes 6 to 9) by high concentrations of imidazole. By contrast, the other secreted proteins, Tir, EspA, EspB/D, and EspC, were not retained on the column as they did not appear in significant amounts in the eluate and appeared only in the flowthrough (lane 2). A small amount of Tir was observed to bind to the His6CesF column (lane 6), but this was not considered significant and likely represented nonspecific interactions with the column. In a control experiment, EspF was not retained on an Ni2+-NTA column that was not prebound with His6CesF (Fig. 5B). These data indicate that there is a specific interaction between CesF and EspF in vitro.
FIG. 5.
Column binding assay showing that CesF specifically binds EspF but not other secreted EPEC proteins. (A) Proteins secreted from EPEC were passed over an Ni2+-NTA column containing prebound His6CesF. EspF was retained on the column and was eluted along with CesF (lanes 6 to 9). In contrast, other secreted proteins (Tir, EspA, EspB, EspC) were not retained and flowed through the column (lane 2), although a small amount of Tir was detected, consistent with a nonspecific interaction with the column (lane 6). (B) EspF is not retained on Ni2+-NTA resin in the absence of CesF. All proteins were detected by Western blotting with specific antiserum as described in the text.
DISCUSSION
CesF fulfills all the criteria necessary to define it as the specific chaperone for EspF in that (i) cesF and espF mutants have similar phenotypes in EPEC virulence assays, (ii) cesF mutants are defective in EspF translocation and accumulate less EspF in the cytoplasm, and (iii) CesF specifically interacts with EspF. The experimental data presented here are also consistent with computational analysis of CesF, which predicted features typical of a chaperone.
In a variety of phenotypic assays used to study EPEC virulence, the phenotypes of a cesF mutant parallel those of an espF mutant (23). Both cesF and espF mutants were unaltered in the ability to adhere to HEp-2 cells, the ability to secrete Tir, EspA, EspD, and EspB, and the ability to form attaching and effacing lesions as determined by the FAS assay. In contrast to the lack of effects on these phenotypes, mutation of either cesF or espF affected the ability of EPEC to disrupt the barrier function of T84 monolayers as determined by measurement of TER. Furthermore, cesF mutants containing cesF cloned on a multicopy plasmid were able to decrease TER to a greater degree than wild-type EPEC, suggesting that the levels of CesF expression parallel the changes in TER. These data indicate that there is a specific link between CesF and TER. CesF is not secreted from the bacterial cell and so is unlikely to act upon the host cell directly. The data presented in this report demonstrate that the phenotypes associated with the cesF mutation were due to the effect of CesF on EspF.
Using EspF-CyaA fusions, we demonstrated that a cesF mutant translocated 50% less EspF into HeLa cells than wild-type EPEC translocated. This observation closely parallels the 62% reduction in TER observed on T84 cell monolayers and indicates that there is a close correlation of CesF production, EspF translocation, and TER. The levels of EspF inside EPEC cells were dramatically reduced in the cesF mutant, but the levels of other Esp proteins were unaffected. Because espF is cotranscribed with espABD (9, 24) and because EspABD levels are not affected by the cesF mutation, these results suggest that cesF does not act on espF transcription but rather acts on EspF stability. Type III secretion chaperones, such as SycE in Yersinia (3, 10), IpgC in Shigella (26), and CesT in EPEC (6), are known to bind to and stabilize effector proteins, characteristics that are functionally consistent with our data regarding CesF. Finally, we demonstrated that CesF bound specifically to EspF and did not bind to Tir or other Esp proteins. These data experimentally demonstrated that CesF has properties consistent with those of a chaperone for EspF.
The experimental evidence that CesF is the EspF chaperone is supported by computational and genomic analyses. Consistent with the properties of other chaperones, CesF is a small, acidic, cytoplasmic protein predicted to be rich in α-helices and contains an amphipathic α-helix. The amino acid sequence of CesF is similar to that of the K99 chaperone FanE. As expected for a chaperone, CesF production is coregulated with production of EspF as transcription of both cesF and espF are activated by Ler, the LEE-encoded regulator (9, 24). Similarly, CesF appears to have coevolved with EspF. If the LEE of EPEC, EHEC O157, and RDEC-1 are compared, EspF is the most variable protein within the LEE, ranging in length from 160 to 248 amino acids and diverging by up to 50% when the entire length is examined (38). Greater variation is observed in LEE-encoded proteins that are secreted and/or interact with the host (30, 38) than in proteins that remain within bacteria, which is consistent with greater evolutionary pressure on proteins exposed to the host immune system. However, because CesF interacts with EspF and is necessary for EspF function, CesF presumably coevolved with the highly divergent protein EspF and so might be expected to have a higher degree of variation than other intracellular LEE-encoded proteins. Such variation is observed, and CesF is more divergent (up to 26%) than most other LEE-encoded proteins and may range in length from 119 to 127 amino acids.
The precise chaperone functions of CesF remain to be determined. Indeed, the role of chaperones in type III secretion remains controversial. Many type III secreted effector proteins lack chaperones but are nonetheless secreted and/or translocated into host cells. Other effectors, such as YopE, possess chaperones that stabilize the protein and are necessary for chaperone-mediated secretion and for translocation into host cells (reviewed in references 4 and 36). However, YopE can also be secreted by a chaperone-independent pathway. It has been proposed that this pathway relies on the secondary structure of the mRNA to direct secretion (2), although most work, including a recent publication (19), suggests that the secretion signal is dependent upon the N-terminal amino acid sequence.
Our data indicate that CesF is clearly necessary for steady-state levels of EspF (implying EspF stability) and for full translocation of EspF into host cells but may not be necessary for EspF secretion. The E2348/69 cesF mutant used in this study is an insertion mutant, and we cannot rule out the possibility that the failure of the cesF mutant to completely abolish translocation or secretion might be due to the residual truncated N-terminal CesF protein produced by the cesF mutant. Nevertheless, it is clear that insertion into the cesF gene is associated with clearly definable phenotypes consistent with those of a chaperone and is supported by other evidence. Furthermore, it is difficult to explain how the possible residual activity is insufficient for EspF stability but has almost no effect on secretion. The present data are consistent with chaperone-independent EspF secretion. In this regard, CesF appears to be most analogous with CesT, the EPEC Tir chaperone (6). Both CesT and CesF are necessary for stability of their target effectors but are not absolutely required for secretion, implying that there is a chaperone-independent secretion pathway in EPEC (2). Recent data from our laboratory (J. A. Crawford and J. B. Kaper, submitted for publication) also suggests that Tir translocation does not absolutely require CesT. It is possible that the residual EspF translocation in the cesF mutant also represents chaperone-independent translocation, but in the absence of complete cesF deletion, this cannot be determined. We are currently investigating these and other issues involved in the secretion and translocation of effector proteins by the EPEC type III system.
Finally, this research supports the recent observation that EspF is absolutely necessary for the EPEC-mediated depolarization of T84 monolayers (23). CesF affects only EspF and EspF-mediated functions and attenuates to the same degree (ca. 50%) EspF translocation into HeLa cells and TER of T84 cells. It remains to be determined what roles EspF and CesF play in other models of diarrhea and in human disease.
Acknowledgments
This work was supported by Public Health Service grants AI-21657 (to J.B.K.), DK50694 (to G.H.), and AI-32074 (to M.S.D.) and by a Merit Review and REAP from the Department of Veterans Affairs (to G.H.).
S.J.E. thanks Kwang Sik Kim, Johns Hopkins University School of Medicine, for his support during the writing of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Abe, A., M. de Grado, R. A. Pfuetzner, C. Sanchez-Sanmartin, R. DeVinney, J. L. Puente, N. C. Strynadka, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162-1175. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24:757-765. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott, S., L. A. Wainwright, T. McDaniel, B. MacNamara, M. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 6.Elliott, S. J., S. W. Hutcheson, M. S. Dubois, J. L. Mellies, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176-1189. [DOI] [PubMed] [Google Scholar]
- 7.Elliott, S. J., and J. B. Kaper. 1997. Role of type 1 fimbriae in EPEC infections. Microb. Pathog. 23:113-118. [DOI] [PubMed] [Google Scholar]
- 8.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott, S. J., V. Sperandio, J. A. Girón, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frithz-Lindsten, E., R. Rosqvist, L. Johansson, and Å. Forsberg. 1995. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensible for targeting to the secretion loci. Mol. Microbiol. 16:635-647. [DOI] [PubMed] [Google Scholar]
- 11.Hartland, E. L., S. J. Daniell, R. M. Delahay, B. C. Neves, T. Wallis, R. K. Shaw, C. Hale, S. Knutton, and G. Frankel. 2000. The type III protein translocation system of enteropathogenic Escherichia coli involves EspA-EspB protein interactions. Mol. Microbiol. 35:1483-1492. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis, K. G., J. A. Girón, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a specialized secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis, K. G., and J. B. Kaper. 1996. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect. Immun. 64:4826-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 15.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 16.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, S. Sotman, and B. Rowe. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed]
- 19.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520-531. [DOI] [PubMed] [Google Scholar]
- 20.Madara, J. L., S. Colgan, A. Nusrat, C. Delp, and C. Parkos. 1992. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil-epithelial interactions. J. Tissue Cult. Methods 14:209-216. [Google Scholar]
- 21.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamara, B. P., and M. S. Donnenberg. 1998. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol. Lett. 166:71-78. [DOI] [PubMed] [Google Scholar]
- 23.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 25.Mellies, J. L., F. Navarro-Garcia, I. Okeke, J. Frederickson, J. P. Nataro, and J. B. Kaper. 2001. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 69:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard, R., P. Sansonetti, C. Parsot, and T. Vasselon. 1994. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79:515-525. [DOI] [PubMed] [Google Scholar]
- 27.Mol, O., H. Fokkema, and B. Oudega. 1996. The Escherichia coli K99 periplasmic chaperone FanE is a monomeric protein. FEMS Microbiol. Lett. 138:185-189. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145-146. [DOI] [PubMed] [Google Scholar]
- 30.Perna, N. T., G. F. Mayhew, G. Pósfai, S. Elliott, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scaletsky, I. C. A., M. L. M. Silva, and L. R. Trabulsi. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45:534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonovic, I., J. Rosenberg, A. Koutsouris, and G. Hecht. 2000. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2:305-315. [DOI] [PubMed] [Google Scholar]
- 33.Spitz, J., R. Yuhan, A. Koutsouris, C. Blatt, J. Alverdy, and G. Hecht. 1995. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am. J. Physiol. 268:G374-G379. [DOI] [PubMed] [Google Scholar]
- 34.Wainwright, L. A., and J. B. Kaper. 1998. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol. Microbiol. 27:1247-1260. [DOI] [PubMed] [Google Scholar]
- 35.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wattiau, P., S. Woestyn, and G. Cornelis. 1996. Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol. 20:255-262. [DOI] [PubMed] [Google Scholar]
- 37.Yuhan, R., A. Koutsouris, S. D. Savkovic, and G. Hecht. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873-1882. [DOI] [PubMed] [Google Scholar]
- 38.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]