Abstract
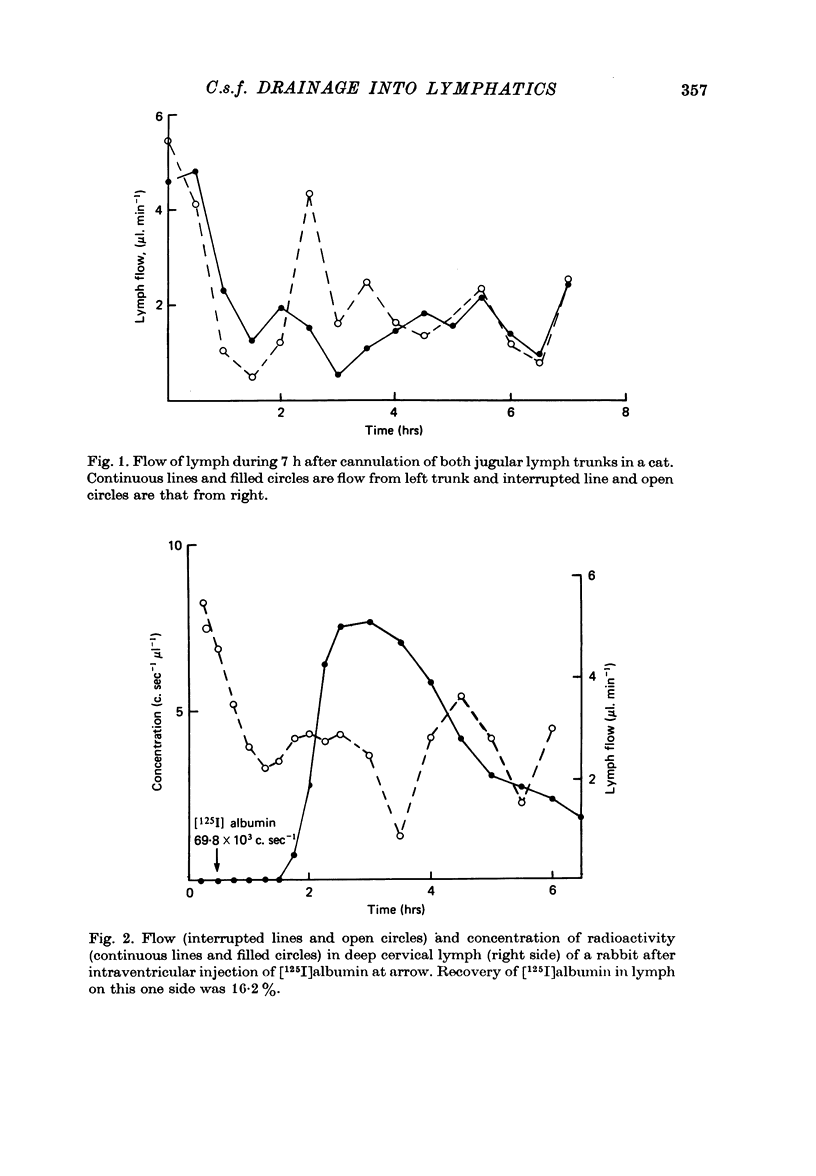
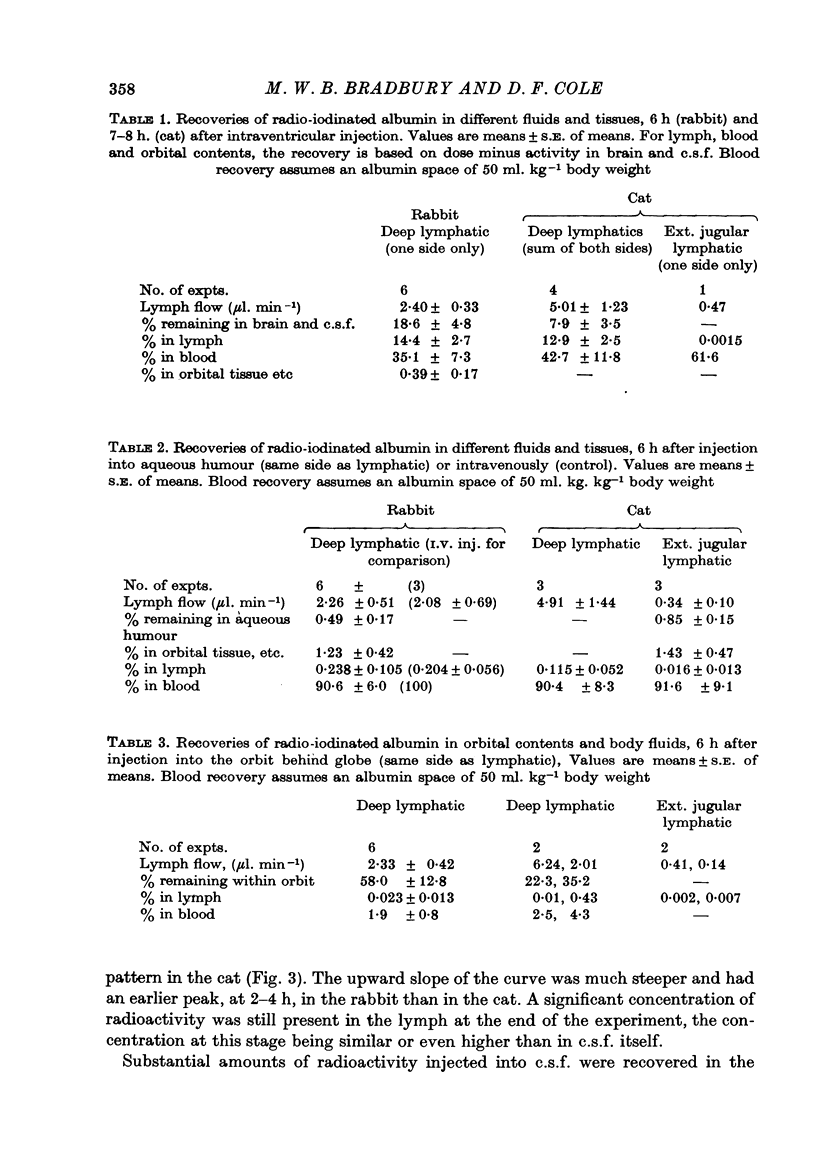
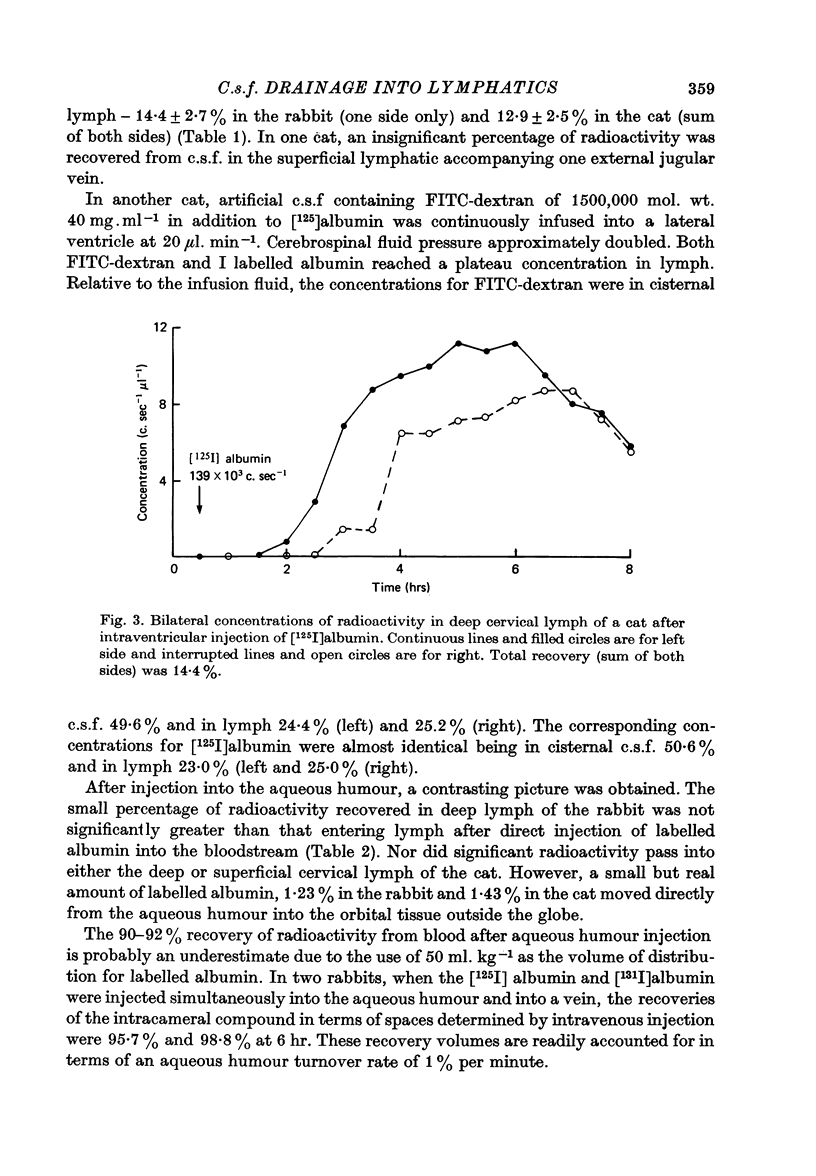
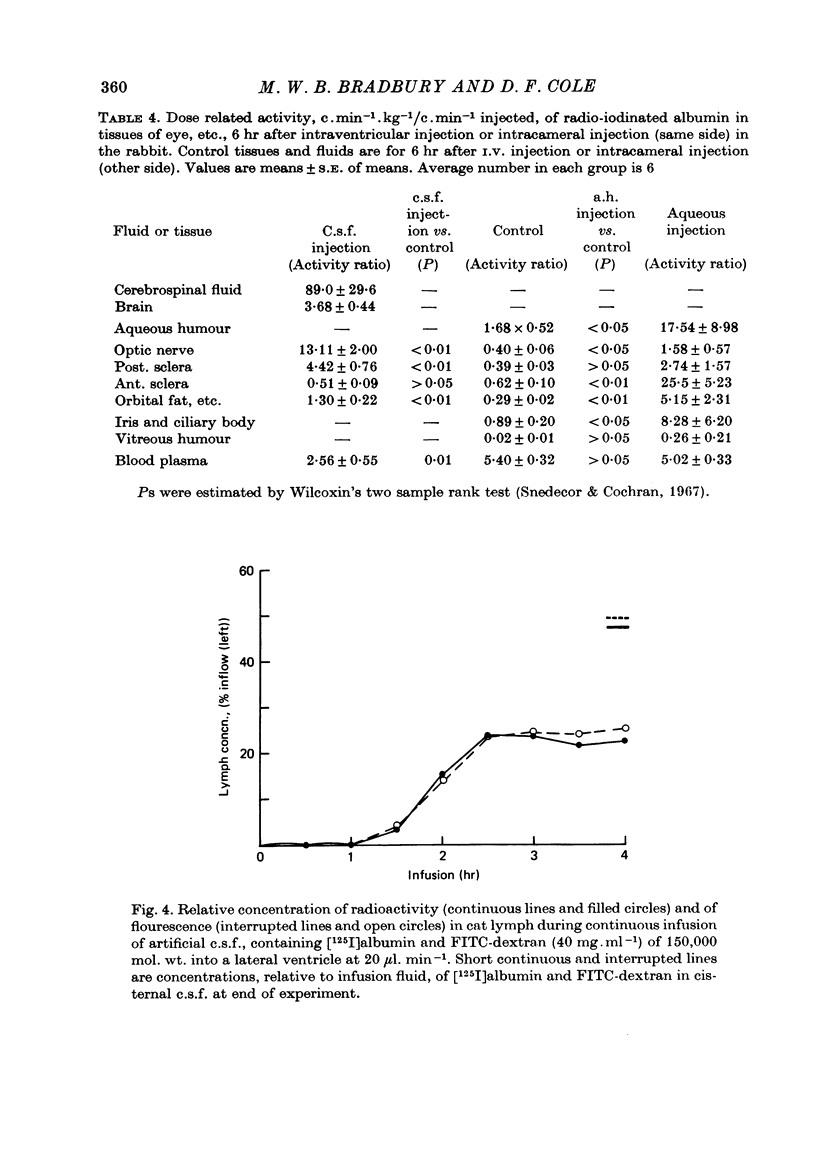
1. The jugular lymphatic trunks were cannulated in anaesthetized rabbits and cats. Over 6-8 hr, the mean lymph flow was 2.3 microliters min-1 in the rabbit (one side only) and 5.0 microliters min-1 in the cat (sum of both sides). 2. After a single injection of radio-iodinated albumin into a lateral cerebral ventricle without significant rise in pressure, a mean of 14.4% of the radioactivity was recovered in deep cervical lymph of one side in the rabbit and of 12.9% in that of both sides in the cat. 3. During slow infusion of [125I]albumin and fluorescent dextran of 150,000 mol. wt. into a lateral ventricle of the cat at 20 microliters min-1, radioactivity and fluorescence reached plateaus in deep cervical lymph at 47.4 and 50.0% of their concentrations in cisternal c.s.f. respectively. 4. No significant radioactivity, other than from blood, was detected in superficial cervical lymph after intraventricular injection of radio-iodinated albumin in the cat. 5. No significant radioactivity, other than from blood, was detected in deep cervical lymph of the rabbit or in deep and superficial cervical lymph of the cat within 6 hr after injection of radio-iodinated albumin into the aqueous humour or orbital fat. 6. Gradients of radioactivity in tissues within the orbit suggested that there is a small flow of c.s.f., 0.05-0.15 microliters min-1 in the rabbit, passing centrifugally along the subarachnoid space of the optic nerve, through the posterior part of the globe and into the orbital tissue. Also a small proportion of aqueous humour, 1-2% or more, drains through the anterior sclera into the surrounding tissue. 7. A substantial quantity of cerebrospinal fluid drains into the deep cervical lymphatic system of the rabbit, 30% or more, and of the cat, 10-15% or more. The small component of aqueous humour drainage passing through the wall of the glove does not enter cervical lymph within 6 hr, if at all.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975 Jul;55(3):383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- Bill A. The routes for bulk drainage of aqueous humour in rabbits with and without cyclodialysis. Doc Ophthalmol. 1966;20:157–169. doi: 10.1007/BF00165414. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Lathem W. A flow of cerebrospinal fluid along the central canal of the spinal cord of the rabbit and communications between this canal and the sacral subarachnoid space. J Physiol. 1965 Dec;181(4):785–800. doi: 10.1113/jphysiol.1965.sp007797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W. Proportion of cerebrospinal fluid draining into jugular lymphatic trunks of the cat [proceedings]. J Physiol. 1978 Mar;276:67P–68P. [PubMed] [Google Scholar]
- Brierley J. B., Field E. J. The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat. 1948 Jul;82(Pt 3):153–166. [PMC free article] [PubMed] [Google Scholar]
- COURTICE F. C., SIMMONDS W. J. The removal of protein from the subarachnoid space. Aust J Exp Biol Med Sci. 1951 Jul;29(4):255–263. doi: 10.1038/icb.1951.30. [DOI] [PubMed] [Google Scholar]
- Cole D. F., Monro P. A. The use of fluorescein-labelled dextrans in investigation of aqueous humour outflow in the rabbit. Exp Eye Res. 1976 Dec;23(6):571–585. doi: 10.1016/0014-4835(76)90215-3. [DOI] [PubMed] [Google Scholar]
- Davson H., Hollingsworth G., Segal M. B. The mechanism of drainage of the cerebrospinal fluid. Brain. 1970;93(4):665–678. doi: 10.1093/brain/93.4.665. [DOI] [PubMed] [Google Scholar]
- FOWLKS W. L. Meridional flow from the corona ciliaris through the pararetinal zone of the rabbit vitreous. Invest Ophthalmol. 1963 Feb;2:63–71. [PubMed] [Google Scholar]
- Grüntzig J., Schicha H., Kiem J., Becker V., Feinendegen L. E. Studien zur Lymphdrainage des Auges. 1. Quantitative Erfassung des Lymphtransportes aus der Orbita des Kaninchens mit radioaktiven Tracern. Klin Monbl Augenheilkd. 1977 May;170(5):713–717. [PubMed] [Google Scholar]
- HAYREH S. S. PATHOGENESIS OF OEDEMA OF THE OPTIC DISC (PAPILLOEDEMA). A PRELIMINARY REPORT. Br J Ophthalmol. 1964 Oct;48:522–543. doi: 10.1136/bjo.48.10.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb J. G., Davson H., Hollingsworth J. R. Attempted separation of blood-brain and blood-cerebrospinal fluid barriers in the rabbit. Exp Eye Res. 1977;25 (Suppl):333–343. doi: 10.1016/s0014-4835(77)80030-4. [DOI] [PubMed] [Google Scholar]
- PATEK P. R., BERNICK S. Extravascular pathways of the eye and orbit. Am J Ophthalmol. 1960 Jan;49:135–141. doi: 10.1016/0002-9394(60)92671-4. [DOI] [PubMed] [Google Scholar]
- Quin J. W., Shannon A. D. The influence of the lymph node on the protein concentration of efferent lymph leaving the node. J Physiol. 1977 Jan;264(2):307–321. doi: 10.1113/jphysiol.1977.sp011670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEET W. H., LOCKSLEY H. B. Formation, flow, and reabsorption of cerebrospinal fluid in man. Proc Soc Exp Biol Med. 1953 Nov;84(2):397–402. doi: 10.3181/00379727-84-20659. [DOI] [PubMed] [Google Scholar]
- VAN WART C. A., DUPONT J. R., KRAINTZ L. Effect of acetazolamide on passage of protein from cerebrospinal fluid to plasma. Proc Soc Exp Biol Med. 1961 Jan;106:113–114. doi: 10.3181/00379727-106-26254. [DOI] [PubMed] [Google Scholar]


