Abstract
We previously demonstrated that butyric acid, an extracellular metabolite from periodontopathic bacteria, induces cytotoxicity and apoptosis in murine thymocytes, splenic T cells, and human Jurkat T cells. In this study, we used a cell-to-cell interaction system to examine the contribution of gingival fibroblasts to the regulation of T-cell death induced by butyric acid. Butyric acid slightly suppressed fibroblast viability in a concentration-dependent fashion. However, DNA fragmentation assays indicated that butyric acid did not induce apoptosis for up to 21 h in human gingival fibroblasts (Gin 1, F41-G, and H. pulp cells). The culture supernatants were assayed for interleukin 1α (IL-1α), IL-1β, IL-6, IL-8, IL-11, tumor necrosis factor alpha, and transforming growth factor β, but only the IL-6, IL-8, and IL-11 levels were significantly increased by addition of butyric acid. Butyric acid- or Fas-induced Jurkat-cell apoptosis was attenuated when Jurkat cells were cocultured with either F41-G or Gin 1 cells that had been preincubated for 6 h with butyric acid. IL-8 slightly stimulated butyric acid- or Fas-induced Jurkat-cell apoptosis in a dose-dependent manner, although a low dose of IL-8 had a mildly inhibitory effect on apoptosis. In contrast, IL-6 and IL-11 significantly suppressed butyric acid- or Fas-induced apoptosis in a dose-dependent fashion. Furthermore, the addition of monoclonal antibodies against human IL-6 and IL-11 to cocultures of gingival fibroblasts and Jurkat cells partially eliminated T-cell recovery. These results suggest that the proinflammatory cytokines such as IL-6 and IL-11, produced in fibroblasts stimulated with butyric acid, are involved in the attenuation of T-cell apoptosis by gingival fibroblasts.
Adult periodontitis is a chronic destructive disease involving host inflammatory responses to gram-negative bacteria. The irreversible tissue destruction associated with the transition from gingivitis to periodontitis is reflected in the intensity and extent of inflammation, the types of immune cells, and the soluble mediators of cell communication and inflammation. A recent study indicated that severe destructive adult periodontitis was caused by a mixed infection and that combinations of certain periodontopathogens, for example, Porphyromonas, Prevotella, and Fusobacterium spp., were important in pathogenesis (30). These bacteria produce an elaborate variety of virulence factors, such as proteases, lipopolysaccharides (LPS), fimbriae, and short-chain fatty acids. One of the short-chain fatty acids, butyric acid, suppresses the in vitro proliferation of a variety of cancer cell lines (8, 15, 36). Our previous study (11) demonstrated that short-chain fatty acids, especially the volatile fatty acids present in the culture filtrates of Porphyromonas gingivalis, Prevotella loescheii, and Fusobacterium nucleatum, markedly inhibited murine T- and B-cell proliferation and cytokine production. Furthermore, we found that butyric acid induced cytotoxicity and apoptosis in murine and human T and B cells (12-14). Emerging evidence indicates that bacterial modulation of apoptosis is an important part of pathogenesis (5). Specific pathogens or their extracellular products may directly induce the apoptosis of host cells (39).
Cell-to-cell interactions also play an important role in pathological conditions in cases where cells that are normally located in different compartments come in close proximity to each other. In inflamed periodontal lesions, dense lymphocytic infiltrations are usually observed in the extravascular periodontal connective tissue, adjacent to gingival fibroblasts. Previous studies revealed that activated lymphocytes can adhere to gingival fibroblasts in vitro (20, 21), and that direct interactions between gingival fibroblasts and lymphoid cells induce the expression of mRNA for proinflammatory cytokines in gingival fibroblasts (22). Other reports indicate that various bacterial products—such as LPS, fimbriae, and proteases (37); interleukin 17 (IL-17) produced by T lymphocytes (18); and IL-18 in synovial fluids (19)—directly affect cytokine production by gingival fibroblasts. Thus, gingival fibroblasts require stimulation by bacterial products, such as LPS and fimbriae; by cytokines from other cells; or by cell-to-cell contact with activated T cells in order to be activated to produce proinflammatory cytokines. It seems that fibroblasts actively participate in immune reactions by producing several immunoregulatory cytokines, which then act on other cell types (2, 31, 33). Indeed, gingival fibroblasts have the ability to produce cytokines, such as IL-1, IL-6, and IL-8. However, little is known about how butyric acid stimulates cytokine production in gingival fibroblasts and how gingival fibroblasts influence butyric acid-induced T-cell apoptosis.
The aims of the present study were to examine the effects of cell-to-cell interactions on butyric acid- or Fas-induced T-cell apoptosis and to elucidate the role of gingival-fibroblast-derived signals in modulating butyric acid- or Fas-induced T-cell apoptosis. We found that interactions between butyric acid-pretreated gingival fibroblasts and T cells prevented butyric acid- or Fas-induced T-cell apoptosis. Furthermore, we found evidence that the increased levels of IL-6 and IL-11 in the supernatants of fibroblasts stimulated with butyric acid influenced the rescue of T cells from apoptosis.
MATERIALS AND METHODS
Reagents.
Highly purified butyric acid was purchased from Sigma (St. Louis, Mo.). Solutions of butyric acid, ranging in concentration from 0.15 to 5 mM, were diluted in Dulbecco's modified Eagle medium (DMEM) (Gibco Laboratories, Grand Island, N.Y.) and adjusted to pH 7.2 with sodium hydroxide. Recombinant human IL-6, IL-8, and IL-11 were purchased from PeproTech (London, England). Neutralizing anti-human IL-6, IL-8, and IL-11 monoclonal antibodies (MAbs) were from R & D Systems Inc. (Minneapolis, Minn.).
Cells.
Human gingival tissue was obtained from periodontally healthy volunteers. Informed consent was obtained from each volunteer prior to tissue resection. Gingival fibroblasts (F41-G) and fibroblasts from tooth pulp (H. pulp) were cultured in a complete medium consisting of 25 mM HEPES-buffered DMEM supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM l-glutamine and used in experiments after 5 to 15 passages. The human gingival fibroblast cell line Gin 1 was obtained from the American Type Culture Collection (Manassas, Va.). The human gingival carcinoma cell line Ca 9-22 was obtained from the Japan Cancer Research Resources Bank. These cell lines were maintained and expanded in complete medium. The human T-lymphoma Jurkat-cell line was kindly provided by Fujisaki Cell Center Hayashibara (Okayama, Japan). The cells were cultured at 37°C in a moist atmosphere of 5% CO2 in a complete medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 0.05 mM 2-mercaptoethanol.
Cell proliferation assay.
Gin 1, F41-G, H. pulp, and Ca 9-22 cells were seeded at a density of 1.0 × 104 cells per well in 0.1 ml of complete medium in flat-bottomed 96-well plates. Butyric acid, dissolved in DMEM, was added at final concentrations ranging between 0.15 and 5 mM; each concentration of butyric acid was tested in quadruplicate. After incubation for 42 h, 20 μl of 5-mg/ml MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide in phosphate-buffered saline (pH 7.2); Sigma] was added to each well. After a further 6 h of incubation, the supernatants were decanted, and the formazan precipitates were solubilized by the addition of 150 μl of 100% dimethyl sulfoxide (Sigma) and placed on a plate shaker for 10 min. The absorbance at 550 nm was determined on an MT32 spectrophotometric microreader (Corona Electric Co., Ibaraki, Japan). The absorbance of the untreated cultures was set at 100%. The mean relative absorbance and the standard error of the mean (SEM) were calculated for each concentration of butyric acid tested.
Cell culture for apoptosis assay.
Gin 1, F41-G, H. pulp, and Ca 9-22 cells (5 × 105 per well) were cultured in 1 ml of complete medium in 24-well tissue-culture plates (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.), along with various concentrations of butyric acid. At the times indicated in the figures, the cells were harvested, centrifuged at 400 × g for 5 min, and washed twice with ice-cold phosphate-buffered saline. The cells were resuspended in 400 μl of hypotonic lysis buffer (0.2% Triton X-100, 10 mM Tris, 1 mM EDTA [pH 8.0]) and centrifuged at 13,800 × g for 15 min (23). The supernatant, which contained small DNA fragments, was divided into two aliquots. One aliquot and the pellet that contained large pieces of DNA and cell debris were used for the diphenylamine (DPA) assay (see below).
Confluent monolayers of F41-G and Gin 1 cells were used between passages 5 and 15. The fibroblasts were removed from their plastic support by exposure to trypsin-EDTA for 5 min and preincubated for 6 h at a density of 2.5 × 105 per well with 5 mM butyric acid. The cells were washed and reseeded in 0.4 ml of complete medium at a density of 105 fibroblasts per well in cell culture inserts (Falcon) in 24-well culture plates with Jurkat T cells (106 per well). The fibroblast-T-cell mixture was preincubated for 1 h and then treated with either 5 mM butyric acid or 10 ng of cytotoxic anti-Fas MAb (CH-11) per ml for 21 h. Harvested T cells were subjected to the DPA assay.
DNA fragmentation assay.
The DPA reaction was performed according to the method of Paradones et al. (26). Perchloric acid (0.5 M) was added to the pellets containing uncut DNA (resuspended in 200 μl of hypotonic lysis buffer) and to the other half of the supernatant containing DNA fragments. Then 2 volumes of a solution containing 0.088 M DPA, 98% (vol/vol) glacial acetic acid, 1.5% (vol/vol) sulfuric acid, and a 0.5% (vol/vol) concentration of 1.6% acetaldehyde solution was added. The samples were stored at 4°C for 48 h. The colorimetric reaction was quantified spectrophotometrically at 575 nm with a model UV-160A UV spectrophotometer (Shimazu Co. Ltd., Tokyo, Japan). The percent fragmentation was calculated as the ratio of DNA in the supernatants to the total DNA.
Cytokine production.
Gin 1, F41-G, and H. pulp cells (2.5 × 105/well) were cultured with 0.15 to 5 mM butyric acid in 24-well tissue culture plates. After a 24-h incubation at 37°C, culture supernatants were centrifuged at 3,000 × g for 5 min, filtered through a 0.22-μm-pore-size filter, and stored at −20°C until assayed for cytokines as described below.
Cytokine assay.
Human IL-1α, IL-1β, IL-6, IL-8, IL-11, tumor necrosis factor alpha (TNF-α), and transforming growth factor β (TGF-β) were measured using enzyme-linked immunosorbent assay kits (Quantikine; R&D Systems) according to the manufacturer's instructions. Cytokine concentrations, from triplicate assays, were expressed in picograms per milliliter.
Statistics.
Multiple-group comparisons were made with a one-way analysis of variance, followed by post hoc intergroup comparisons using the Bonferroni-Dunn test. Where appropriate, Student's t test was used to assess the statistical significance of differences between two groups.
RESULTS
Effect of butyric acid on cell proliferation and apoptosis.
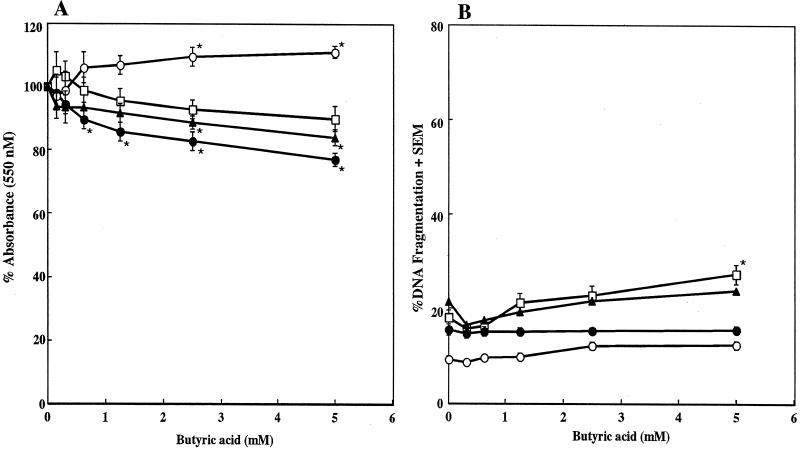
We examined the effects of various concentrations of butyric acid on the proliferation of human fibroblast Gin 1, F41-G, and H. pulp cells and human carcinoma Ca 9-22 cells. The proliferation rates, assessed by MTT assay, of Gin 1, F41-G, and Ca 9-22 cells in response to 5 mM butyric acid were reduced by 10.0, 23.0, and 16.0%, respectively (Fig. 1A). Although butyric acid exhibited a dose-dependent inhibition of F41-G and Ca 9-22 cell proliferation, this effect was minor compared to our previous results with T and B cells (12, 13). Furthermore, when the fibroblasts and carcinoma cells were cultured in the presence of 0.31 to 5.0 mM butyric acid for 21 h, no dose-dependent increase in DNA fragmentation was seen, except in the case of Gin 1 cells (Fig. 1B). Although 5 mM butyric acid augmented DNA fragmentation in Gin 1 cells by 9.1% compared to nontreated cells, this increase was not statistically significant. These results indicate that human fibroblast proliferation and apoptosis were scarcely affected by the butyric acid treatment.
FIG. 1.
Effect of butyric acid on cell proliferation and apoptosis. Gin 1 (□), F41-G (•), H. pulp (○), and Ca 9-22 (▴) cells were cultured with the indicated concentrations of butyric acid for 48 h (A) or 21 h (B). (A) Cellular proliferation was determined by an MTT assay and is expressed as the percentage of the control absorbance, obtained in the absence of butyric acid. (B) The DNA fragmentation assay was performed by DPA assay of harvested cells. The results are expressed as the means ± SEMs (error bars) of three different experiments with triplicate cultures. Values that were significantly different from those of the corresponding negative controls at P < 0.05 are indicated by asterisks.
Effect of butyric acid on cytokine production.
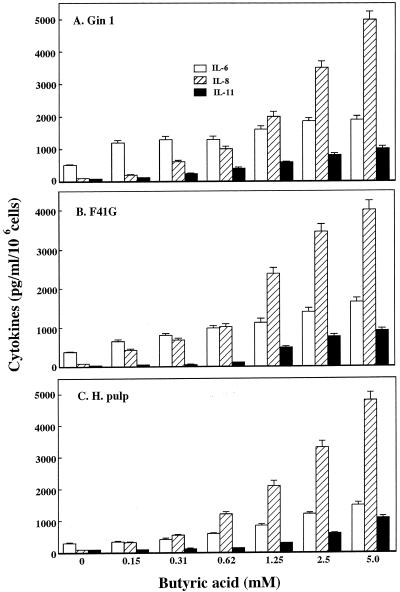
It has been previously shown that various bacterial products, such as LPS, fimbriae, and proteases, affect cytokine production from gingival fibroblasts (37). Therefore, we examined the effects of butyric acid on the production of IL-1α, IL-1β, IL-6, IL-8, IL-11, TNF-α, and TGF-β in Gin 1, F41-G, and H. pulp cells. The IL-1α, IL-1β, TNF-α, and TGF-β levels in fibroblast culture supernatants were unchanged 24 h after the addition of 5 mM butyric acid, although the addition of LPS to cultured fibroblasts significantly induced the production of these cytokines (data not shown). In addition, butyric acid induced dose-dependent increases in IL-6, IL-8, and IL-11 in the fibroblast culture supernatants (Fig. 2). Maximal cytokine production levels were noted 24 h after the addition of 5 mM butyric acid to Gin 1, F41-G, and H. pulp cells. The observed levels corresponded to 1,500 to 1,900 pg/ml (IL-6), 4,000 to 5,000 pg/ml (IL-8), and 900 to 1,100 pg/ml (IL-11) (Fig. 2). The increases in cytokine levels compared to unstimulated fibroblast cultures were 3.8- to 4.9-fold (IL-6), 50.0- to 58.8-fold (IL-8), and 11.3- to 28.8-fold (IL-11). These results indicate that butyric acid significantly induced proinflammatory cytokine production in gingival fibroblasts.
FIG. 2.
Effect of butyric acid on cytokine production by gingival and tooth pulp fibroblasts. Gin 1 (A), F41-G (B), and H. pulp (C) cells were cultured with the indicated concentrations of butyric acid for 24 h. Cytokine levels in the culture supernatants were measured by enzyme-linked immunosorbent assay. The results are expressed as the means + SEMs (error bars) of three different experiments with triplicate cultures.
Fibroblasts rescue T cells from apoptosis.
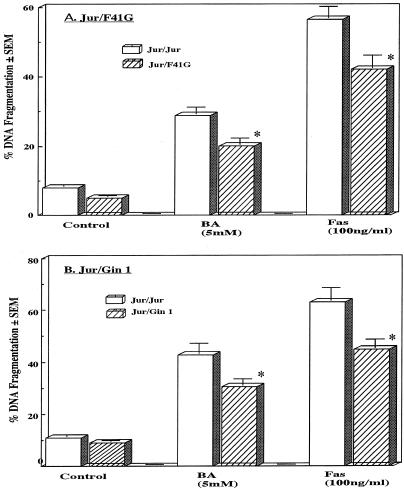
In order to examine how the interactions between T cells and fibroblasts affected T-cell apoptosis, Jurkat T cells were cultured either together with other Jurkat cells as positive controls or in combination with fibroblasts (F41-G and Gin 1) that had been presensitized with 5 mM butyric acid. The T-cell cultures were grown in the presence or absence of 5 mM butyric acid or 10-ng/ml anti-Fas MAb (CH-11) for 24 h. Coculturing Jurkat cells with F41-G or Gin 1 cells in pore-filled culture inserts (intercups) partially rescued butyric acid- or Fas-induced Jurkat-cell apoptosis (Fig. 3). DNA fragmentation in Jurkat cells decreased by 30.4 or 28.5% when these cells were cocultured in 5 mM butyric acid with F41-G or Gin 1 cells, respectively. Furthermore, even in the presence of anti-Fas (CH-11) MAb, DNA fragmentation in Jurkat cells decreased by 25.3 or 28.9% when the cells were cocultured with F41-G or Gin 1 cells, respectively. Since similar results were obtained when peripheral blood mononuclear-T cells were cocultured with these fibroblasts, our results suggest that butyric acid induces the release of a soluble factor from fibroblasts that inhibits T-cell apoptosis.
FIG. 3.
Effect of fibroblast coculture on butyric acid- or Fas-induced T-cell apoptosis. Jurkat cells were indirectly cocultured with F41-G (A) or Gin 1 (B) cells that had been presensitized with 5 mM butyric acid, using pore-filled cell culture inserts (intercup) for 1 h, and then cultured with 5 mM butyric acid or 10-ng/ml cytotoxic anti-Fas MAb (CH-11) for 21 h. Harvested cells were subjected to the DPA assay. The data represent the means ± SEMs (error bars) of three different experiments with triplicate cultures. Values that were significantly different from corresponding fibroblast-free butyric acid values at P < 0.05 are indicated by asterisks.
IL-6 and IL-11 rescue T cells from apoptosis.
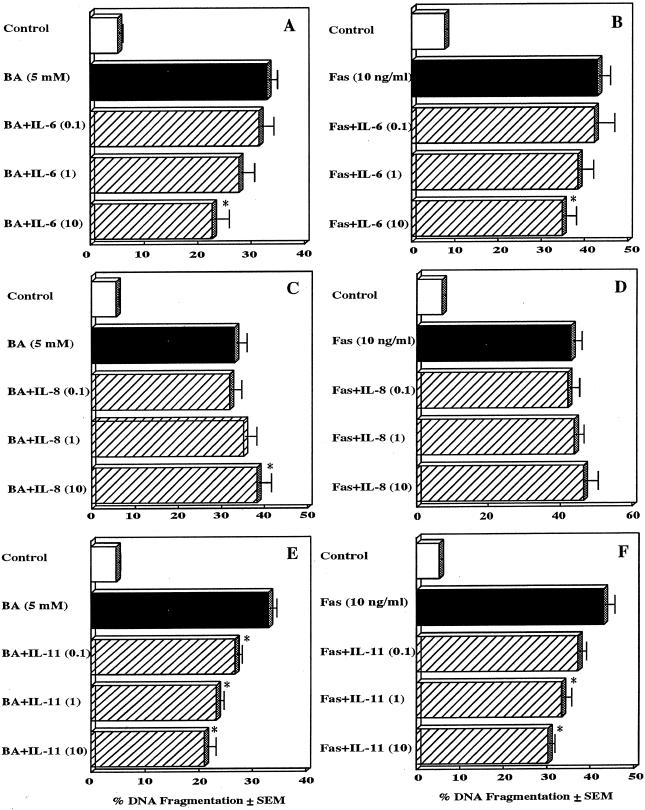
To determine the nature of the putative soluble factor that was released from butyric acid-stimulated fibroblasts and blocked T-cell apoptosis, we examined whether cytokines produced by butyric acid-stimulated gingival fibroblasts inhibited butyric acid-induced T-cell apoptosis. Since butyric acid significantly increased IL-6, IL-8, and IL-11 levels in the supernatants of human gingival fibroblasts (Fig. 2), the effects of these cytokines on butyric acid- or Fas-induced Jurkat-cell apoptosis were examined (Fig. 4). The addition of IL-8 slightly increased butyric acid-induced or Fas-induced Jurkat-cell apoptosis in a dose-dependent fashion. Although a low dose (0.1 ng/ml) of IL-8 slightly inhibited this apoptosis, the differences were not statistically significant. In contrast, IL-6 and IL-11 inhibited butyric acid- or Fas-induced Jurkat-cell apoptosis in a dose-dependent manner (P < 0.01). Maximal inhibition, i.e., 31.2% for IL-6 and 36.4% for IL-11 in the case of butyric acid induction and 20.1% for IL-6 and 30.2% for IL-11 in the case of Fas induction, was noted at the 10-μg/ml concentration of either IL-6 or IL-11. The addition of IL-6, IL-8 or IL-11 alone had no effect on the levels of DNA fragmentation in Jurkat cells. The addition of IL-1, which was not increased in gingival fibroblasts by butyric acid, did not show any effect on butyric acid- or Fas-induced Jurkat-cell apoptosis (data not shown).
FIG. 4.
Effects of proinflammatory cytokines on butyric acid-induced T-cell apoptosis. Jurkat cells were preincubated with the indicated concentrations of IL-6 (A and B), IL-8 (C and D), or IL-11 (E and F) for 1 h and were then incubated with 5 mM butyric acid (BA) (A, C, and E) or 10 ng of cytotoxic anti-Fas MAb (Fas) (B, D, and F) for 21 h. Harvested cells were analyzed using the DPA assay. The results are expressed as the means + SEMs (error bars) of three different experiments with triplicate cultures. Values significantly different from the corresponding cytokine-free butyric acid or anti-Fas antibody values at P < 0.05 are indicated by asterisks.
We performed additional experiments, in which butyric acid-treated T cells were cocultured with gingival fibroblasts together with MAbs for each of the cytokines (i.e., IL-6, IL-8, and IL-11) that were increased in butyric acid-treated fibroblasts. The addition of anti-IL-6 or anti-IL-11 MAbs in cocultures of gingival fibroblasts and Jurkat cells partially eliminated T-cell recovery (Table 1).
TABLE 1.
Monoclonal antibodies against human IL-6 and IL-11 eliminate T-cell recovery by coculture of gingival fibroblasts and Jurkat cellsa
| Coculture | Treatment | % DNA fragmentation (meanb ± SD) |
|---|---|---|
| Jur + Jur | BA | 28.9 ± 1.9 |
| Jur + F41-G | BA | 20.1 ± 1.8 |
| Jur + F41-G | BA + anti-IL-6 | 27.6 ± 1.2c |
| Jur + F41-G | BA + anti-IL-11 | 27.2 ± 2.0c |
| Jur + F41-G | BA + anti-IL-8 | 19.4 ± 2.1 |
| Jur + F41-G | BA + anti-IL-6, -IL-11, -IL-8 | 29.3 ± 1.9c |
Jurkat cells were indirectly cocultured with F41-G cells that had been presensitized with 5 mM butyric acid (BA), using pore-filled cell culture insert (intercup), for 1 h in the absence or presence of neutralizing MAbs (10 μg/ml) against IL-6, IL-8, and IL-11 and then cultured with 5 mM butyric acid for 21 h. Harvested cells were assayed by the DPA assay.
Mean of three experiments carried out in triplicate.
Significantly different from the corresponding MAb-free butyric acid values at a P of <0.05.
DISCUSSION
Human chronic periodontitis is usually characterized by dense inflammatory cell infiltrations in the periodontal connective tissues (16, 24, 28). T lymphocytes are known to play an important role in modulating local immune responses at periodontal lesions (10, 24). Since most T cells that penetrate periodontal lesions are adjacent to gingival fibroblasts, it is worth considering how these two cell types interact. Therefore, we assessed the role of gingival fibroblast in the regulation of butyric acid-induced T-cell death using a coculturing system (intercup method). On the basis of our previous results showing that 13.3 to 26.8 mM butyric acid was detected in culture filtrates from P. gingivalis, P. loescheii, and F. nucleatum (11), along with a previous study showing that butyric acid concentrations in subgingival plaque from a periodontitis site could reach 14.4 to 20 mM [17; C. Naleway, H. Chou, T. Manos, C. Goodman, P. Robinson, and R. Singer, abstract from the International Association for Dental Research 1989, J. Dent. Res. 68(Suppl.):121, 1989] and that its concentration in periodontal pockets has been shown to correlate with the severity of periodontal disease (4), butyric acid can be recognized as an important virulence factor of these periodontopathogens. Therefore, in this study, cells were cultured with 5 mM butyric acid, which induced a maximal increase in DNA fragmentation after 21 h of culture (12-14). In this study, butyric acid slightly suppressed the viability of gingival fibroblasts in a dose-dependent manner. However, butyric acid-induced apoptosis in fibroblasts was negligible compared to our previous data, which documented the effects of butyric acid on T and B cells (12, 13). The results obtained with gingival fibroblasts were similar to those seen when human epithelial KB cells or HeLa cells were used (data not shown). From this we conclude that hematopoietic and nonhematopoietic cells differ in their sensitivities to butyric acid.
Butyric acid increased the production levels of proinflammatory cytokines, such as IL-6, IL-8, and IL-11, in gingival fibroblasts in a dose-dependent manner. Several virulence factors produced by periodontopathic bacteria, such as LPS (31), fimbriae (7), bacterial DNA (32), and outer membrane proteins (9), stimulate the production of proinflammatory cytokines. Furthermore, many studies have suggested that inflammatory cytokines triggered by bacterial infection play central roles in the pathological processes taking place in diseased periodontal tissues. IL-6 is a bone resorptive cytokine that is produced by many cell types, including macrophages, T cells, and fibroblasts (38). IL-8 is strongly chemotactic for polymorphonuclear leukocytes and T cells (3) and has been implicated in fibrotic diseases of the lung and kidney (29). Proinflammatory cytokines, such as IL-6 and IL-8, are believed to be the major pathological mediators in periodontal disease. Therefore, butyric acid, by inducing IL-6 and IL-8 production, may also be involved in the resorption of alveolar bone and gingival inflammation that leads to periodontal destruction.
We also examined how butyric acid-stimulated gingival fibroblasts, with their increased production of proinflammatory cytokines, could affect butyric acid- or Fas-induced T-cell apoptosis. Coculturing Jurkat cells with presensitized F41-G or Gin 1 cells in pore-filled culture inserts (intercups) attenuated butyric acid- or Fas-induced Jurkat-cell apoptosis. Jurkat cells cultured with other Jurkat cells as positive controls were susceptible to butyric acid- or Fas-induced apoptosis at levels similar to those observed with intercup-free Jurkat cells (data not shown), which suggested that a butyric acid-induced soluble factor from fibroblasts might affect butyric acid- or Fas-induced Jurkat-cell apoptosis. Therefore, we examined the effect on Jurkat-cell apoptosis of those proinflammatory cytokines (IL-6, IL-8, and IL-11) whose production was significantly increased by butyric acid stimulation of gingival fibroblasts. IL-8 slightly stimulated, and IL-6 and IL-11 significantly suppressed, butyric acid- or Fas-induced Jurkat-cell apoptosis in dose-dependent manners. It has been shown that IL-6, IL-8, and IL-11 affected mammalian cell apoptosis. For instance, IL-6 rescued resting murine T cells from apoptosis (34) and activation-induced cell death (1). IL-11 also prevented apoptosis in UVB-irradiated mouse skin (27) and small intestinal mucosa cells (25). In contrast, IL-8 increased apoptosis in activated endothelial cells (35) and inhibited apoptosis in polymorphonuclear neutrophils (6). Furthermore, the addition of anti-IL-6 or anti-IL-11 MAbs to cocultures of gingival fibroblasts and Jurkat cells partially eliminated T-cell recovery. Therefore, our results suggest that the attenuation of butyric acid- or Fas-induced T-cell apoptosis by gingival fibroblasts resulted from the effects of proinflammatory cytokines, such as IL-6 and IL-11, which were produced in fibroblasts stimulated with butyric acid.
In conclusion, our data indicate that while the proinflammatory cytokines produced by gingival fibroblasts in response to stimulation with butyric acid contribute to the pathology of periodontal disease, they eliminate butyric acid- or Fas-induced T-cell apoptosis.
Acknowledgments
This work was supported by a Frontier Science Research grant; by a scientific research grant-in-aid (20130594) from the Ministry of Education, Science, and Culture of Japan; and by a Suzuki Memorial Grant (01-1003) from the Nihon University School of Dentistry at Matsudo.
Editor: J. D. Clements
REFERENCES
- 1.Ayroldi, E., O. Zollo, L. Cannarile, F. D'Adamio, U. Grohmann, D. V. Delfino, and C. Riccardi. 1998. Interleukin-6 (IL-6) prevents activation-induced cell death: IL-2-independent inhibition of Fas/fasL expression and cell death. Blood 92:4212-4219. [PubMed] [Google Scholar]
- 2.Bartold, P., and D. Haynes. 1991. Interleukin-6 production by human gingival fibroblasts. J. Periodont. Res. 26:339-345. [DOI] [PubMed] [Google Scholar]
- 3.Bickel, M. 1993. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodont. 64:456-460. [PubMed] [Google Scholar]
- 4.Botta, G. A., L. Radin, A. Costa, G. Schito, and G. Blasi. 1985. Gas-liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J. Periodont. Res. 20:450-457. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., and A. Zychlinsky. 1994. Apoptosis induced by bacterial pathogens. Microb. Pathog. 17:203-212. [DOI] [PubMed] [Google Scholar]
- 6.Dunican, A. L., S. J. Leuenroth, P. Grutkoski, A. Ayala, and H. H. Simms. 2000. TNFalpha-induced suppression of PMN apoptosis is mediated through interleukin-8 production. Shock 14:284-288. [DOI] [PubMed] [Google Scholar]
- 7.Hanazawa, S., Y. Murakami, K. Hirose, S. Amano, and S. Kitano. 1991. Porphyromonas (Bacteroides) gingivalis fimbriae activate mouse periodontal macrophages and induce gene expression and production of interleukin-1. Infect. Immun. 59:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodin, R. A., S. Meng, S. Archer, and R. Tang. 1996. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ. 7:647-653. [PubMed] [Google Scholar]
- 9.Imatani, T., T. Kato, and K. Okuda. 2001. Production of inflammatory cytokines by human gingival fibroblasts stimulated by cell-surface preparations of Porphyromonas gingivalis. Oral Microbiol. Immunol. 16:65-72. [DOI] [PubMed] [Google Scholar]
- 10.Ito, H., Y. Harada, T. Matsuo, S. Ebisu, and H. Okada. 1988. Possible role of T cells in the establishment of Ig G plasma cell-rich periodontal lesion. Augmentation of Ig G synthesis in the polyclonal B cell activation response by autoreactive T cells. J. Periodont. Res. 23:39-45. [DOI] [PubMed] [Google Scholar]
- 11.Kurita-Ochiai, T., K. Fukushima, and K. Ochiai. 1995. Volatile fatty acids, metabolic by-products of periodontopathic bacteria, inhibit lymphocyte proliferation and cytokine production. J. Dent. Res. 74:1367-1373. [DOI] [PubMed] [Google Scholar]
- 12.Kurita-Ochiai, T., K. Fukushima, and K. Ochiai. 1997. Butyric acid-induced apoptosis of murine thymocytes, splenic T cells, and human Jurkat T cells. Infect. Immun. 65:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurita-Ochiai, T., K. Ochiai, and K. Fukushima. 1998. Volatile fatty acid, a metabolic by-product of periodontopathic bacteria, induces apoptosis in WEHI231 and RAJI B lymphoma cells and splenic B cells. Infect. Immun. 66:2587-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurita-Ochiai, T., K. Fukushima, and K. Ochiai. 1999. Lipopolysaccharide stimulates butyric acid-induced apoptosis in human peripheral blood mononuclear cells. Infect. Immun. 67:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landon, S. P., M. M. Hawkes, F. G. Hay, S. S. Lawrie, D. J. Schol, J. Hilgers, R. C. F. Leonard, and J. F. Smith. 1998. Effect of sodium butyrate and other differentiation inducers on poorly differentiated human ovarian adenocarcinoma cell lines. Cancer Res. 48:6161-6165. [PubMed] [Google Scholar]
- 16.Mackler, B. F., K. B. Frostad, P. B. Robertson, and B. M. Levy. 1977. Immunoglobulin-bearing lymphocytes and plasma cells in human periodontal disease. J. Periodont. Res. 12:37-45. [DOI] [PubMed] [Google Scholar]
- 17.Margolis, H. C., J. H. Duckworth, and E. C. Moreno. 1988. Composition and buffer capacity of pooled starved plaque fluid from caries-free and caries-susceptible individuals. J. Dent. Res. 67:1476-1482. [DOI] [PubMed] [Google Scholar]
- 18.Molet, S., Q. Hamid, F. Davoine, E. Nutku, R. Taha, N. Page, R. Olivenstein, J. Elias, and J. Chakir. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108:430-438. [DOI] [PubMed] [Google Scholar]
- 19.Morel, J. C., C. C. Park, P. Kumar, and A. E. Koch. 2001. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NF kappa B activation. Lab. Investig. 81:1371-1383. [DOI] [PubMed] [Google Scholar]
- 20.Murakami, S., T. Sano, Y. Shimabukuro, R. Isoda, Y. Miki, and H. Okada. 1993a. Very late antigen integrins are involved in the adhesive interaction of lymphoid cells to human gingival fibroblasts. Immunology 79:425-433. [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami, S., T. Saho, A. Asari, E. Hino, D. Kasai, Y. Shimabukuro, and H. Okada. 1996. CD44-hyaluronate interaction participates in the adherence of T lymphocytes to gingival fibroblasts. J. Dent. Res. 75:1545-1552. [DOI] [PubMed] [Google Scholar]
- 22.Murakami, S., E. Hino, Y. Shimabukuro, T. Nozaki, Y. Kusumoto, T. Saho, F. Hirano, H. Hirano, and H. Okada. 1999. Direct interaction between gingival fibroblasts and lymphoid cells induces inflammatory cytokine mRNA expression in gingival fibroblasts. J. Dent. Res. 78:69-76. [DOI] [PubMed] [Google Scholar]
- 23.Newell, M. K., L. J. Haughn, C. R. Maroun, and M. H. Julius. 1990. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature 347:286-289. [DOI] [PubMed] [Google Scholar]
- 24.Okada, H., T. Kida, and H. Yamagami. 1983. Identification and distribution of immunocompetent cells inflamed gingiva of human chronic periodontitis. Infect. Immun. 41:365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orazi, A., X. X. Du, Z. X. Yang, M. Kashai, and D. A. Williams. 1996. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab. Investig. 75:33-42. [PubMed] [Google Scholar]
- 26.Paradones, C. E., V. A. Illera, D. Peckham, L. L. Stunz, and R. F. Ashman. 1993. Regulation of apoptosis in-vitro in mature spleen T cells. J. Immunol. 151:3521-3529. [PubMed] [Google Scholar]
- 27.Scordi, I. A., M. Nassiri, A. J. Hanly, and V. Vincek. 1999. Interleukin-11 rescues apoptosis in UVB-irradiated mouse skin. Dermatology 199:296-301. [DOI] [PubMed] [Google Scholar]
- 28.Seymour, G. J., and J. S. Greenspan. 1979. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J. Periodont. Res. 14:39-46. [DOI] [PubMed] [Google Scholar]
- 29.Silver, R. M. 1995. Intestinal lung disease of systemic sclerosis. Int. Rev. Immunol. 12:281-291. [DOI] [PubMed] [Google Scholar]
- 30.Soder, P. O., L. J. Jin, and B. Soder. 1993. DNA probe detection of periodontopathogens in advanced periodontitis. Scand. J. Dent. Res. 101:363-370. [DOI] [PubMed] [Google Scholar]
- 31.Takada, H., J. Mihara, I. Morisaki, and S. Hamada. 1991. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect. Immun. 59:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeshita, A., K. Imai, and S. Hanazawa. 1999. CpG motifs in Porphyromonas gingivalis DNA stimulate interleukin-6 expression in human gingival fibroblast. Infect. Immun. 67:4340-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura, M., M. Tokuda, S. Nagaoka, and H. Takada. 1992. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect. Immun. 60:4932-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teague, T. K., P. Marrack, J. W. Kappler, and A. T. Vella. 1997. IL-6 rescues resting mouse T cells from apoptosis. J. Immunol. 158:5791-5796. [PubMed] [Google Scholar]
- 35.Terui, Y., M. Ikeda, H. Tomizuka, T. Kasahara, T. Ohtsuki, M. Uwai, M. Mori, T. Itoh, M. Tanaka, M. Yamada, S. Shimamura, Y. Ishizaka, K. Ikeda, K. Ozawa, Y. Miura, and K. Hatake. 1998. Activated endothelial cells induce apoptosis in leukemic cells by endothelial interleukin-8. Blood 92:2672-2680. [PubMed] [Google Scholar]
- 36.Tsutsumi, T., A. Ido, K. Nakao, K. Hamasaki, Y. Kato, A. Ohtsuru, K. Nakata, T. Tamaoki, and S. Nagataki. 1994. Reciprocal regulation of alpha-fetoprotein and albumin gene expression by butyrate in human hepatoma cells. Gastroenterology 107:499-504. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, M., K. Reddi, and B. Henderson. 1996. Cytokine-inducing components of periodontopathic bacteria. J. Periodont. Res. 31:393-407. [DOI] [PubMed] [Google Scholar]
- 38.Wong, G. C., and S. C. Clark. 1988. Multiple actions of interleukin-6 within a cytokine network. Immunol. Today 9:137-139. [DOI] [PubMed] [Google Scholar]
- 39.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]