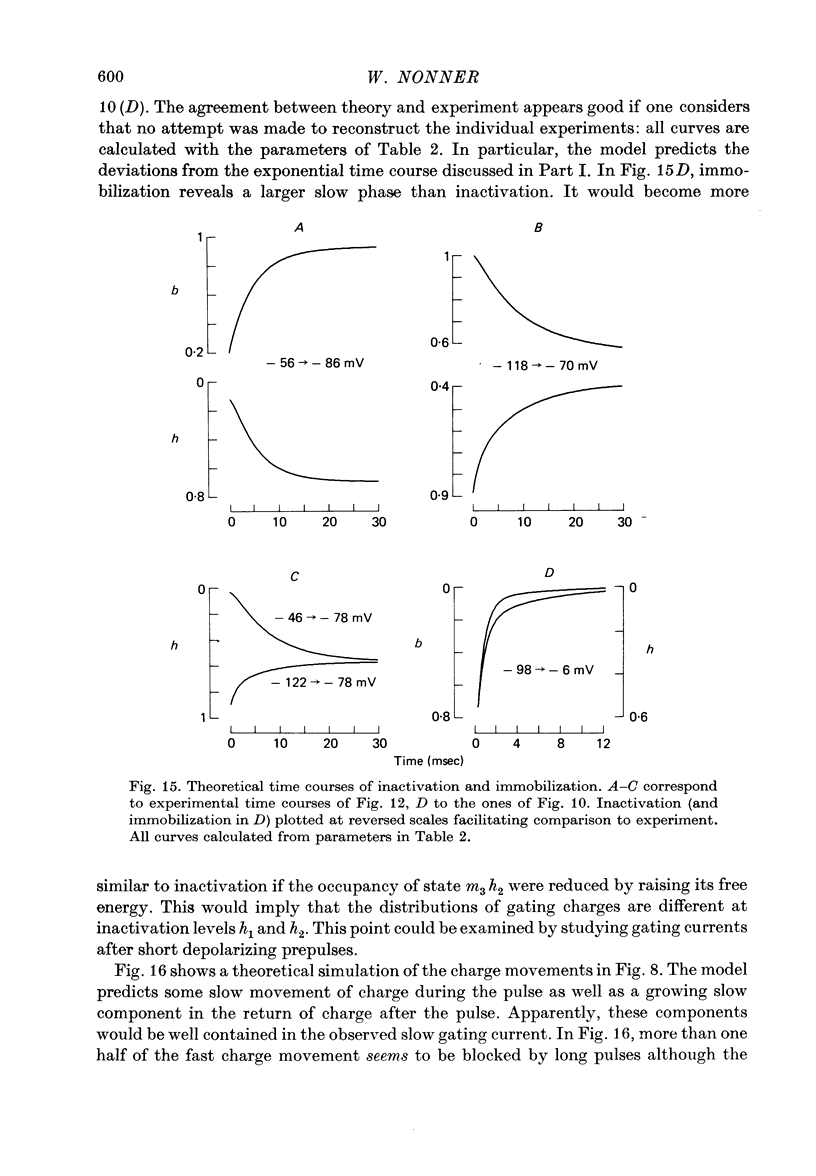
Abstract
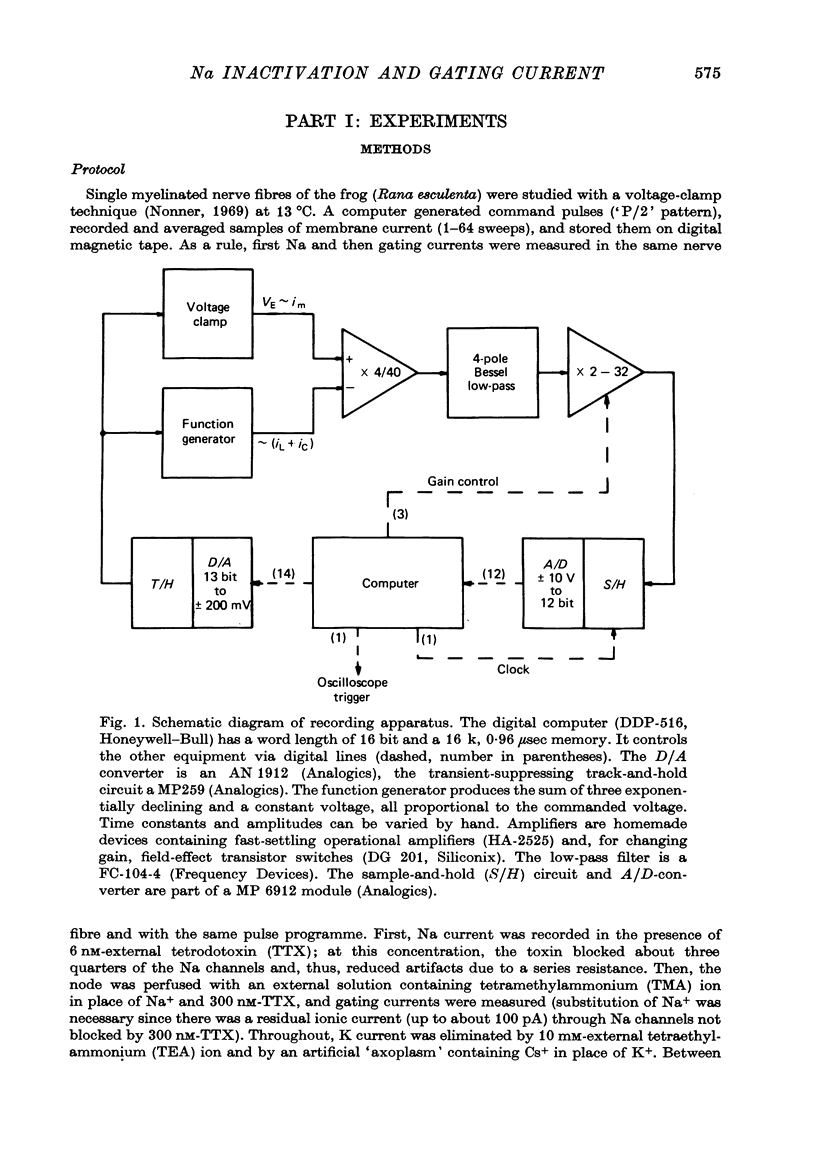
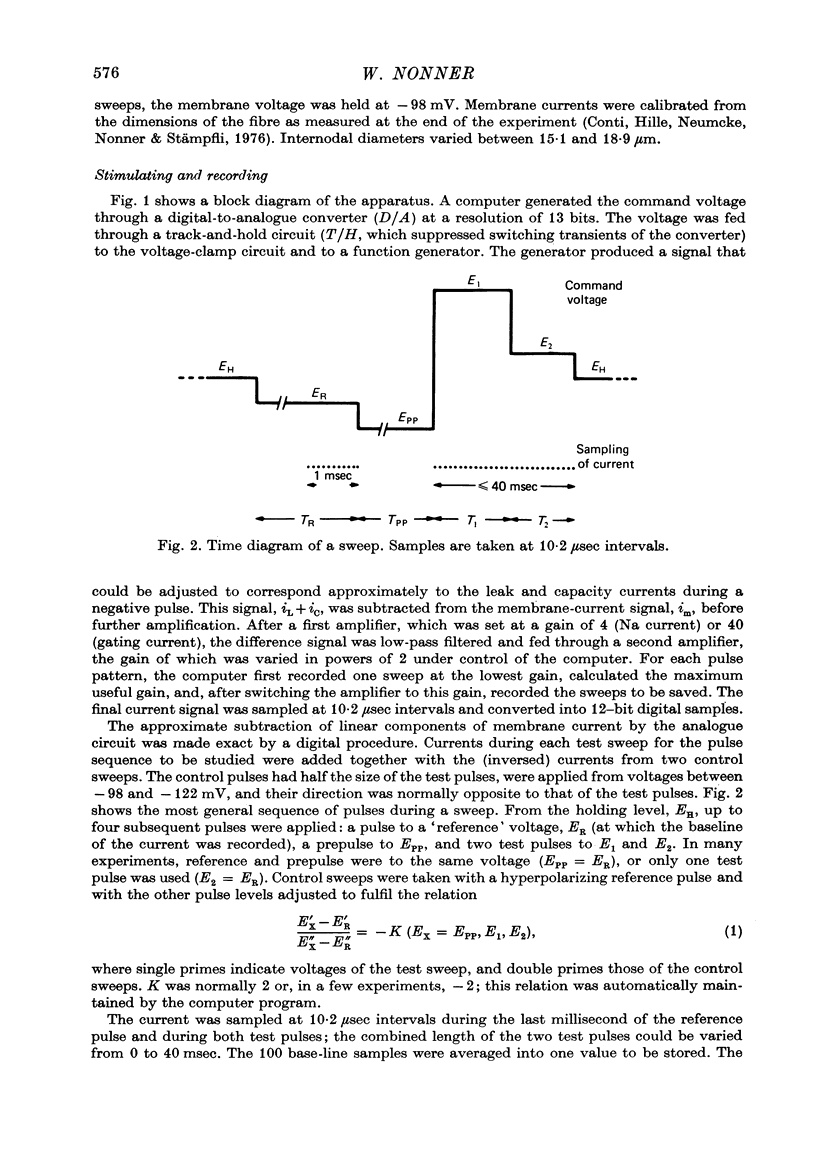
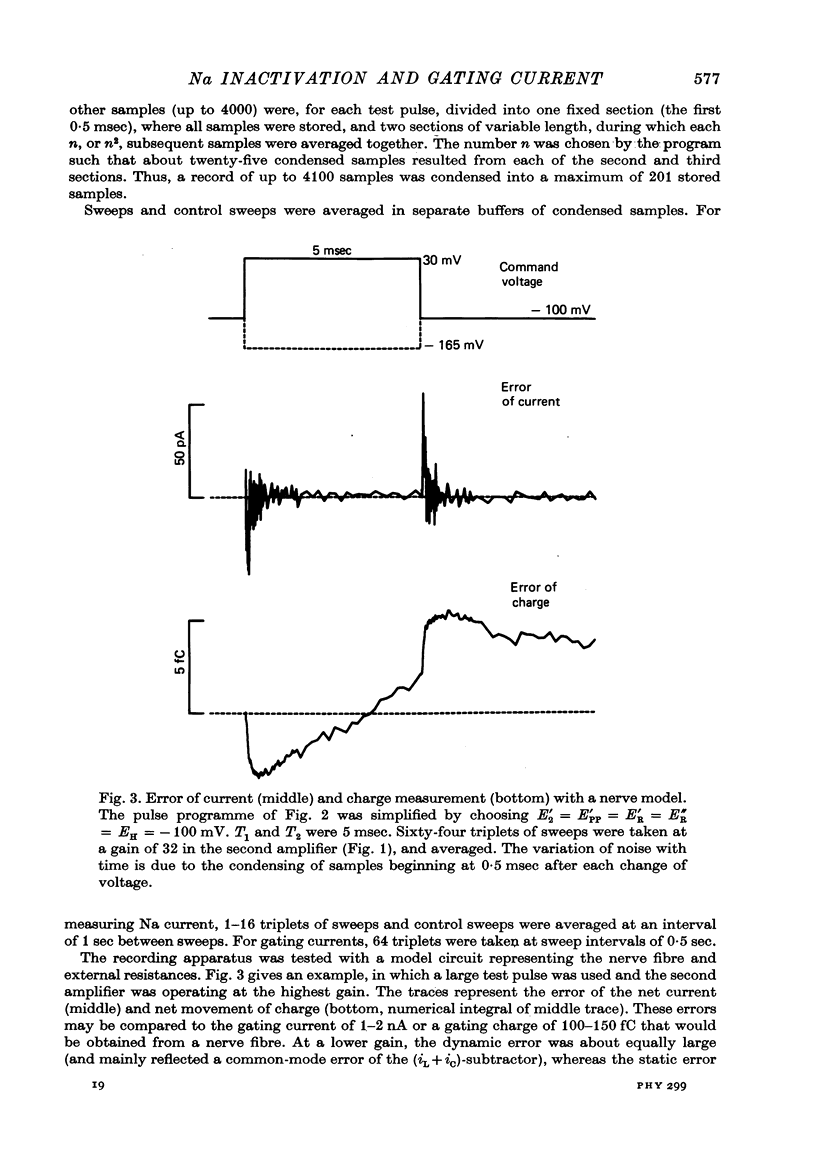
1. Single, voltage-clamped nerve fibres of Rana esculenta were stimulated with `P/2' pulse patterns for measuring Na and gating currents at 13 °C.
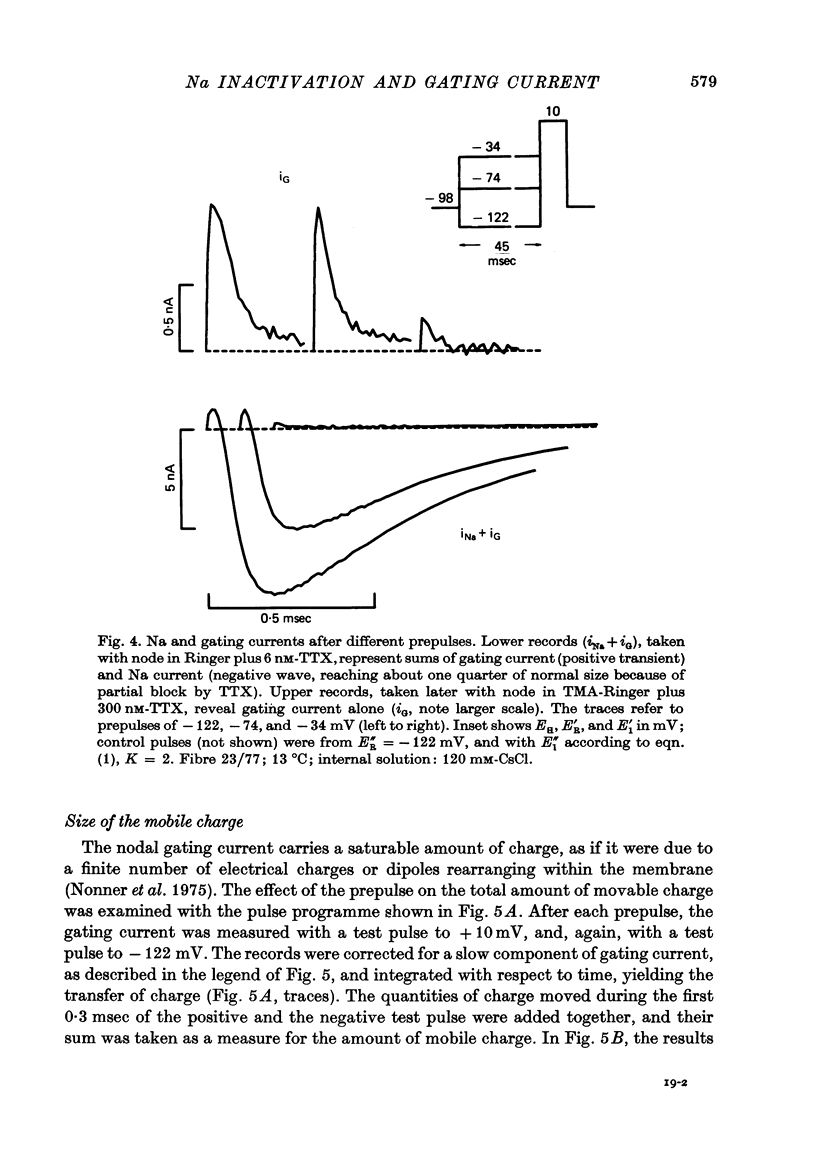
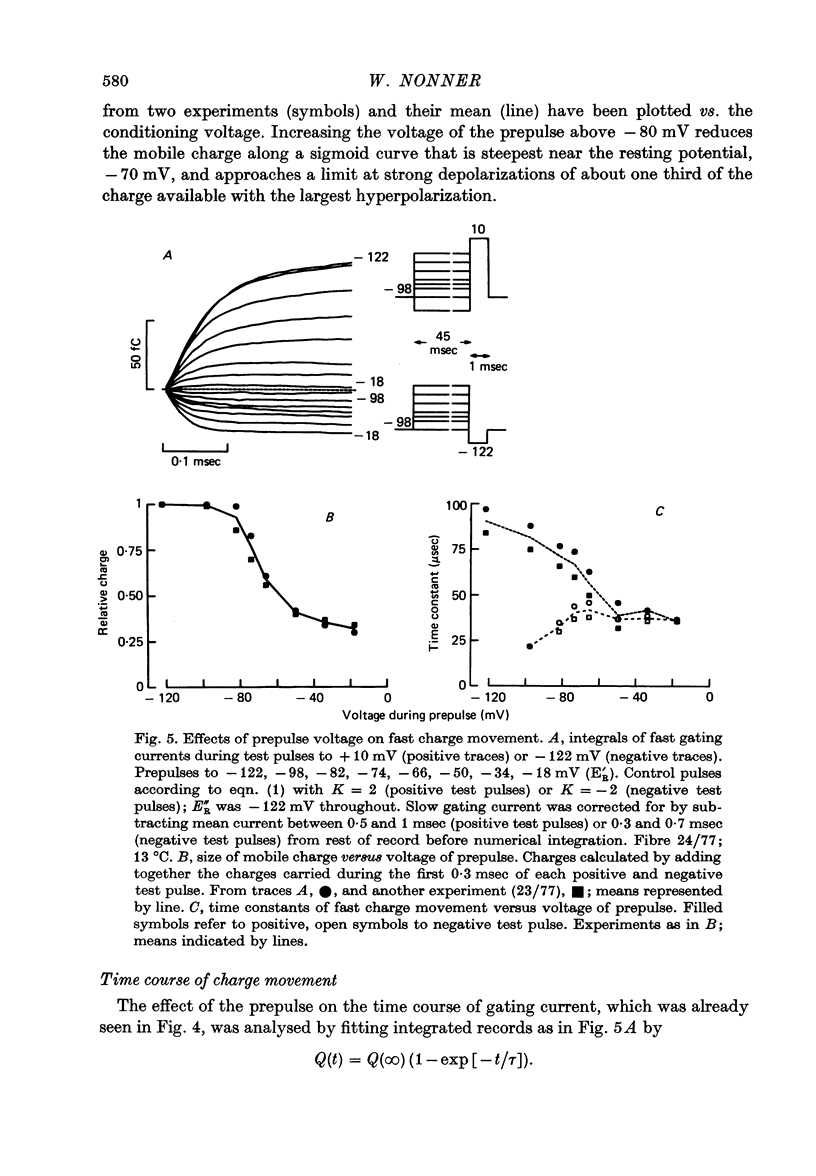
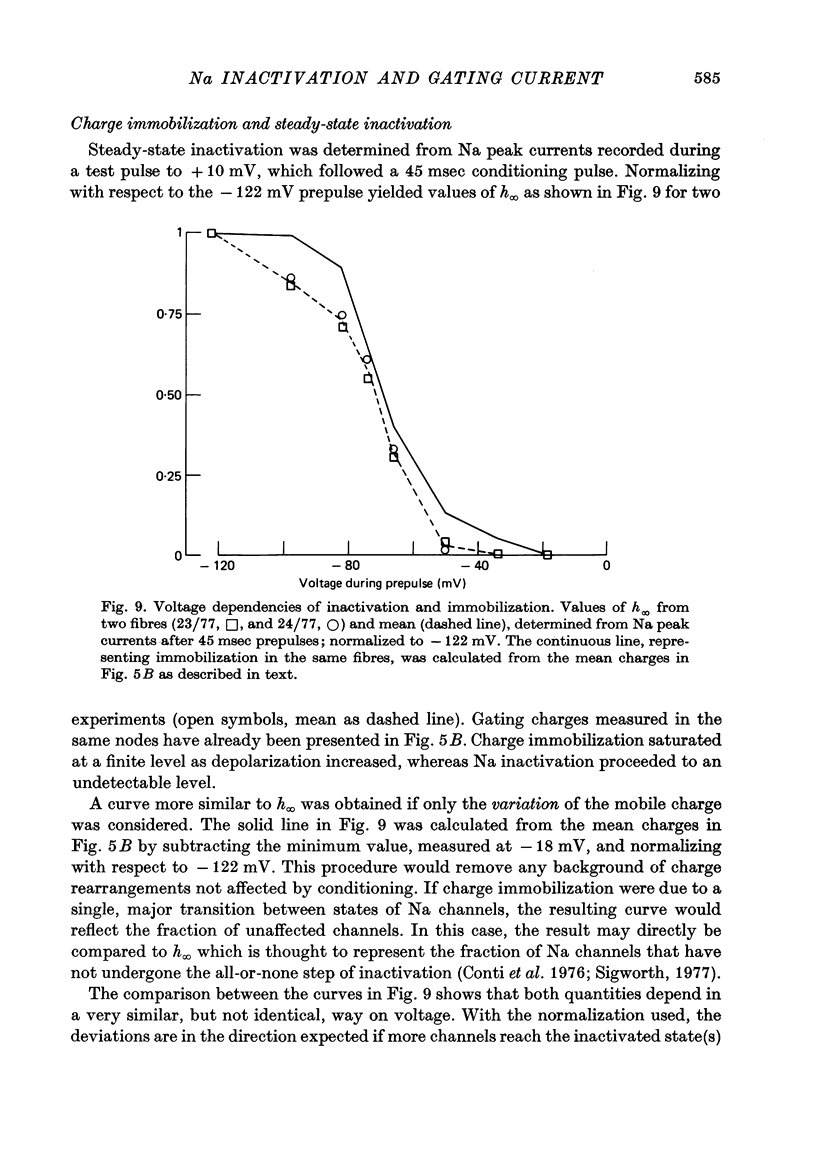
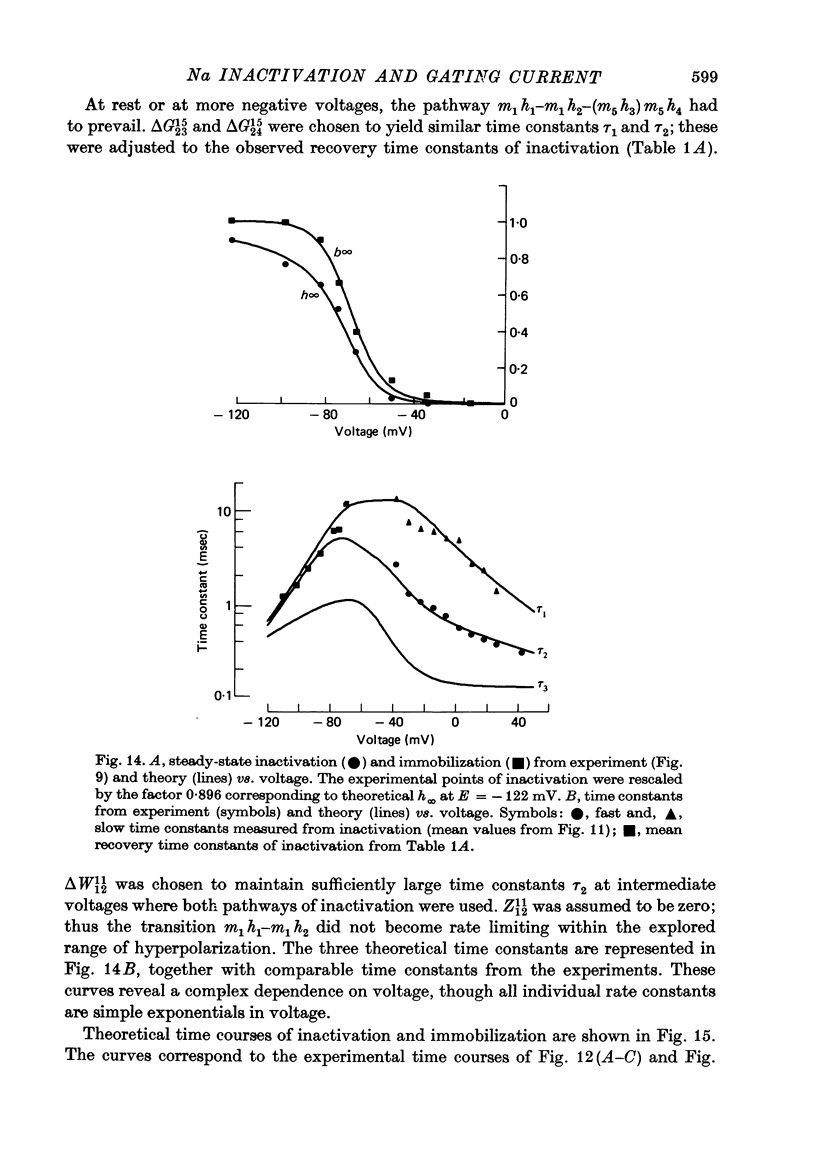
2. Gating currents during test pulses to - 122 or + 10 mV were measured after 45 msec conditioning steps to voltages between - 122 and - 18 mV. As the conditioning voltage was made more positive than - 80 mV, the movable gating charge diminished along a sigmoid curve, approaching a value of nearly one third of the maximum charge. On the other hand, Na inactivation began at a more negative potential and proceeded to undetectable levels.
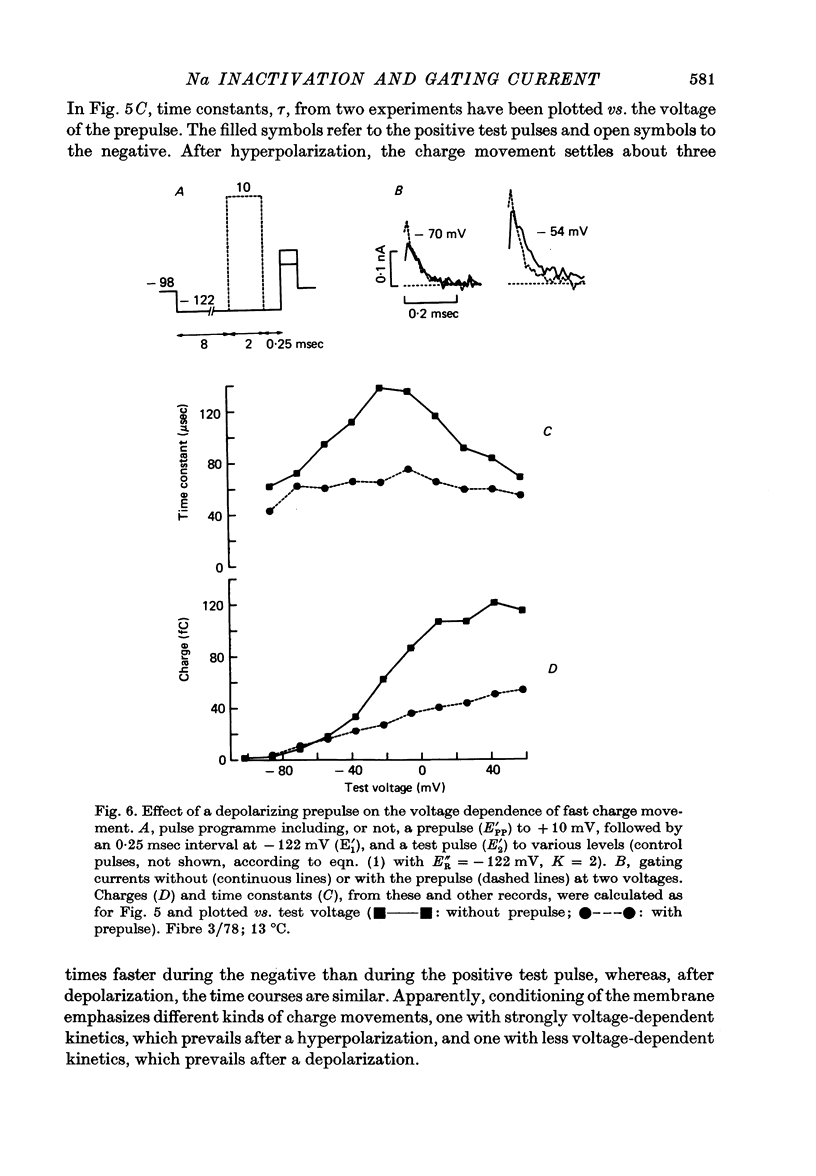
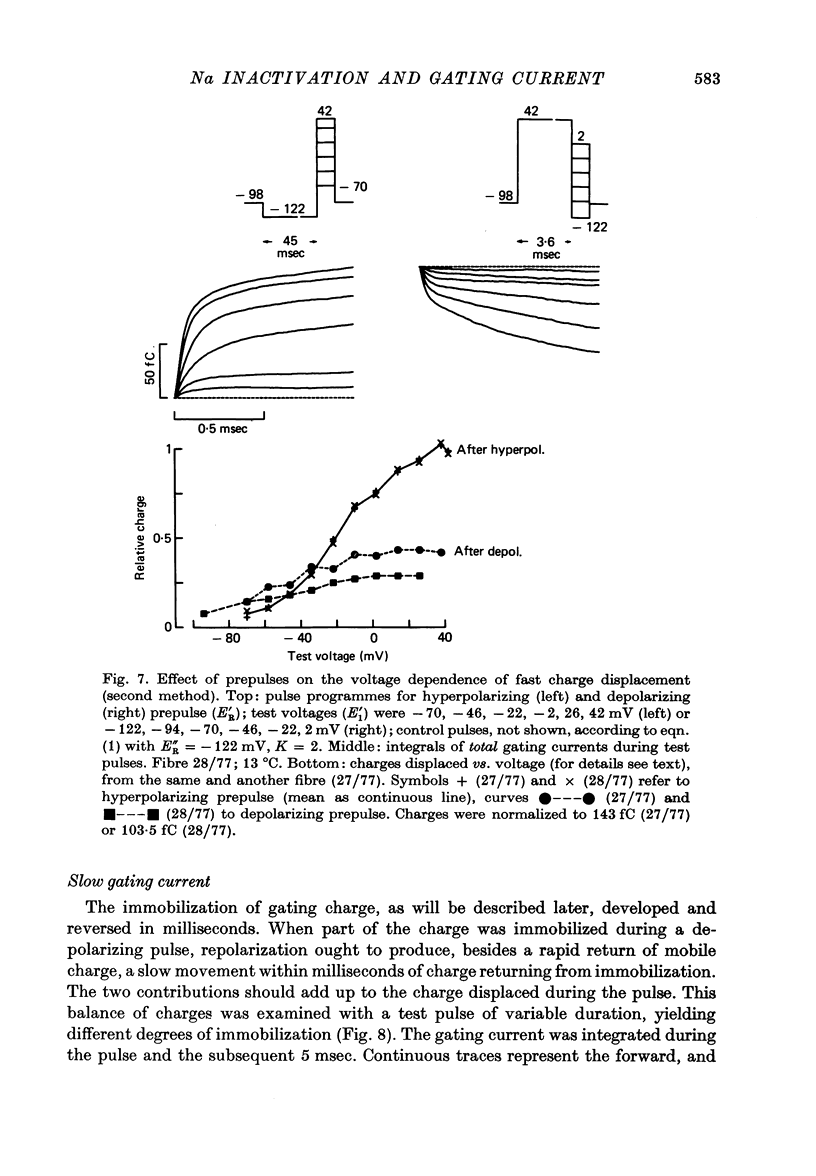
3. After a depolarizing prepulse, both time constant and size of the charge movment depended less steeply on the test voltage than normally. The prepulse reduced gating currents associated with steps from - 122 to test voltages ≥ - 40 mV, but enhanced gating currents obtained with test voltages < - 40 mV.
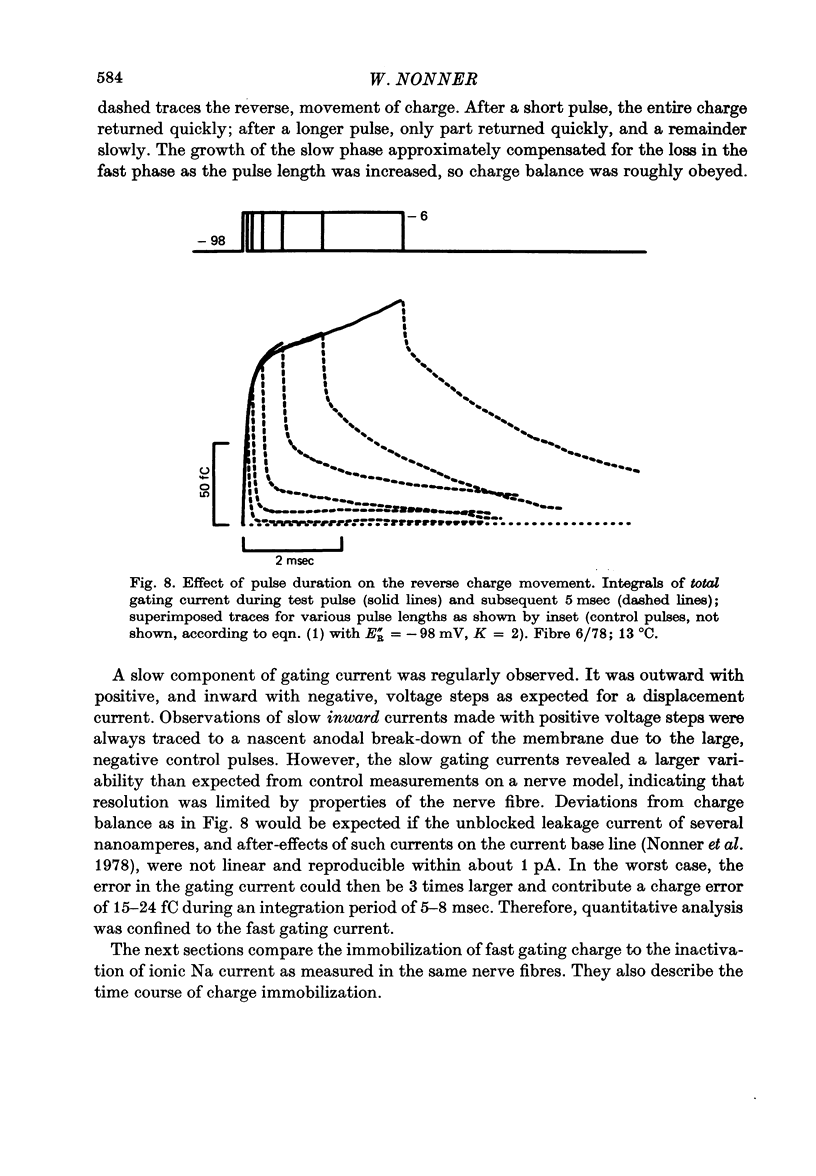
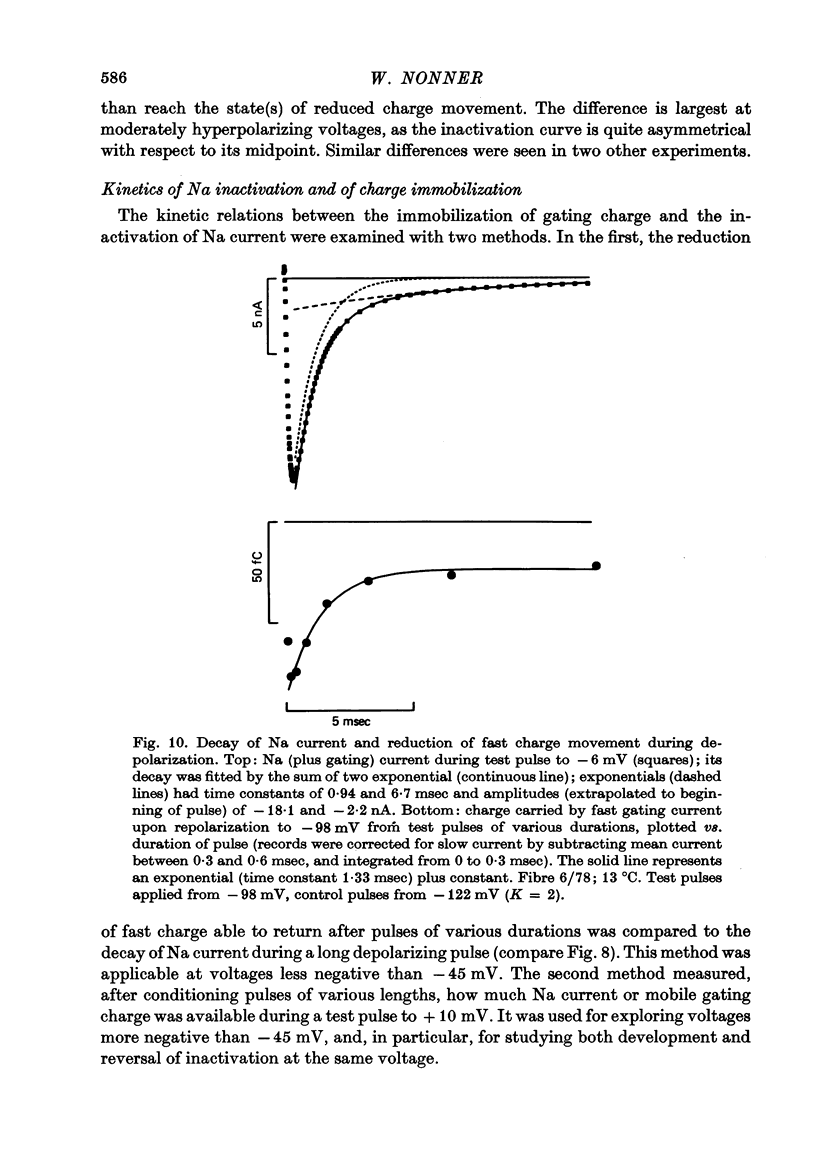
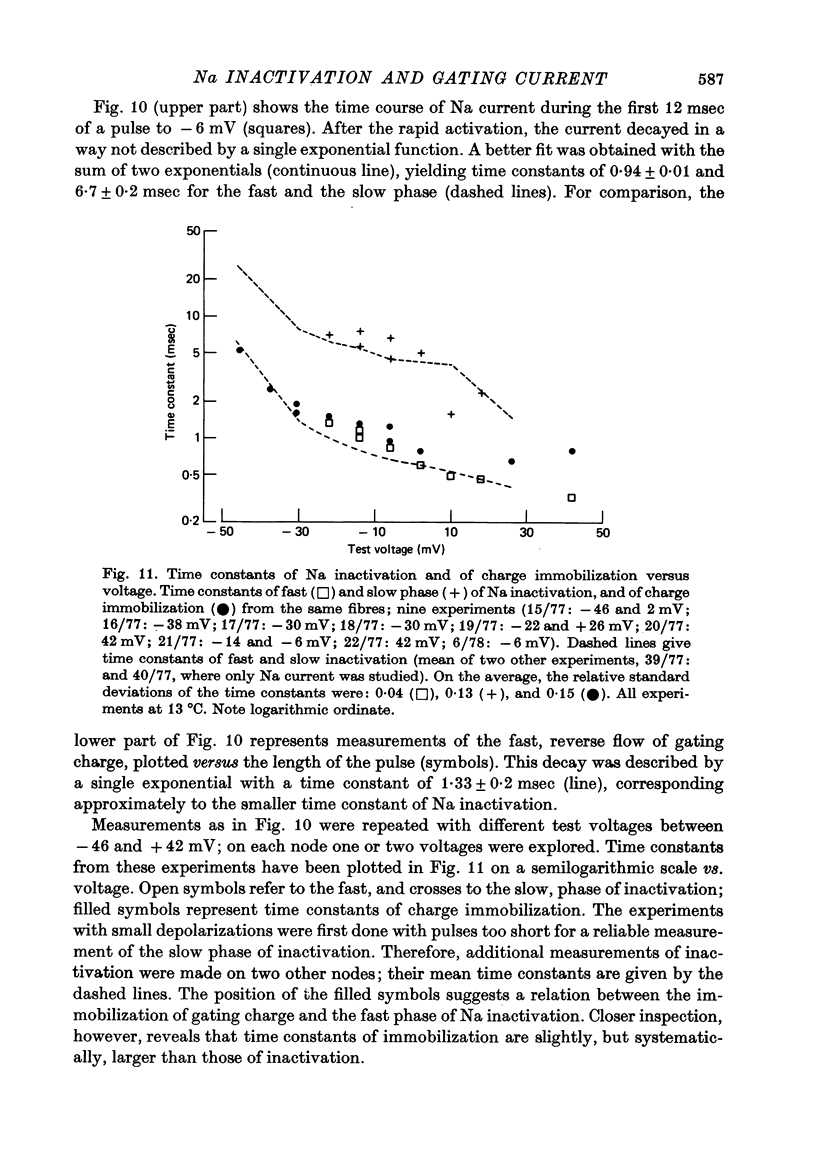
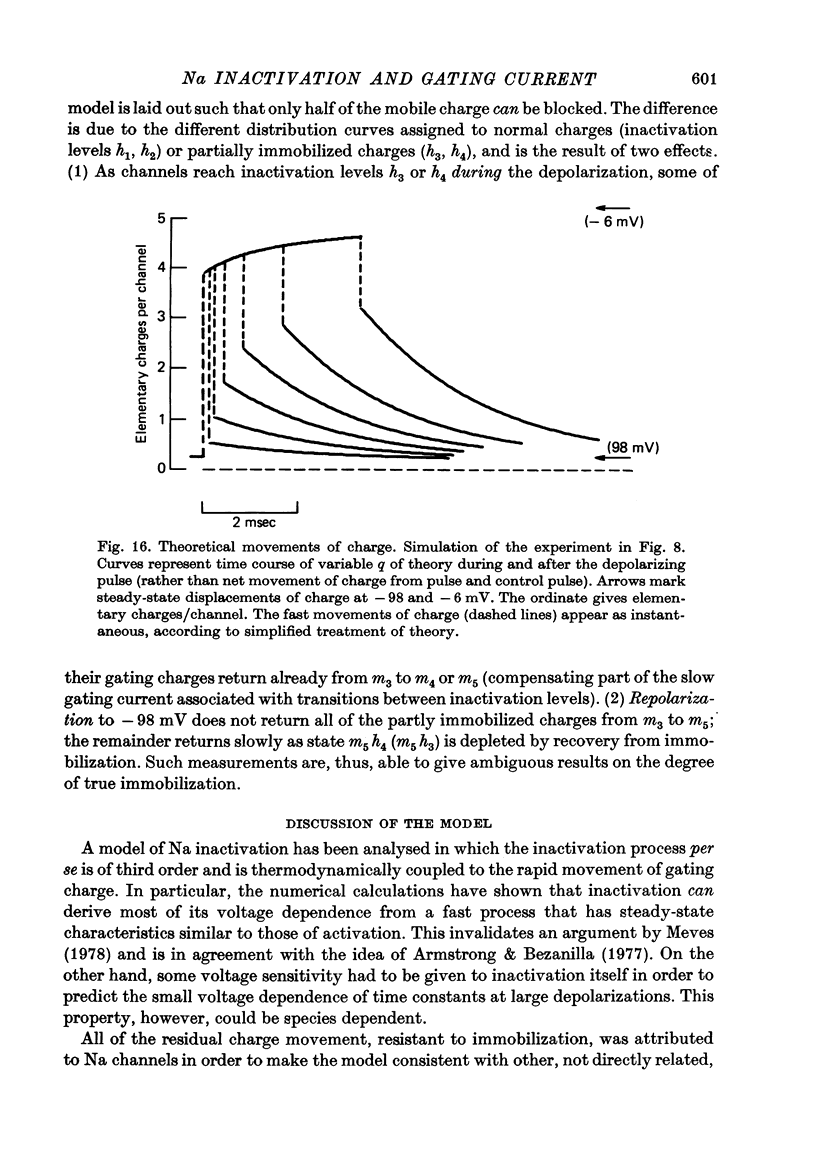
4. Increasing the duration of a depolarizing pulse (- 54 to + 42 mV) reduced the fast `off' gating current at the end of the pulse and enhanced a slow component. Their total charge corresponded approximately to that carried during the pulse. During depolarization, Na current inactivated in a fast and a slow phase. The fast phase was also reflected in the loss of fast charge movement (immobilization) as seen after the pulse was interrupted at various durations.
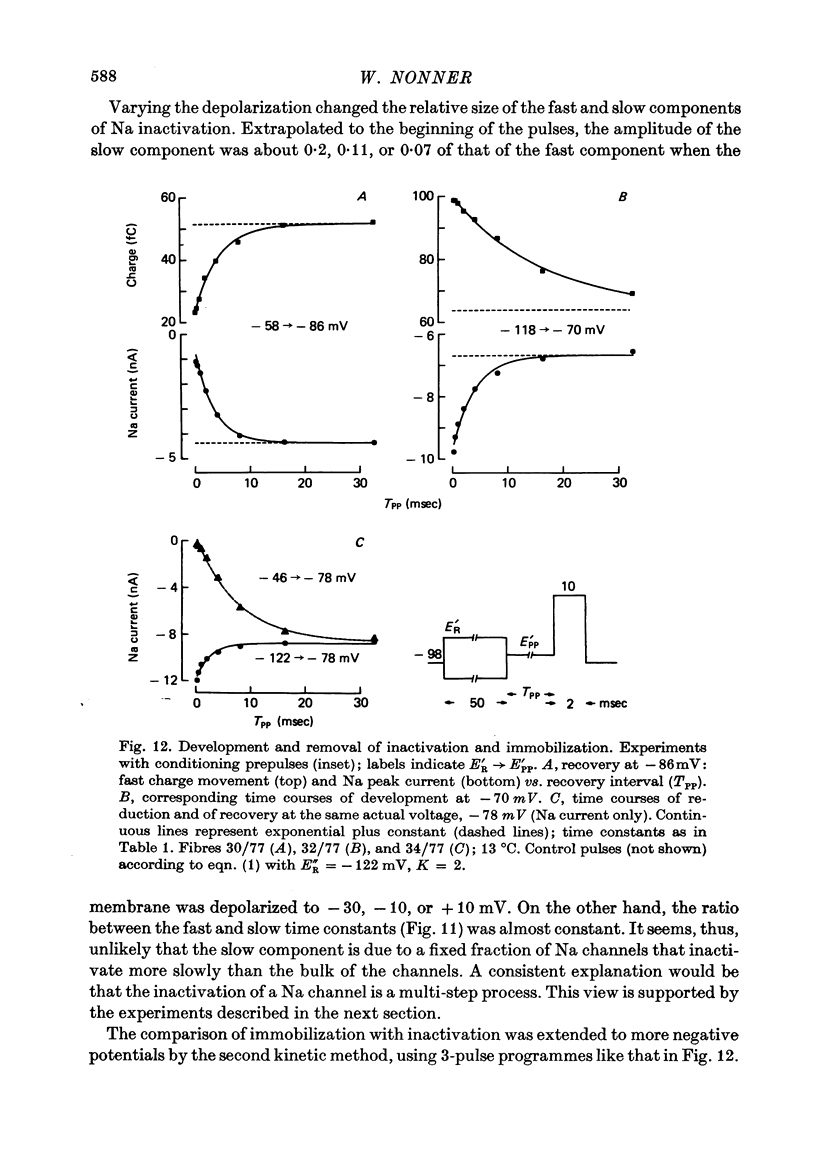
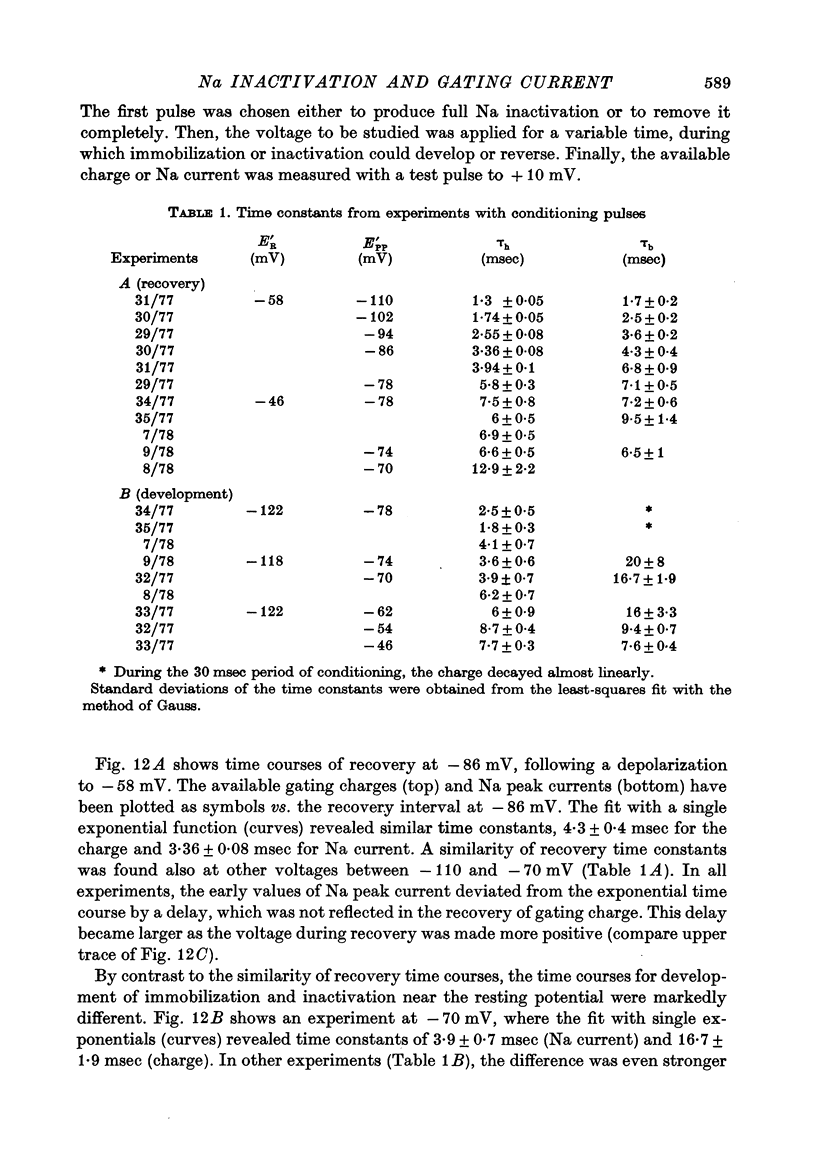
5. The available Na current and the fast movement of gating charge diminished in parallel during prepulses more positive than - 54 mV, and recovered in parallel upon repolarization to levels between - 102 and - 46 mV. During prepulses between - 62 and - 78 mV, however, Na inactivation occurred up to 4 times faster than charge immobilization. Also, at - 78 mV, Na current was inactivated 3 times faster than it recovered.
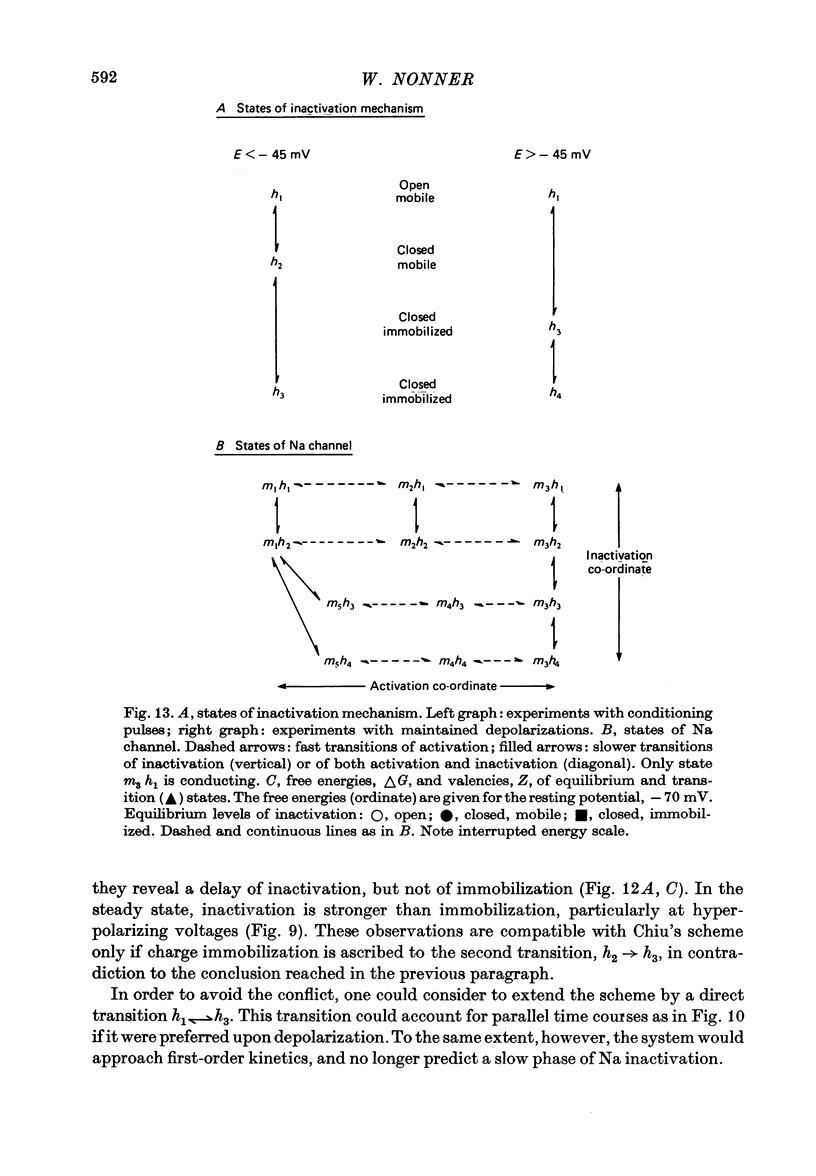
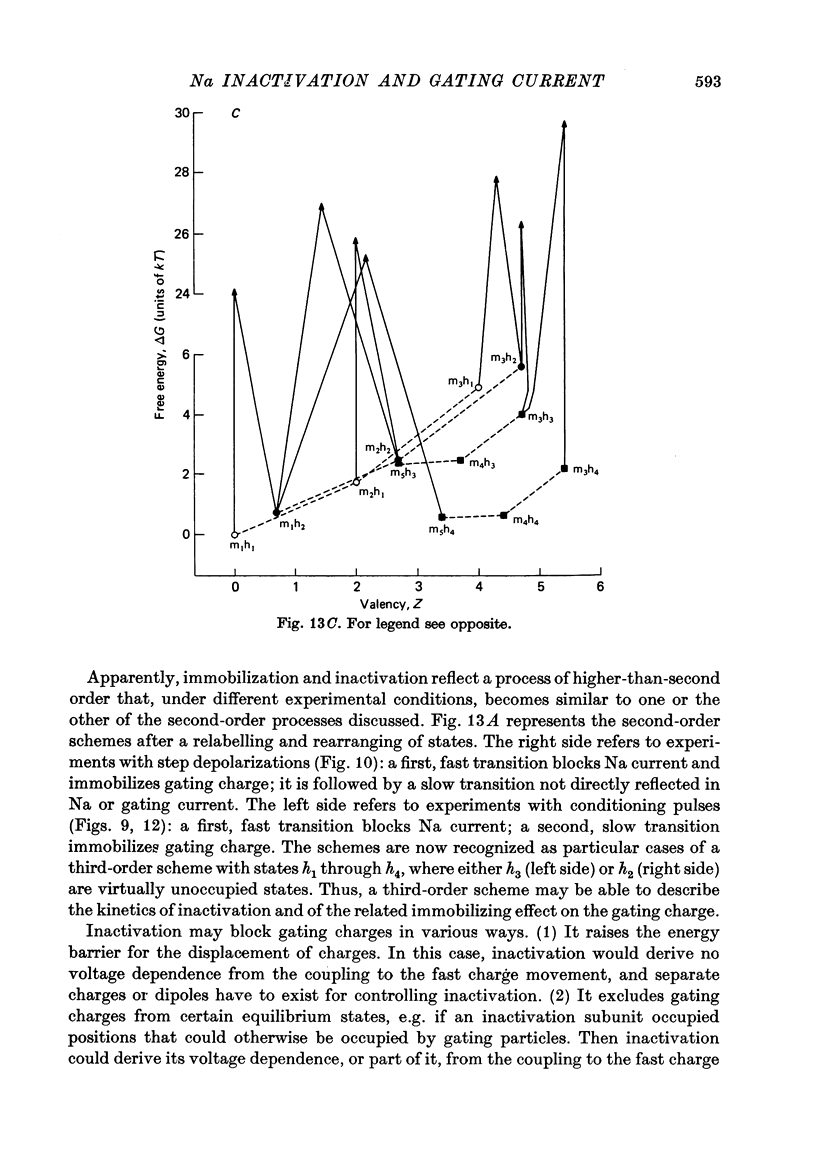
6. These findings indicate that Na inactivation and charge immobilization are linked, but proceed with high-order kinetics. The simplest scheme that accounts for their relation is [Formula: see text] Depending on voltage, either state h2 (E > - 45 mV) or h3 (E < - 45 mV) becomes kinetically undetectable.
7. A model of the Na channel is developed in which inactivation gains most of its voltage dependence by a coupling to the fast charge movement (activation). The model is shown to be quantitatively consistent with the results. In particular, the change of kinetics observed near - 45 mV can be explained as an effect of the redistribution of charges on the inactivation process.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Block of sodium conductance and gating current in squid giant axons poisoned with quaternary strychnine. Biophys J. 1979 Jul;27(1):57–73. doi: 10.1016/S0006-3495(79)85202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Measurement of the conductance of the sodium channel from current fluctuations at the node of Ranvier. J Physiol. 1976 Nov;262(3):699–727. doi: 10.1113/jphysiol.1976.sp011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Inactivation of the asymmetrical displacement current in giant axons of Loligo forbesi. J Physiol. 1977 May;267(2):377–393. doi: 10.1113/jphysiol.1977.sp011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Nonner W., Stämpfli R. Asymmetrical displacement current and its relation with the activation of sodium current in the membrane of frog myelinated nerve. Pflugers Arch. 1976 Jun 22;363(3):193–203. doi: 10.1007/BF00594601. [DOI] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli R. Asymmetrical displacement currents in the membrane of frog myelinated nerve: early time course and effects of membrane potential. Pflugers Arch. 1978 Jun 21;375(1):75–85. doi: 10.1007/BF00584151. [DOI] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli R. Gating currents in the node of Ranvier: voltage and time dependence. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):483–492. doi: 10.1098/rstb.1975.0024. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Sodium channels in nerve apparently have two conductance states. Nature. 1977 Nov 17;270(5634):265–267. doi: 10.1038/270265a0. [DOI] [PubMed] [Google Scholar]



