Abstract
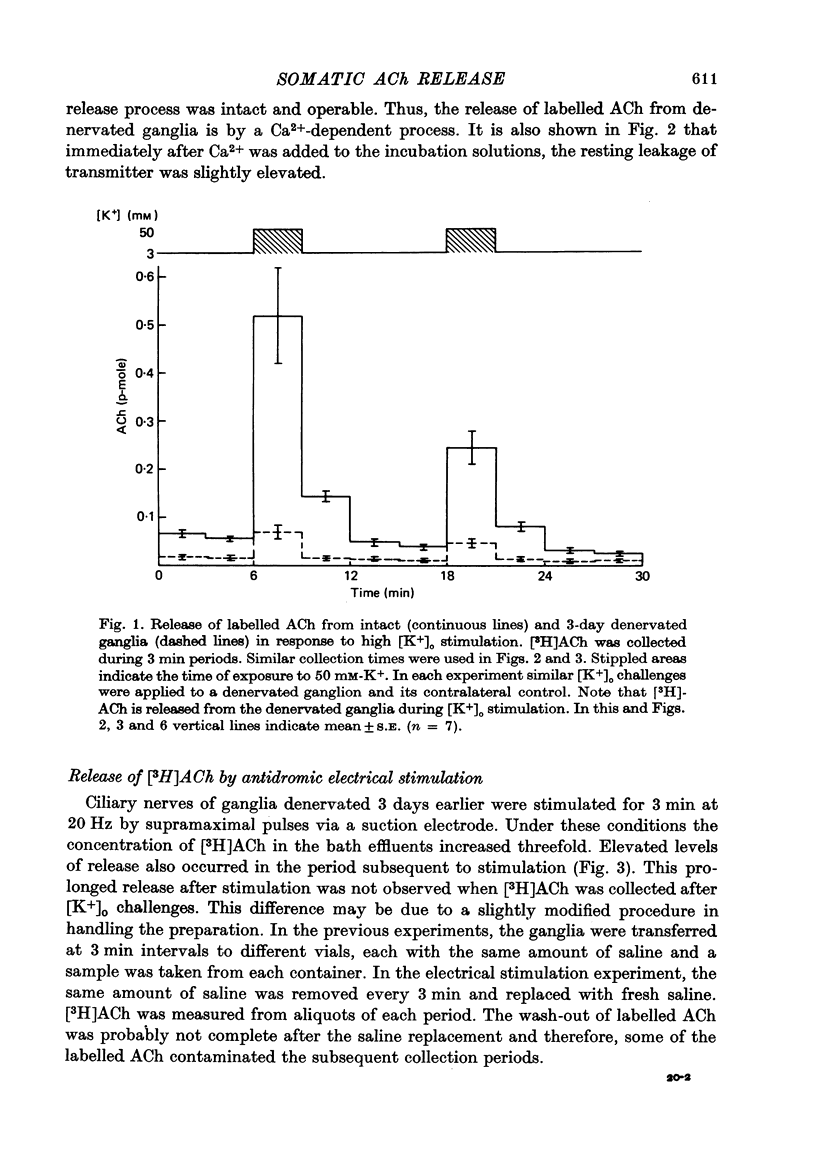
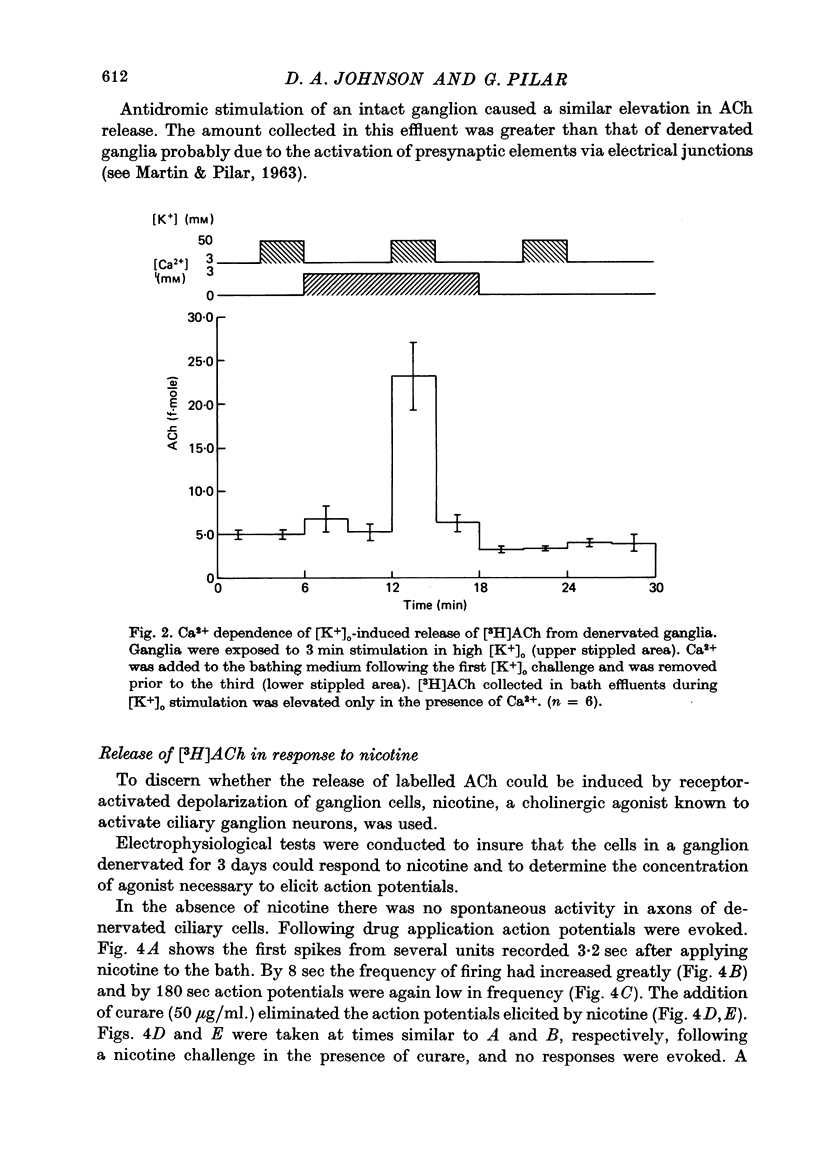
1. Acetylcholine (Ach) release from parasympathetic ganglia cell somata was investigated in denervated avian ciliary ganglia. Three days after the input to the ganglion (the oculomotor nerve) was sectioned, all presynaptic nerve terminals had degenerated. 2. Denervated ganglia were shown to contain endogenous ACh and to be capable of synthesizing [3H]ACh from [3H]choline added to the incubation medium. 3. In response to depolarization induced by incubation in 50 mM-[K+]o, denervated ganglia released [3H]ACh into bath effluents in amounts approximately 15% of the non-denervated contralateral control. This release was shown to be Ca2+ dependent in both intact and denervated ganglia. 4. Antidromic electrical stimulation of ciliary nerves also elicited [3H]ACh release. Nicotine (1 microgram/microliter.) depolarized denervated ciliary ganglion cells and evoked release of the transmitter and this release was antagonized by curare. 5. It is concluded that the ganglionic cell bodies sysnthesized ACh and released the transmitter in response to K+ depolarization, antidromic stimulation and cholinergic agonists, despite the lack of morphological specializations usually associated with stimulus-induced release of neurotransmitter. The evidence suggests the existence of a mechanism of transmitter release which is Ca2+ dependent, probably from a cytoplasmic pool and therefore distinct from the usual vesicular release at the nerve terminal.
Full text
PDF





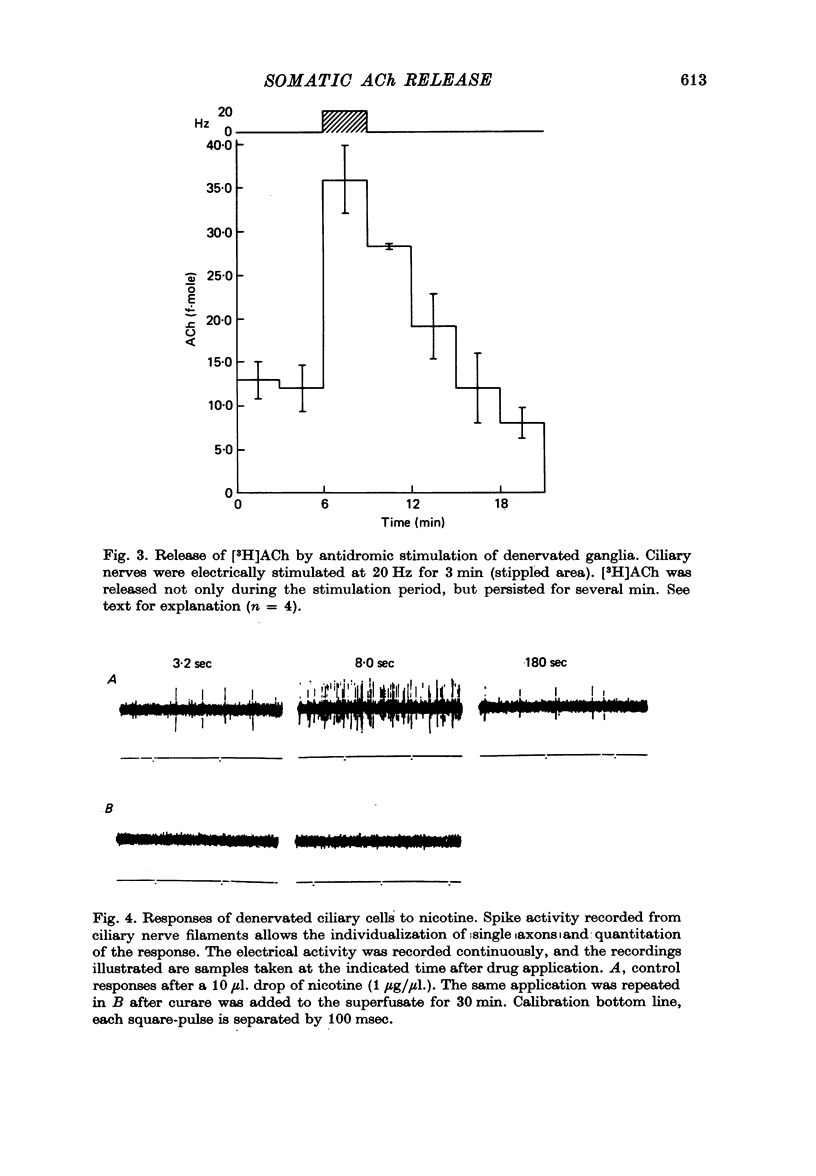
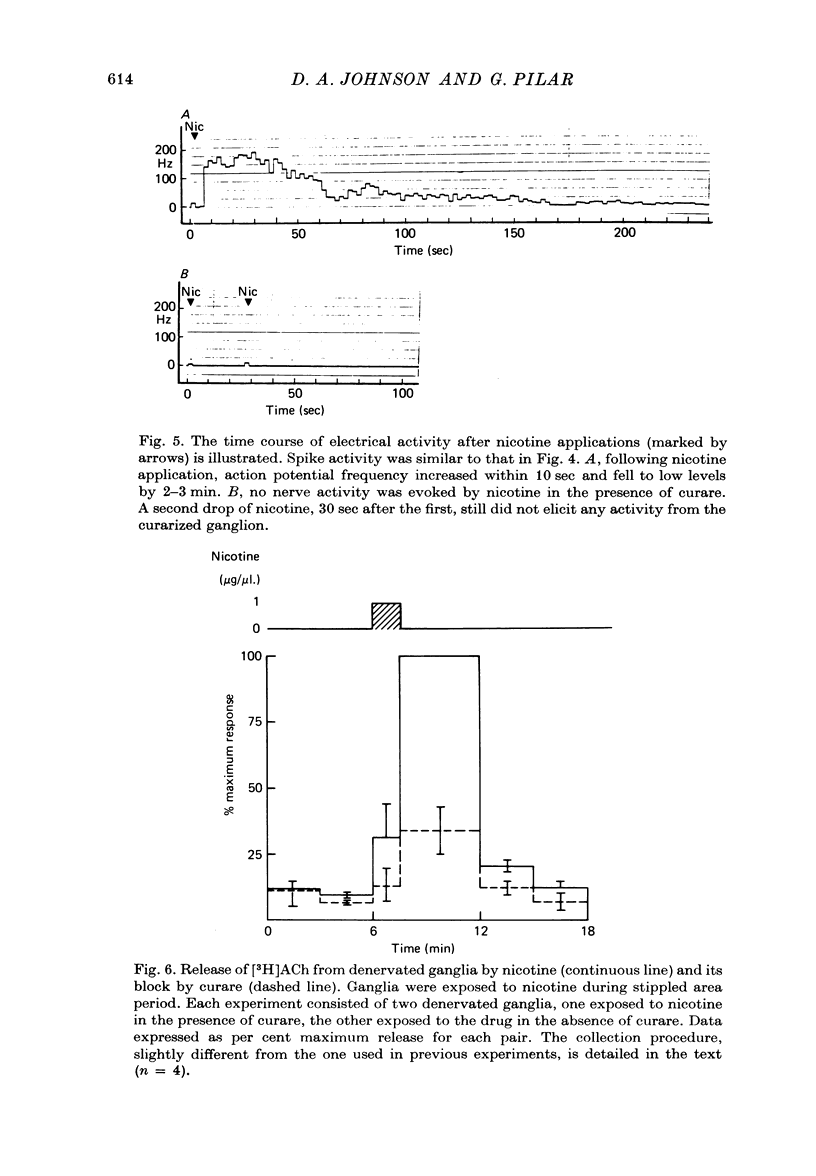




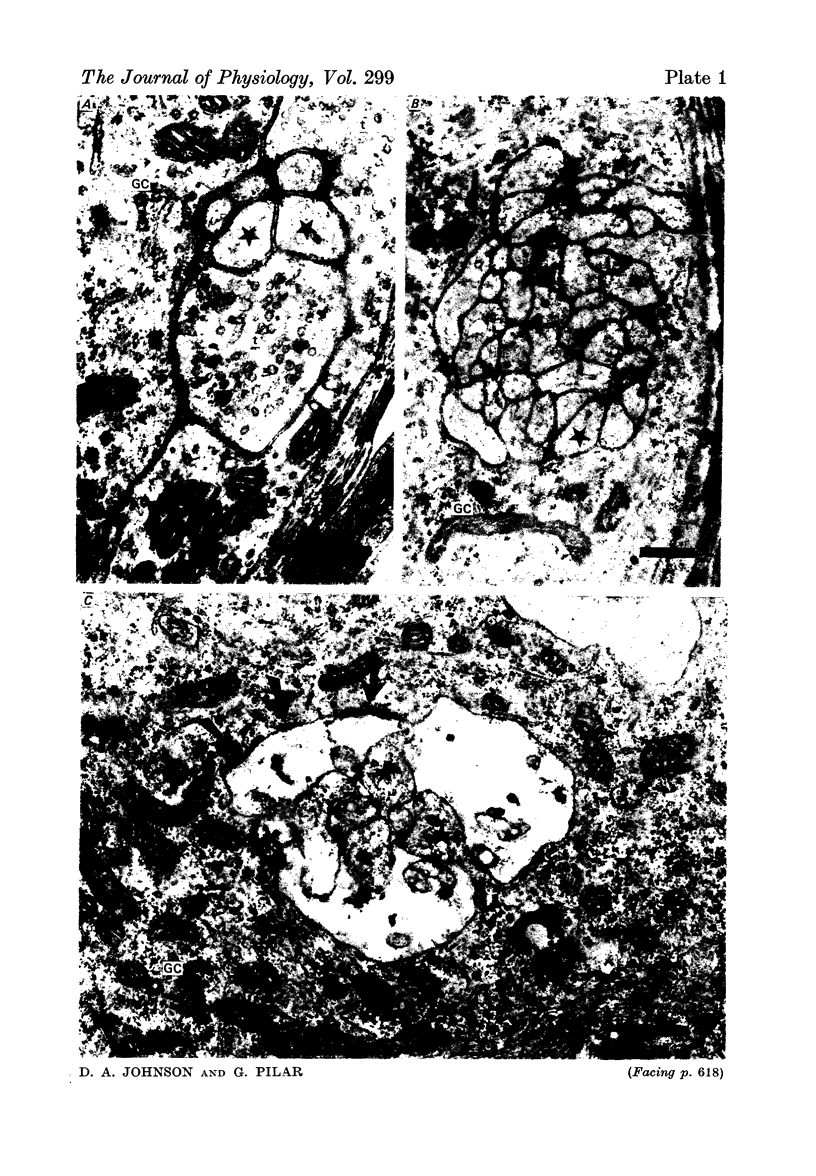


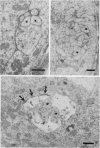
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Grampp W., Miledi R. Properties of spontaneous potentials at denervated motor endplates of the frog. Proc R Soc Lond B Biol Sci. 1976 Oct 15;194(1115):195–210. doi: 10.1098/rspb.1976.0073. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Fitch J. G. Storage and release of acetylcholine in a sympathetic ganglion. J Physiol. 1974 Jul;240(1):125–134. doi: 10.1113/jphysiol.1974.sp010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. The relationship of transmitter release and storage to fine structure in a sympathetic ganglion. J Neurocytol. 1974 Jun;3(2):133–160. doi: 10.1007/BF01098386. [DOI] [PubMed] [Google Scholar]
- Cantino D., Mugnaini E. The structural basis for electrotonic coupling in the avian ciliary ganglion. A study with thin sectioning and freeze-fracturing. J Neurocytol. 1975 Oct;4(5):505–536. doi: 10.1007/BF01351535. [DOI] [PubMed] [Google Scholar]
- DE LORENZO A. J. The fine structure of synapses in the ciliary ganglion of the chick. J Biophys Biochem Cytol. 1960 Feb;7:31–36. doi: 10.1083/jcb.7.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dail W. G., Evan A. P. Effects of chronic deafferentation on adrenergic ganglion cells and small intensely fluorescent cells. J Neurocytol. 1978 Feb;7(1):25–37. doi: 10.1007/BF01213458. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Electrically induced release of acetylcholine from denervated Schwann cells. J Physiol. 1974 Mar;237(2):431–452. doi: 10.1113/jphysiol.1974.sp010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F., Frizell M., Sjöstrand J. Transport, turnover and distribution of choline acetyltransferase and acetylcholinesterase in the vagus and hypoglossal nerves of the rabbit. J Neurochem. 1973 Nov;21(5):1109–1120. doi: 10.1111/j.1471-4159.1973.tb07565.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Isolation of choline esters from aqueous solutions by extraction with sodium tetraphenylboron in organic solvents. Biochem J. 1969 Jun;113(2):291–298. doi: 10.1042/bj1130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E., Pilar G., Suszkiw J., Uchimura H. Normal distribution and denervation changes of neurotransmitter related enzymes in cholinergic neurones. J Physiol. 1979 Jan;286:233–253. doi: 10.1113/jphysiol.1979.sp012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich D. R. Partial purification and properties of choline kinase (EC 2. 7. 1. 32) from rabbit brain: measurement of acetylcholine. J Neurochem. 1973 Aug;21(2):315–328. doi: 10.1111/j.1471-4159.1973.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Ito Y., Miledi R. The effect of calcium-ionophores on acetylcholine release from Schwann cells. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):51–58. doi: 10.1098/rspb.1977.0028. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarelli R., Ciesielski-Treska J., Ebel A., Mandel P. Choline uptake in glial cell cultures. Brain Res. 1974 Dec 6;81(2):361–363. doi: 10.1016/0006-8993(74)90953-6. [DOI] [PubMed] [Google Scholar]
- McCaman R. E., Stetzler J. Radiochemical assay for ACh: modifications for sub-picomole measurements. J Neurochem. 1977 Mar;28(3):669–671. doi: 10.1111/j.1471-4159.1977.tb10442.x. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Murrin L. C., Kuhar M. J. Activation of high-affinity choline uptake in vitro by depolarizing agents. Mol Pharmacol. 1976 Nov;12(6):1082–1090. [PubMed] [Google Scholar]
- Pilar G., Jenden D. J., Campbell B. Distribution of acetylcholine in the normal and denervated pigeon ciliary ganglion. Brain Res. 1973 Jan 30;49(2):245–256. doi: 10.1016/0006-8993(73)90421-6. [DOI] [PubMed] [Google Scholar]
- Sargent P. B., Dennis M. J. Formation of synapses between parasympathetic neurones deprived of preganglionic innervation. Nature. 1977 Aug 4;268(5619):456–458. doi: 10.1038/268456a0. [DOI] [PubMed] [Google Scholar]
- Shea P. A., Aprison M. H. An enzymatic method for measuring picomole quantities of acetylcholine and choline in CNS tissue. Anal Biochem. 1973 Nov;56(1):165–177. doi: 10.1016/0003-2697(73)90181-4. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., Beach R. L., Pilar G. R. Choline uptake by cholinergic neuron cell somas. J Neurochem. 1976 Jun;26(6):1123–1131. doi: 10.1111/j.1471-4159.1976.tb06995.x. [DOI] [PubMed] [Google Scholar]
- Szerb J. C. Endogenous acetylcholine release and labelled acetylcholine formation from [3H]choline in the myenteric plexus of the guinea-pig ileum. Can J Physiol Pharmacol. 1975 Aug;53(4):566–574. doi: 10.1139/y75-080. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Schwartz J. H. Injection of radioactive materials into an identified axon of Aplysia. Brain Res. 1974 Mar 22;68(2):358–364. doi: 10.1016/0006-8993(74)90405-3. [DOI] [PubMed] [Google Scholar]
- Vaca K., Pilar G. Mechanisms controlling choline transport and acetylcholine synthesis in motor nerve terminals during electrical stimulation. J Gen Physiol. 1979 May;73(5):605–628. doi: 10.1085/jgp.73.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]