Abstract
A recent model for cytolysin-mediated translocation in Streptococcus pyogenes proposes that NAD-glycohydrolase is translocated through streptolysin O-generated pores into a host cell (J. Madden, N. Ruiz, and M. Caparon, Cell 104:143-152, 2001). This model also assumes that the NAD-glycohydrolase (nga) and streptolysin O (slo) genes that code for these products are organized in an operon-like structure expressed from a single promoter only (nga). We expand this model by showing that slo possesses its own autonomous promoter, which is located 155 bp upstream of the slo gene. Under experimental conditions in which S. pyogenes is grown in THY medium, the strength of the slo promoter, as measured by the activity of a lacZ reporter gene, resulted in low but highly reproducible values. Finally, we demonstrated that sloR, a S. pyogenes gene that closely resembles the Clostridium perfringens pfoR gene, exerts a negative effect on the expression of the slo gene.
Streptolysin O (SLO) is an extracellular protein produced by most strains of Streptococcus pyogenes. It belongs to a large group of highly conserved cholesterol-dependent cytolysins found in species of four genera, including Streptococcus, Clostridium, Listeria, and Bacillus (2). SLO exerts its cytolytic function by forming large homopolymeric pores in the membranes of the targeted cells. The pores are formed by binding of SLO monomers to cholesterol receptors in the cell membrane followed by their aggregation into supramolecular complexes containing up to 50 monomers (11, 13).
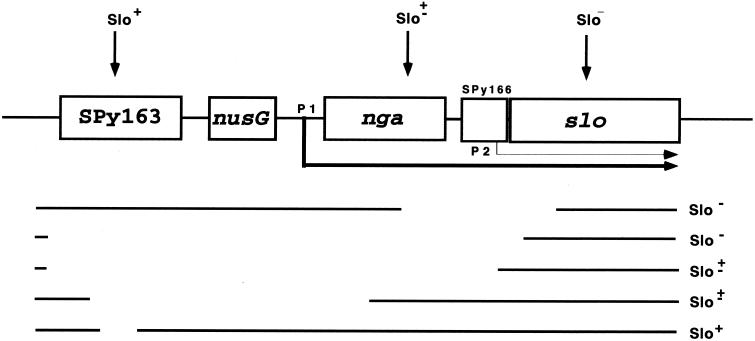
In contrast to rather extensive research on the SLO toxin and its mode of action, little information is available on the regulation of the slo gene. The original studies of Kehoe, Timmis, and colleagues were centered mainly on the cloning, sequencing, and analysis of the slo gene in comparison with other cytolysin-coding genes. However, these studies also provided some information on the regulatory role of a certain DNA segment in the upstream region of the slo gene (6, 7). Their deletion and transposon mutagenesis analysis of the slo region produced three distinct phenotypes: Slo+, Slo−, and Slo±, the last of which was obtained by transposon insertion into the chromosome region later shown to be occupied by the nga gene and deletions which cover the nga promoter but not the slo promoter (Fig. 1). These results suggested the existence of a weak slo promoter (6) located, as has been predicted, 155 bp upstream of the structural gene (7). The most recent report by Caparon and colleagues describes for the first time a cytolysin-mediated translocation system in gram-positive bacteria which is a functional equivalent to the type III secretion system found in gram-negative bacteria (8). Their data support a model in which the effector NAD-glycohydrolase, encoded by nga (spn), the gene immediately upstream of the slo gene (4) (Fig. 1), is transported through the SLO pore into the host cell (8). Results of that study also suggest that the slo gene is a part of a bicistronic nga-slo operon transcribed from a promoter immediately upstream of the nga gene, implying that they are regulated in common at the transcriptional level (8) (Fig. 1). Here we show that the regulation of these two products is possibly more complicated and that the slo gene, in accordance with the reports cited in references 6 and 7, may also possess its own independently regulated promoter.
FIG. 1.
Schematic representation of the nga-slo region of the S. pyogenes chromosome (4). Arrowed horizontal lines represent transcripts emerging from the nga (P1, thick line) and slo (P2, thin line) promoters. Transposon inserts (vertical arrows) and horizontal interrupted lines (with gaps representing deleted material), both with specific phenotypes related to SLO synthesis, were adapted from Kehoe and Timmis (6) and superimposed on the current nga-slo region map (4).
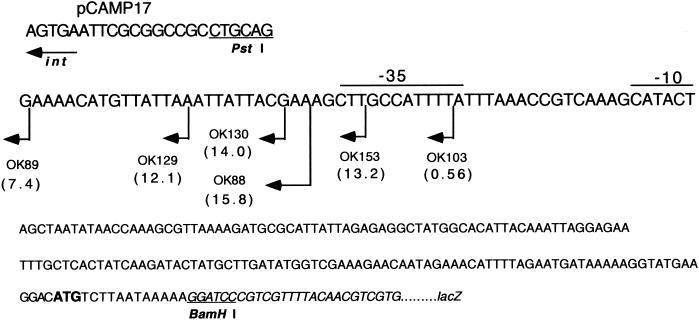
To test the authenticity of the previously predicted slo promoter (7), a set of strains carrying fragments of different lengths of the slo upstream region fused to the lacZ reporter gene of vector pCAMP17 was constructed. The pCAMP17 vector contains the attachment site for and the integrase gene of S. pyogenes phage T12, which enable it to integrate irreversibly into a specific site in the S. pyogenes chromosome (5). The fragments were obtained by PCR amplification of chromosomal DNA from strain NZ131 (M49) with mutagenic oligonucleotides containing PstI and BamHI restriction sites. Upon treatment with PstI and BamHI enzymes, the fragments were cloned into the PstI- and BamHI-treated vector pCAMP17. The BamHI restriction site is found at locations inside both the lacZ and slo genes, and the obtained products encompass the ribosome binding site, the ATG initiation codon, and the first four codons of the slo gene (Fig. 2). All constructs (data not presented) were confirmed by DNA sequence analysis before they were integrated into the chromosome of strain NZ131 (the resulting strains: OK88, OK89, OK103, OK129, OK130, and OK153) (Fig. 2). The DNA recombinant techniques and measurement of β-galactosidase (β-Gal) activity were performed as described previously (10). The advantage of this system is that the slo upstream DNA in all constructs ends far below the hypothetical Spy166 gene promoter (4) (Fig. 1). Also, the int gene on pCAMP17 is positioned in an opposite orientation to that of the lacZ reporter gene (Fig. 2). Consequently, all detected activity of the reporter gene should be attributed to the putative slo promoter. The results demonstrate low but reproducible expression of the lacZ gene in all deletion mutants, with the exception of control strain NZ131, with no plasmid (data not presented), and strain OK103, in which the −35 sequence of the putative slo promoter is deleted (zero β-Gal activity) (Fig. 2). The low activity values obtained may be the result of sensitivity of the slo promoter to local DNA conformations (pCAMP17 inserts into the chromosome at the opposite site of the chromosome relative to the native site of the slo gene, more specifically between the open reading frames Spy1289 and Spy1290 (4), lack of a specific environmental signal, sharing of a hypothetical positive regulator with the native slo regulatory region, weakness of the slo promoter, or the combined effects of several factors. We conclude that expression of the reporter gene in strains with β-Gal activity is not the result of some spurious transcript coming from vector DNA and that the slo gene possesses its own genuine promoter as predicted before (7).
FIG. 2.
Schematic representation of the slo gene upstream region with constructed deletion ends indicated (vertical lines with arrows oriented to the left). The material to the left of deletion ends is spliced to the PstI site of the pCAMP17 DNA (upper row), and the bottom three nucleotide sequences are contiguous to the −10 box. Numbers in parentheses denote average values in Miller units obtained in three independent measurements. Lines above the main nucleotide sequence represent the predicted −10 and −35 promoter sequences of the slo gene (7). The initiation codon of the slo gene is given in bold letters, and the lacZ DNA sequence is given in italics. The PstI site in the pCAMP17 vector and BamHI site at the junction of the slo and lacZ DNA sequences are underlined. For a more detailed description of pCAMP17-based constructs and their mode of integration into the chromosome, see the text.
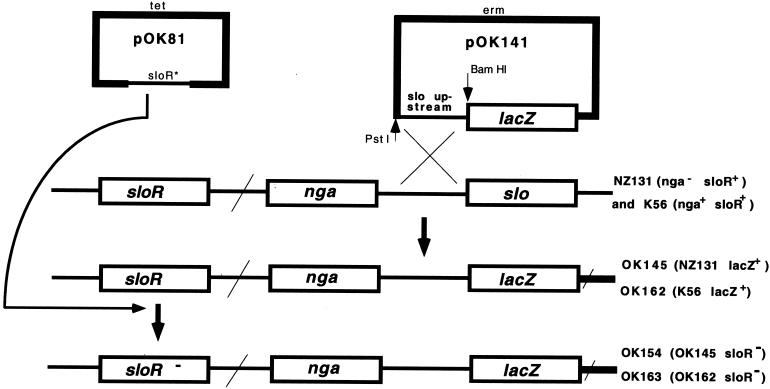
A similar experiment to that carried out with plasmid constructs as described above was performed with a set of mutants in which the reporter lacZ gene was inserted into the chromosome at its natural site. For that purpose, a mutant of pCAMP17 (pCAMP17-1) which had lost the ability to integrate at its specific chromosomal site (5) was used. As in the previously described construction procedure (Fig. 2), a piece of the slo upstream region (approximately 0.6 kb) was amplified by PCR, treated with PstI and BamHI enzymes, and ligated into the pCAMP17-1 mutant vector. The construct obtained (pOK141) (Fig. 3) was inserted into strain NZ131 by transformation. Confirmation that insertion in the chosen Ermr transformant occurred at the slo locus was accomplished by PCR with appropriate primers. In this way, the lacZ reporter and slo upstream region in the new strain OK145 were brought into continuity with the immediate upstream genes and the rest of the chromosome (Fig. 3). Previous results indicate that the nga gene is inactive in strain NZ131 (our unpublished results and reference 1). The nature of the nga mutation in strain NZ131 is unknown. However, similar absolute values of β-Gal activity obtained for deletion strains devoid of any incoming transcripts from the upstream DNA (Fig. 2) and strain OK145 (Fig. 4), as opposed to the β-Gal activity values obtained for strain OK162 with a wild-type allele of the nga gene, point to the polar nature of the nga mutation in strain NZ131 and its derivative OK145. These data suggest that all, or almost all, transcripts coming into the lacZ gene originate from the slo promoter.
FIG. 3.
Construction of strains OK145 and OK162 by integration of the recombinant plasmid pOK141 carrying the slo upstream region into the slo regions of the recipients NZ131 and K56, respectively. In the second step, the sloR derivatives of strain OK145 and strain OK162 (strains OK154 and OK163, respectively) were created by insertional inactivation by using the recombinant plasmid pOK81. The designation sloR* indicates that pOK81 carries an incomplete copy of the sloR gene (see the text). As indicated for the NZ131 genotype, strain NZ131 and its derivatives (OK145 and OK154) harbor the nga mutant allele. The scheme of strains OK154 and OK163, which are related to the sloR allele, is presented in simplified form, without inserted plasmid material and with two incomplete copies of the sloR gene. For details, see the text. The drawing is not to scale.
FIG. 4.
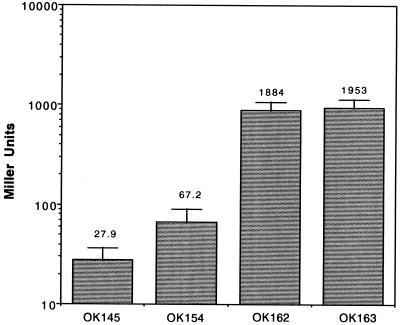
Comparison of lacZ reporter gene activities among strains with the wild-type or mutated alleles of the sloR gene. For a description of strain genotypes, see Fig. 3. The numbers above the columns denote average values in Miller units obtained in 10 (strains OK145 and OK154) or 5 (strains OK162 and OK163) independent experiments.
It has been shown that the phoR gene of Clostridium perfringens may regulate expression of the phoA gene as an inducer (12), although the hypothesis regarding the mechanism of action of the PhoR protein in regulation of the phoA gene was later questioned (3). The phoA gene encodes perfringolysin O, which together with SLO belongs to a single family of CDC pore-forming cytolysins (13). Our analysis of the S. pyogenes genome demonstrated a high degree of DNA sequence homology between the sloR gene (Spy146) of S. pyogenes, which maps to a location approximately 19 kb from the slo gene (4), and the phoR gene from C. perfringens. To test whether the sloR gene influences expression of the slo gene, the activities of β-Gal were compared for strains OK145 (nga sloR+) and OK154 (nga sloR). Strain OK154 was made by insertional inactivation as follows. The central part (around 800 bp) of the approximately 1-kb-long sloR gene was amplified with appropriate primers by using DNA from strain NZ131 as a substrate, cloned into pGEM-T Easy vector (Promega Corp.), and recloned into the insertional vector p7tet (9). The resulting construct (pOK81) (Fig. 3) was inserted by transformation into the OK145 recipient, and its insertion into the sloR gene was verified by PCR with appropriate primers (data not presented; Fig. 3). The resulting data demonstrate a 2.4-fold-higher level of activity of the reporter gene in strain OK154 (sloR) compared to that of the OK145 strain with the wild-type allele of the sloR gene (Fig. 4), indicating the repressor role of the SloR protein in regulation of the slo gene. This result is in contrast to the inducer role of pfoR gene in C. perfringens (12). As mentioned, a more detailed study of the role of the pfoR gene in the regulation of expression of the phoA gene (3) and the solution of the problem posed thereby await the isolation and analysis of a pfoR mutant in C. perfringens.
A pair of mutants with the same characteristics as those described above was made by using strain K56, which, in contrast to NZ131, possesses the wild-type allele of the nga gene (OK162 [nga+ sloR+] and OK163 [nga+ sloR]) (Fig. 3). As shown in Fig. 4, the β-Gal activities detected for these strains attained much higher values than those for strains OK145 and OK154. Also, no substantial difference was observed between strains carrying sloR+ or sloR alleles (Fig. 4). The levels of expression for the strains in the NZ131 background (OK145 versus OK154) differed significantly (P < 0.001), while those for the strains in the K56 background (OK162 versus OK163) did not (P > 1). These data confirm that the nga and slo genes are organized in an operon structure expressed from the strong common promoter upstream from the nga gene (8). They also indicate that the product of the sloR gene, which has been shown to be a DNA binding protein (12), does not affect transcription from the nga promoter but does affect expression of the slo promoter, which confirms ipso facto the previous results (Fig. 2) and points to the existence of an autonomous slo promoter. We explain the lack of significant differences in β-Gal activity between strains OK162 and OK163 and strains OK145 and OK154 by the relative strengths of the nga and slo promoters; that is, the overwhelming majority of mRNA coming into the lacZ gene in these strains possibly originates from the nga promoter, obscuring the effect of the sloR gene product at the weak slo promoter. That the sloR gene does not affect transcription from the nga promoter was confirmed in an independent experiment, in which no difference was found in NADase activity between strains K56 and OK156 (K56 sloR) (results not presented). The results presented here are in agreement with earlier findings (6, 7) and in contrast with the report of Caparon and colleagues, whose data indicated that a polar mutation in the nga gene reduces expression of the slo gene to undetectable levels (8). A detailed analysis of RNA transcripts in this region, including determination of the status of the hypothetical gene Spy166 (4), should provide an explanation for these disparate views.
The genetic data we present support the hypothesis of the existence of an internal slo promoter within the operon-like organized nga and slo genes which is regulated in a negative manner by the product of the sloR gene. The weak activity of this promoter does not necessarily reflect its potential under different physiological circumstances; it only reflects its activity during growth in Todd Hewitt broth with 0.2% yeast extract (THY) medium. The biological implication of the existence of an autonomous slo promoter is that it would increase genetic flexibility by providing the cell with a potential to divorce the SLO and NGA synthesis processes when necessary. That is, it is not difficult to imagine the SLO toxin as having another function in the infection process and pathogenesis besides its role in the NGA translocation system. Further studies of the regulatory mechanisms are needed to elucidate the functional relationship between these two genes.
Acknowledgments
We are grateful to C. Primaux for excellent technical assistance.
This work was supported by Public Health Service grants AI19304 and AI38406 from the National Institutes of Health to J.J.F.
Editor: E. I. Toumanen
REFERENCES
- 1.Ajdic, D., W. M. McShan, D. J. Savic, D. Gerlach, and J. J. Ferretti. 2000. The NAD-glycohydrolase (nga) gene of Streptococcus pyogenes. FEMS Microbiol. Lett. 191:235-241. [DOI] [PubMed] [Google Scholar]
- 2.Aluof, J. E. 1980. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxins). Pharmacol. Ther. 11:616-717. [DOI] [PubMed] [Google Scholar]
- 3.Cheung, J., and J. I. Rood. 2000. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J. Bacteriol. 182:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gase, K., J. J. Ferretti, C. Primeaux, and M. W. McShan. 1999. Identification, cloning, and expression of the CAMP factor gene (cfa) of group A streptococci. Infect. Immun. 67:4725-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehoe, M., and K. N. Timmis. 1984. Cloning and expression in Escherichia coli of the streptolysin O determinant from Streptococcus pyogenes: characterization of the cloned streptolysin O determinant and demonstration of the absence of substantial homology with determinants of other thiol-activated toxins. Infect. Immun. 43:804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehoe, M. A., L. Miller, J. Walker, and G. J. Boulnois. 1987. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging thiol-acivated toxins. Infect. Immun. 55:3228-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madden, J. C., N. Tuiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 9.McShan, M. W., R. W. McLaughlin, A. Norstrand, and J. J. Ferretti. 1998. Vectors containing streptococcal bacteriophage integrases for site-specific gene insertion. Methods Cell Sci. 20:51-57. [Google Scholar]
- 10.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Shatursky, O., A. P. Heuck, L. A. Shepard, J. Rossjohn, M. W. Parker, A. E. Johnson, and R. K. Tweten. 2000. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99:293-299. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu, T., A. Okabe, J. Minami, and H. Hayashi. 1991. An upstream regulatory sequence stimulates expression of the perfringolysin O gene of Clostridium perfringens. Infect. Immun. 59:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tweten, R. K. 1995. Pore-forming toxins of gram-positive bacteria, p. 207-229. In J. A. Roth et al. (ed.), Virulence mechanisms of bacterial pathogens, 2nd ed. ASM Press, Washington, D.C.