Abstract
The cadherin/catenin complex serves as an important structural component of adherens junctions in epithelial cells. Under certain conditions, β-catenin can be released from this complex and interact with transcription factors in the nucleus to stimulate the expression of genes that regulate apoptosis and cell cycle control. While studying the effects of the bacterial pathogen Chlamydia trachomatis on human cervical epithelial cells in culture, we observed that C. trachomatis caused the epithelial cells to separate from each other without detaching from their growing surface. The objective of the present study was to determine if this effect might involve the disruption of the cadherin/catenin complex. Primary cultures of human cervical epithelial cells or HeLa cells were infected with C. trachomatis serovar E. Cadherin-like immunoreactive materials and β-catenin were visualized by immunofluorescence. Preliminary studies showed that N-cadherin was the primary cadherin expressed in both the primary cultures and the HeLa cells. In noninfected cells, N-cadherin and β-catenin were colocalized at the intercellular junctional complexes. By contrast, the infected cells showed a marked loss of both N-cadherin and β-catenin labeling from the junctional complexes and the concomitant appearance of intense β-catenin labeling associated with the chlamydial inclusion. The results of Western blot analyses of extracts of C. trachomatis showed no evidence of cross-reactivity with the β-catenin antibody. These results indicate that C. trachomatis causes the breakdown of the N-cadherin/β-catenin complex and that the organism can sequester β-catenin within the chlamydial inclusion. This could represent an important mechanism by which C. trachomatis alters epithelial cell function.
Chlamydia trachomatis is an obligate intracellular bacterial pathogen that typically infects columnar epithelial cells but which may also infect a variety of other types of mammalian cells (24). The organism can exist as a metabolically dormant elementary body and an active reticulate body. The initial infection involves the attachment of the elementary body to the cell surface and the subsequent internalization of the organism. Inside the cell, the elementary body differentiates to form reticulate bodies that replicate within a vacuole, or “inclusion,” in the cytoplasm. Chlamydiae depend on their host cell for nutritional support and can alter cellular function to evade various intracellular defense mechanisms, inhibit apoptosis, direct vesicular traffic, disrupt cytoskeletal arrangements, and alter host cell signaling mechanisms (for reviews, see references 24 and 25).
Despite extensive research efforts, the exact mechanisms by which C. trachomatis causes derangements of host cell physiology remain poorly understood. During the course of studies to address this problem, we observed that infection of primary cultures of human cervical epithelial cells with C. trachomatis caused the cells to separate from each other without detaching from their growing surface. This suggested to us that C. trachomatis might somehow affect one or more of the molecular elements that maintain epithelial cell-cell adhesion.
One of the most important molecular determinants of epithelial cell-cell adhesion is the cadherin/catenin complex. The cadherins represent a diverse family of Ca2+-dependent cell adhesion molecules (for reviews, see references 23, 42, 51, and 56). Thus far, over 40 cadherins have been described, with the best characterized being the classical cadherins, such as E-, P-, and N-cadherin. These classical cadherins are integral Ca2+-binding glycoproteins that are usually localized at the adhering junctional complexes in epithelial cells (12, 16, 61). The cadherins are transmembrane proteins that contain an extracellular domain, a transmembrane domain, and an intracellular domain. The extracellular domain contains the Ca2+-binding sites, as well as the adhesive regions of the molecule. The binding of extracellular Ca2+ causes the molecule to assume a more rigid conformation and orients the adhesive regions so that the cadherin from one cell can interact, in a homologous manner, with a similar cadherin from an adjacent cell (1, 4, 40, 47, 48, 54). The intracellular domain of the cadherin is linked to β-catenin, which is also bound to α-catenin, which in turn links the entire complex to the actin cytoskeleton (2, 27, 36, 41, 43, 44, 52). In this context, the cadherin/catenin complex serves as a key structural component of adherens-type junctions
In addition to our observation that infection with C. trachomatis causes cervical epithelial cells to separate from each other, several other lines of evidence suggest that C. trachomatis might affect the cadherin/catenin complex. Majeed et al. (32-34) have shown that the infection of epithelial cells with C. trachomatis and the translocation of the bacterium within the cells involves Ca2+ ions and a reorganization of the actin cytoskeleton, both of which could directly or indirectly affect the cadherin/catenin complex. Moreover, while there is no direct information regarding the effects of chlamydiae on the cadherin/catenin complex, a large number of recent studies have shown that other bacterial pathogens, including various species of Listeria, Salmonella, Shigella, and Helicobacter, can interact with the cadherins, the catenins, or their associated proteins and that this mechanism may play a critical role in microbial infection and subsequent changes in cell physiology (28, 30, 31, 37, 39, 53, 57, 58). In light of these observations, we felt that it would be interesting to examine the effects of C. trachomatis on the integrity of the cadherin/catenin complex and the localization of β-catenin in human cervical epithelial cells.
MATERIALS AND METHODS
Cell culture and infection with C. trachomatis.
C. trachomatis serovar E (E-BOUR Y17 H8) was obtained from Todd Cotter and was derived from a seed stock originally supplied to him by Harlan Caldwell at Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, Mont. Stock organisms were propagated in our laboratory in HeLa 229 cells and purified by differential centrifugation as described previously (14). The stocks were assessed for viable chlamydiae by culture of sequential 10-fold dilutions in HeLa 229 monolayers and subsequent enumeration of inclusions by indirect immunofluorescence (17, 18). The remaining samples were frozen at −70°C in SPG buffer (0.25 M sucrose, 10 mM phosphate, and 5 mM l-glutamic acid, pH 7.3) until they were needed. Primary cultures of human cervical epithelial cells (CrEC-Ec) were purchased from Clonetics Inc. (San Diego, Calif.) and maintained in monolayer cultures in CC-3118 epithelial growth medium (Clonetics Inc.). In most experiments, the cervical epithelial cells or HeLa 229 cells were seeded onto glass coverslips in 24-well plates and grown to 80 to 90% confluence. The cells were then inoculated with C. trachomatis at a multiplicity of infection of 0.3, centrifuged at 1,500 × g at 36°C for 1 h, and incubated for 24 h in a humidified environment of 5% CO2. Following fixation with methanol, the monolayers were processed for fluorescence microscopy. All experiments included control samples in which cell monolayers were treated in the same manner except that C. trachomatis was omitted from the inoculation solution.
Fluorescence microscopy.
Preliminary immunofluorescence studies employing a panel of antibodies that specifically recognize either E-, P-, or N-cadherin, as well as a broad-spectrum pancadherin antibody, indicated that the primary cadherin that was expressed in both the primary cultures of the cervical epithelial cells and the HeLa cells was N-cadherin. For the present studies, N-cadherin was visualized by indirect immunofluorescence using the following procedure. Cells on glass coverslips were infected with C. trachomatis as described above. After 24 h, the samples were fixed in methanol at −20°C for 10 min. They were then incubated in 3% goat serum for 15 min (primary blocking serum). The samples were then incubated for 40 min with the primary antibody, a mouse anti-human N-cadherin (Transduction Labs, Lexington, Ky.), washed in phosphate-buffered saline (PBS), and incubated for 40 min in the secondary antibody, a fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G (IgG; Sigma, St. Louis, Mo.). The samples were washed in deionized water, mounted on glass slides in Aqua Polymount (Polysciences Inc., Warrington, Pa.), and viewed with a fluorescence microscope. β-Catenin and Chlamydia inclusions were visualized by indirect immunofluorescence using a dual-labeling procedure. Samples were fixed and permeabilized in −20°C methanol for 10 min and then blocked with 10% goat serum in PBS for 10 min. The samples were then incubated for 45 min at 37°C in PBS containing the primary antibodies, a polyclonal rabbit anti β-catenin (Zymed Laboratories, South San Francisco, Calif.) at 10 μg/ml and a mouse anti-Chlamydia genus-specific monoclonal antibody (14 M-3-B9) that recognizes a genus-specific epitope of the chlamydial lipopolysaccharide at a 1:300 dilution (18). To assess background labeling, some samples were incubated in the same manner except that the primary antibodies were omitted from the solution. The samples were rinsed quickly in PBS and then incubated for 30 min at 37°C in PBS containing the secondary antibodies, a tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG (Sigma) at a dilution of 1:100 and an FITC-conjugated goat anti-mouse IgG (Calbiochem, San Diego, Calif.) at a dilution of 1:60. To visualize DNA in the same samples, 0.1 μg of 4,6-diamido-2-phenylindole (DAPI)/ml was also included in the solution. The samples were rinsed successively in PBS and deionized water and then were mounted on glass slides in Aqua Polymount. The samples were allowed to dry in the dark and were viewed with a 100× objective lens on a Nikon Eclipse E400 epifluorescence microscope. Digital images of the cells were captured with a Spot 2 digital camera (Diagnostic Instruments Inc., Sterling Heights, Mich.) using constant exposure times and gain settings. For the labeling of β-catenin, the Chlamydia-specific antigen, and DAPI-reactive materials, the exposure times were 0.8 s (red), 1.0 s (green), and 0.8 s (blue), with a gain setting of 8. For the labeling of N-cadherin in the primary cell culture, the same exposure times were used, but the gain was set at 16. For the labeling of N-cadherin in the HeLa cells, the exposure times were increased threefold. The digital images were processed using the Image-Pro Plus image analysis software package (Media Cybernetics, Inc., Silver Spring, Md.). All experiments were repeated at least three times and were highly reproducible.
Western blot analysis of β-catenin.
To examine the possibility that C. trachomatis contains proteins that could cross-react with the β-catenin antibody, protein extracts of C. trachomatis, as well as extracts from the human cervical epithelial cells, were subjected to Western blot analysis. Chlamydia elementary bodies were purified from HeLa 229 cells by differential centrifugation followed by ultracentrifugation over a Renograffin density gradient as previously described (14). The purified elementary bodies were then inactivated with UV light (50), standardized according to protein content (BCA Protein Assay kit; Pierce Chemical Co., Rockford, Ill.), and frozen in sterile PBS at −80°C until they were used. Cervical epithelial cells and the isolated C. trachomatis cells were lysed with 1 ml of 4× lithium dodecyl sulfate sample buffer (Invitrogen, Carlsbad, Calif.) containing 0.5 M dithiothreitol (Invitrogen) at 90°C for 4 min. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis using a 4% stacking gel, a 10% separating gel, the standard Laemmli buffer system, and BlueRanger prestained protein molecular weight standards (Pierce Inc.). The separated proteins were electrophoretically transferred from the gels to polyvinylidene difluoride membranes. The membranes were blocked in 1% fat-free bovine serum albumin (Sigma)-10 mM Tris-100 mM NaCl-0.1% Tween 20 for 2 h and incubated with the mouse anti-β-catenin antibody dilution for 2 h at room temperature. The membrane was then incubated in an anti-mouse IgG peroxidase conjugate (Sigma) at a dilution of 1:20,000 for 2 h at room temperature. The β-catenin probe was visualized using Super Signal West Pico chemiluminescent substrate (Pierce), and the membrane was exposed to X-ray film for 10 s. To visualize total protein, the membrane was stained with GelCode Blue stain reagent (Pierce) according to the manufacturer's protocol.
RESULTS
C. trachomatis causes cervical epithelial cells to separate from each other.
Figure 1 shows the effect of C. trachomatis on the general morphology of the primary cultures of cervical epithelial cells. As may be seen in the phase-contrast image in Fig. 1A, the normal cells were closely associated with each other and exhibited an epithelium-like phenotype, although some of the cells were irregularly shaped and contained elongated processes that extended over adjacent cells. These normal cells showed no labeling with the Chlamydia-specific antibody (Fig. 1B). As may be seen in Fig. 1C, the Chlamydia-infected cells appeared to have separated from each other, as evidenced by the appearance of gaps between the cells. Labeling with the Chlamydia-specific antibody showed that approximately 25 to 30% of the cells contained a single large inclusion.
FIG. 1.
C. trachomatis causes cervical epithelial cells to separate from each other. Cells were infected with C. trachomatis and processed for the visualization of the Chlamydia antigen as described in Materials and Methods. (A) Phase-contrast view of normal cells; (B) fluorescence view showing a lack of Chlamydia labeling in the normal cells; (C) phase-contrast view of Chlamydia-infected cells; (D) fluorescence labeling of Chlamydia antigen in infected cells. The arrows indicate the gaps between the Chlamydia-infected cells. Original magnification, ×525.
Effects of C. trachomatis on localization of N-cadherin.
The results of preliminary studies (not shown) had indicated that the predominant cadherin that was expressed in the cervical epithelial cells was N-cadherin. As may be seen in Fig. 2A, N-cadherin was primarily localized at the contacts between the cells. Infection of the cells with C. trachomatis caused a marked loss of N-cadherin from the cell-cell contacts, as well as the retraction of cells from each other and the appearance of gaps between the cells (Fig. 2B). Other samples from this experiment that were labeled with the Chlamydia-specific antibody indicated that about 30% of the cells were infected (not shown).
FIG. 2.
Effect of C. trachomatis on the localization of N-cadherin. Normal and Chlamydia-infected cells were processed for the visualization of N-cadherin, as described in Materials and Methods. (A) Control cells; (B) Chlamydia-infected cells. Original magnification, ×525.
C. trachomatis accumulates β-catenin in the intracellular inclusion bodies.
Figure 3 shows the effects of C. trachomatis on the localization of β-catenin in primary cultures of human cervical epithelial cells. In the normal cells (Fig. 3A), β-catenin was localized at the contacts between the cells, with some diffuse labeling present in the cytoplasm and little or no labeling in the nuclei. As may be seen in Fig. 3B, infection with C. trachomatis caused a reduction in the intensity of β-catenin labeling at the cell-cell contacts. Of even greater importance was the observation that some of the cells showed intense intracellular labeling in well-defined structures that appeared to be shaped like the Chlamydia inclusions. Viewing the same samples with filter configurations to highlight the labeling with the Chlamydia-specific antibody (Fig. 2C and D) or the DAPI nucleic acid labeling (Fig. 2E and F) also indicated that the β-catenin was associated with the inclusion. The DAPI labeling was especially interesting, since it highlighted both the inclusions and the nuclei of the cervical epithelial cells.
FIG. 3.
C. trachomatis sequesters β-catenin. Normal cells and Chlamydia-infected cells were processed for the visualization of β-catenin, the Chlamydia-specific antigen, and DNA as described in Materials and Methods. (A) Control cells with β-catenin; (B) Chlamydia-infected cells with β-catenin; (C) control cells with Chlamydia-specific antibody; (D) Chlamydia-infected cells with Chlamydia-specific antibody; (E) control cells with DAPI labeling of DNA; (F) Chlamydia-infected cells with DAPI labeling of DNA. The arrows in panel F indicate the labeling of the DNA in the Chlamydia inclusions. Original magnification, ×525.
In order to rule out the possibility that the apparent β-catenin labeling of the inclusions resulted from fluorescence “spillover” when the FITC-labeled Chlamydia samples were viewed with the TRITC filter configuration used to visualize β-catenin, some samples of the Chlamydia-infected cells were labeled with the Chlamydia-specific antibody and the FITC tag and then viewed using the TRITC filter configuration. Under these conditions, the FITC-labeled Chlamydia tag exhibited no fluorescence (not shown), indicating that the apparent β-catenin labeling in the inclusion did not result from any fluorescence spillover.
To examine the possibility that the β-catenin labeling in the inclusions resulted from the cross-reactivity of the β-catenin antibody with a chlamydial protein(s), we first performed BLASTIN and BLASTX sequence similarity searches (5) using human β-catenin (GenBank accession number NM001904) as the query and the C. trachomatis genome. The results of these searches indicated that there were no statistically significant regions of similarity. In addition, extracts from concentrated isolates of C. trachomatis, as well as the cervical epithelial cells, were subjected to Western blot analysis using the β-catenin antibody as the probe. As may be seen in Fig. 4, protein extracts from the cervical epithelial cells showed pronounced β-catenin labeling at an apparent molecular mass of 98 kDa along with unidentified bands at 79 and <27 kDa. By contrast, no β-catenin labeling was evident in the extracts from C. trachomatis. The absence of labeling in the Chlamydia extracts indicates that there is no cross-reactivity between the β-catenin antibody and any chlamydial proteins. This strongly suggests that the β-catenin labeling of the inclusion is, in fact, due to the sequestering of mammalian β-catenin.
FIG. 4.
Western blot analysis of β-catenin. Protein extracts containing 3 μg of protein from concentrated isolates of C. trachomatis and cervical epithelial cells were subjected to Western blot analysis using the β-catenin probe as described in Materials and Methods. Protein extracts from the cervical epithelial cells showed pronounced β-catenin labeling at an apparent molecular mass of 98 kDa. In contrast, no β-catenin labeling was evident in the extracts from C. trachomatis.
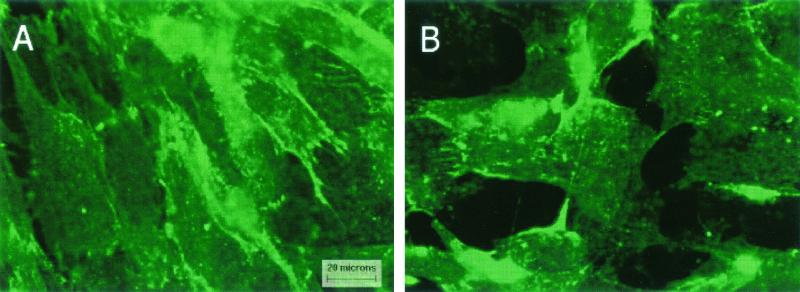
While this study was under review, the company from which we had purchased the primary cultures of cervical epithelial cells (Clonetics) instituted several changes in their policies for the distribution of primary cell cultures. Due to a delay in the implementation of the new policies, the primary cultures of the cervical epithelial cells were temporarily unavailable. In order to identify an alternate model system for our studies, we examined the effects of C. trachomatis in HeLa cells. As may be seen in Fig. 5, the normal HeLa cells expressed both N-cadherin (Fig. 5A) and β-catenin (Fig. 5C), which were colocalized at the cell-cell contacts, although the intensity of the labeling was slightly less than in the primary cell cultures. As in previous studies with primary cell cultures, infection of the HeLa cells with C. trachomatis caused a marked loss of both N-cadherin (Fig. 5B) and β-catenin (Fig. 5D) from the cell-cell contacts and the concomitant accumulation of β-catenin labeling in the inclusions (Fig. 5D and F).
FIG. 5.
Effects of C. trachomatis on N-cadherin and β-catenin in HeLa cells. HeLa 229 cells were infected with C. trachomatis as described in Materials and Methods and incubated for 36 h. Some of the samples were processed for the visualization of N-cadherin, whereas other samples were processed for the visualization of β-catenin and the Chlamydia-specific antigen by the dual-labeling procedure described in Materials and Methods. (A) Control cells with N-cadherin; (B) Chlamydia-infected cells with N-cadherin; (C) control cells with β-catenin; (D) Chlamydia-infected cells with β-catenin; (E) control cells with Chlamydia-specific antigen; (F) Chlamydia-infected cells with Chlamydia-specific antigen. Original magnification, ×525.
DISCUSSION
The results of these studies indicate that C. trachomatis disrupts N-cadherin-dependent cell-cell junctions and causes the breakdown of the N-cadherin/β-catenin complex in primary cultures of human cervical epithelial cells and in HeLa cells. Moreover, C. trachomatis appears to sequester β-catenin in its cytoplasmic inclusions. Each of these findings could have important implications regarding the mechanisms by which C. trachomatis produces its pathophysiologic effects. For example, the loss of N-cadherin-mediated cell-cell adhesion and the subsequent increase in epithelial paracellular permeability could help to explain some of the physiologic manifestations of acute Chlamydia infections, including inflammation, secretory response (i.e., watery discharge), and the associated increased susceptibility to infections by other pathogens, such as human immunodeficiency virus (59).
In considering the possible significance of our findings, it is important to note that in addition to serving as a structural component of adherens junctions, β-catenin functions as an important component of the so-called wingless, or Wnt, signaling pathway in the regulation of gene expression. Originally described in Drosophila, this pathway appears to be highly conserved in mammalian cells. The details of the Wnt/β-catenin signaling pathway and the possible roles that it may play in developmental biology and in the initiation and progression of cancer have recently been discussed in several excellent reviews (7, 9, 11, 13, 19, 49). The key elements of this signaling pathway are shown schematically in Fig. 6. Under certain conditions, β-catenin can be released from the junctional complexes into the cytosol, where it may be targeted for proteosomal degradation in a complex process that involves the adenomatous polyposis coli (APC) gene product, or it can be translocated to the nucleus, where it can bind to transcription factors, such as TCF/LEF1, and stimulate the expression of various genes that regulate apoptosis and cell cycle control (10, 11, 13, 19). Activation of β-catenin signaling has been implicated as a key regulator of a variety of physiologic processes, including cell growth and differentiation, the induction of apoptosis, and carcinogenesis (9, 10, 11, 13, 45).
FIG. 6.
Role of β-catenin in the wingless/Wnt nuclear signaling pathway.
In light of the importance of β-catenin as an intracellular signaling molecule, the apparent ability of C. trachomatis to accumulate β-catenin in the intracellular inclusions could be especially significant. One of the more intriguing questions that arises from this observation is, what biological advantage might C. trachomatis gain by sequestering β-catenin? While we can only speculate at this time, one possibility might be to prevent the infected cells from undergoing apoptosis. Previous studies have shown that cells infected with C. trachomatis become resistant to apoptotic stimuli (21, 22). At the same time, other studies have shown that the release of β-catenin from junctional complexes into the cytosol may be an important step in the initiation of apoptotic death in several types of cells (3, 15, 20, 29, 46, 60, 62). Perhaps, by binding β-catenin and disrupting one of the pathways that leads to apoptotic cell death, C. trachomatis might prolong the life of the infected cell so that the organism can develop and mature.
It should be noted that along with the decrease in junction-associated β-catenin in the infected cells, noninfected cells also showed a decrease in the amount of β-catenin at the cell-cell contacts. The most likely explanation for this phenomenon is that the disruption of cadherin/catenin-mediated adhesion in one cell indirectly affects adjacent cells. In noninfected cells that are adjacent to an infected cell, there would be nothing for the cadherin/catenin complex to adhere to on the surface of the infected cell. Since each cell is normally attached to many others, infection of just a few cells could affect catenin localization in many cells. Whether this has any functional consequences for β-catenin signaling in noninfected cells remains to be seen.
Another interesting question that arises is whether the effects of C. trachomatis on β-catenin may be related to the increased risk of cervical cancer that has been associated with C. trachomatis infections (6). The β-catenin-mediated nuclear signaling system has been implicated in a variety of existing cancers, and activation of this system has been suggested to play a role in the induction of oncogenes and the carcinogenic transformation of cells (7, 9, 11, 26, 35, 38, 55). Intuitively, it might seem that the ability of C. trachomatis to sequester β-catenin in the inclusion bodies would inhibit β-catenin-mediated nuclear signaling and, if anything, prevent the carcinogenic transformation of cells. However, it is important to note that the present study focused on the early stages of chlamydial infection and did not explore the long-term fate of the Chlamydia-β-catenin interaction. Specifically, chlamydial infection of a host cell in vivo may not always result in host cell lysis, as is normally observed in most in vitro systems. As reviewed by Beatty et al. (8), stressors, such as heat, cytokines, nutrient deprivation, or suboptimal antimicrobial therapy, may induce persistent nonlytic chlamydial infections of host cells and elaborate a structurally and physiologically altered form of the organism. Could such persistent infections prevent host cell lysis or apoptosis and thereby eventually allow a transformation event to occur? While we do not yet know the fate of β-catenin during the later stages of active or persistent infection, this would be an interesting area for future research.
While this study was under revision, Belland et al. (10) published a report showing that the C. trachomatis strains MoPn and D caused HeLa cells to separate from each other and that this effect may be mediated by a specific chlamydial cytotoxin that resembles known clostridial cytotoxins. At present, it is not clear whether this cytotoxin is involved in mediating the effects of C. trachomatis on N-cadherin and β-catenin, although this too would be an interesting area for future research.
Acknowledgments
Portions of this work were supported by Grant R01 ES06478 from the National Institute of Environmental Health Sciences.
We thank Victoria Seas for her help with the manuscript.
Editor: E. I. Tuomanen
REFERENCES
- 1.Adams, C. L., and W. J. Nelson. 1998. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10:152-157. [DOI] [PubMed] [Google Scholar]
- 2.Adams, C. L., W. J. Nelson, and S. J. Smith. 1996. Quantitative analysis of cadherin-catenin actin reorganization during development of cell-cell adhesion. J. Cell Biol. 135:1899-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed, Y., S. Hayashi, A. Levine, and E. Wieschaus. 1998. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93:1171-1182. [DOI] [PubMed] [Google Scholar]
- 4.Alattia, J. R., H. Kurokawa, and M. Ikura. 1999. Structural view of cadherin-mediated cell-cell adhesion. Cell. Mol. Life Sci. 55:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman,. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anttila, T., P. Saikku, P. Koskela, A. Bloigu, J. Dillner, I. Ikaheimo, E. Jellum, M. Lehtinen, P. Lenner, T. Hakulinen, A. Narvanen, E. Pukkala, S. Thoreson, L. Youngman, and J. Paavonen. 2001. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 285:47-51. [DOI] [PubMed] [Google Scholar]
- 7.Barker, N., P. Morin, and H. Clevers. 2000. The yin-yang of TCF/β-catenin signaling. Adv. Cancer Res. 77:1-24. [DOI] [PubMed] [Google Scholar]
- 8.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beavon, I. R. G. 2000. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur. J. Cancer 36:1607-1620. [DOI] [PubMed] [Google Scholar]
- 10.Belland, R. J., M. A., Scidmore, D. B. Crane, D. M. Hogan, W. Whitmire, G. McClarty, and H. D. Caldwell. 2001. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc. Natl. Acad. Sci. USA 98:13984-13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ze'ev, A., M. Shtutman, and J. Zhurinsky. 2000. The integration of cell adhesion with gene expression: the role of β-catenin. Exp. Cell Res. 261:75-82. [DOI] [PubMed] [Google Scholar]
- 12.Boller, K., D. Vestweber, and R. Kemler. 1985. Cell adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J. Cell Biol. 100:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeliet, P., M. G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, A. Zanetti, A. Angellilo, V. Mattot, D. Nuyens, E. Lutgens, F. Clotmann, M. C. de Ruiter, A. Gittenberger-de Groot, R. Poelmann, F. Lupu, J. M. Herbert, D. Collen, and E. Dejana. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147-157. [DOI] [PubMed] [Google Scholar]
- 16.Collares-Buzato, C. B., G. T. A. McEwan, M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Paracellular barrier and junctional protein distribution depend on basolateral Ca2+ in cultured epithelia. Biochim. Biophys. Acta 1222:147-158. [DOI] [PubMed] [Google Scholar]
- 17.Cotter, T. W., Q. Meng, Z. Shen, Y. Zhang, H. Su, and H. D. Caldwell. 1996. Protective efficacy of major outer membrane protein specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotter, T. W., G. S. Miranupuri, K. H. Ramsey, C. E. Poulsen, and G. I. Byrne. 1997. Reactivation of chlamydial genital tract infection in mice. Infect. Immun. 65:2067-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale, T. C. 1998. Signal transduction by the wnt family of ligands. Biochem. 329:209-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damalas, A., A. Ben-Ze'ev, I. Simcha, M. Shtutman, J. F. Leal, J. Zhurinsky, B. Geiger, and M. Oren. 1999. Excess beta-catenin promotes accumulation of transcriptionally active p53. EMBO J. 18:3054-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69:2442-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallin, W. J. 1998. Evolution of the “classical” cadherin family of cell adhesion molecules in vertebrates. Mol. Biol. E 15:1099-1107. [DOI] [PubMed] [Google Scholar]
- 24.Hackstadt, T. 1999. Cell Biology, p. 101-138. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 25.Hatch, T. 1999. Developmental biology, p. 29-67. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 26.He, T.-C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 27.Herrenknecht, K., M. Ozawa, C. Esckerskorn, F. Lottsperch, M. Lenter, and R. Kemler. 1991. The uvomorulin-anchorage protein alpha-catenin is a vinculin homologue. Proc. Natl. Acad. Sci. USA 88:9156-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jepson, M. A., C. B. Collares-Buzato, M. A. Clark, B. H. Hirst, and S. L. Simmons. 1995. Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect. Immun. 63:356-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, K., K. M. Pang, M. Evans, and E. D. Hay. 2000. Over-expression of β-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol. Biol. Cell 11:3509-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecuit, M., R. Hurme, J. Pizarro-Cerda, H. Ohayon, B. Geiger, and P. Cossant. 2000. A role for α and β-catenins in bacterial uptake. Proc. Natl. Acad. Sci. USA 97:10008-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecuit, M., S. Vandermael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 32.Majeed, M., and E. Kihlstrom. 1991. Mobilization of F-actin and clathrin during redistribution of Chlamydia trachomatis to an intracellular site in eucaryotic cells. Infect. Immun. 59:4465-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majeed, M., J. D. Ernst, K.-E. Magnusson, E. Kihlstrom, and O. Stendahl. 1994. Selective translocation of annexins during intracellular redistribution of Chlamydia trachomatis in HeLa and McCoy cells. Infect. Immun. 62:126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majeed, M., M. Gustafsson, E. Kihlstrom, and O. Stendahl. 1993. Roles of Ca2+ and F-actin in intracellular aggregation of Chlamydia trachomatis in eucaryotic cells. Infect. Immun. 61:1406-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann, B., M. Gelso, A. Siedow, M. L. Hanski, A. Grachev, M. Ilyas, W. F. Bodmer, M. P. Moyer, E. O. Riecken, H. J. Buhr, and C. Hanski. 1999. Target genes of β-catenin-T-cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 96:1603-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrea, P. D., C. W. Turck, and B. Gumbiner. 1991. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1350-1361. [DOI] [PubMed] [Google Scholar]
- 37.Mengaud, J., H. Ohayon, P. Gounon, R.-M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 38.Morin, P. J., A. B. Sparks, V. Korinek, N. Baker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275:1787-1790. [DOI] [PubMed] [Google Scholar]
- 39.Mounier, J., T. Vasselon, R. Hellio, M. Lesourd, and P. J. Sansonetti. 1992. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect. Immun. 60:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagar, B., M. Overduin, M. Ikura, and J. M. Rini. 1996. Structural basis of cadmium induced E-cadherin rigidification and dimerization. Nature 380:360-364. [DOI] [PubMed] [Google Scholar]
- 41.Nelson, W. J., E. M. Shore, A. Z. Wang, and R. W. Hammerton. 1990. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 110:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nollet, F., P. Kools, and F. van Roy. 2000. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 299:551-572. [DOI] [PubMed] [Google Scholar]
- 43.Norvell, S. M., and K. J. Green. 1998. Contributions of extracellular and intracellular domains of full length and chimeric cadherin molecules to junction assembly in epithelial cells. J. Cell Sci. 111:1305-1318. [DOI] [PubMed] [Google Scholar]
- 44.Ozawa, M., and R. Kemler. 1992. Molecular organization of the uvomorulin-catenin complex. J. Cell Biol. 116:989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson, C. A., and W. C. Prozialeck. 2001. E-cadherin, beta-catenin and cadmium carcinogenesis. Med. Hypotheses 56:573-581. [DOI] [PubMed] [Google Scholar]
- 46.Peluso, J. P., A. Pappalardo, and S. A. Hess. 2000. Effect of disrupting cell contact on the nuclear accumulation of β-catenin and subsequent apoptosis of rat ovarian surface epithelial cells in vitro. Endocrine 12:295-302. [DOI] [PubMed] [Google Scholar]
- 47.Pertz, O., D. Bozic, A. W. Koch, C. Fauser, A. Brancaccio, and J. Engel. 1999. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 18:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pokutta, S., K. Herrenknecht, R. Kemler, and J. Engel. 1994. Conformational changes in the recombinant extracellular domain of E-cadherin upon calcium binding. Eur. J. Biochem. 223:1019-1026. [DOI] [PubMed] [Google Scholar]
- 49.Potter, E., C. Bergwitz, and G. Brabant. 1999. The cadherin-catenin system: implications for growth and differentiation of endocrine tissues. Endocr. Rev. 20:207-239. [DOI] [PubMed] [Google Scholar]
- 50.Ramsey, K. H., W. J. Newhall, and R. G. Rank. 1989. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect. Immun. 57:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redies, C. 2000. Cadherins in the central nervous system. Prog. Neurobiol. 61:611-648. [DOI] [PubMed] [Google Scholar]
- 52.Ringwald, M., R. Schuh, D. Vestweber, H. Eistetter, F. Lottsperch, J. Engel, R. Dolz, F. Jahnig, J. Epplen, S. Mayer, C. Muller, and R. Kemler. 1987. The structure of cell adhesion molecule uvomorulin. Insights into the mechanisms of Ca2+-dependent cell adhesion. EMBO J. 6:3647-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sansonetti, J. P., J. Mounier, M. C. Prevost, and R.-M. Mege. 1994. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell 76:829-839. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro, L., A. M. Fannon, P. D. Kwong, A. Thompson, M. S. Lehmann, G. Grubel, J. F. Legrund, J. Als-Nielson, D. R. Coleman, and W. A. Hendrickson. 1995. Structural basis of cell-cell adhesion by cadherins. Nature 374:327-337. [DOI] [PubMed] [Google Scholar]
- 55.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeichi, M. 1990. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59:237-252. [DOI] [PubMed] [Google Scholar]
- 57.Terres, A. M., J. M. Pajares, D. O'Toole, S. Ahern, and D. Kelleher. 1998. H. pylori infection is associated with down regulation of E-cadherin, a molecule involved in epithelial cell adhesion and proliferation control. J. Clin. Pathol. 51:410-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran Van Nhieu, G., A. Ben-Ze'ev, and P. J. Sansonetti. 1997. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 16:2717-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 60.Wong, M. H., B. Rubinfeld, and J. I. Gordon. 1998. Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J. Cell Biol. 141:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yap, A. S., W. M. Brieher, and B. M. Gumbiner. 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 113:119-146. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, Z., H. Hartmann, V. M. Do, D. Abramowski, C. Sturchler-Pierrat, M. Staufenbiel, B. Sommer, M. van de Wetering, H. Clevers, P. Saftig, B. De Strooper, X. He, and B. A. Yankner. 1998. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395:698-702. [DOI] [PubMed] [Google Scholar]