Abstract
It was recently reported that the complement system may be critically involved in the febrile response of guinea pigs to systemic, particularly intraperitoneally (i.p.) injected, lipopolysaccharides (LPS). The present study was designed to identify which component(s) of the complement cascade may be specifically critical. To this end, we used mice with C3, C5, and CR2 gene deletions. To assess preliminarily the suitability of mice for such a study, we replicated our earlier studies with guinea pigs. Thus, to verify initially whether complement is similarly involved in the febrile response of wild-type (C57BL/6J) mice to i.p. LPS (Escherichia coli, 1 μg/mouse), we depleted complement with cobra venom factor (CVF; 7 U/mouse, intravenously [i.v.]). These animals did not develop fever, whereas the core temperature (Tc) of CVF vehicle-treated controls rose ∼1°C by 80 min postinjection and then gradually abated over the following 2.5 h, confirming the involvement of complement in fever production after i.p. LPS injection and the suitability of this species for these studies. C3- and C5-sufficient (C3+/+ and C5+/+) mice also developed 1°C fevers within 80 min after i.p. LPS (1 or 2 μg/mouse) injection. These fevers were totally prevented by CVF (10 U/mouse, i.v.) pretreatment. C3- and C5-deficient (C3−/− and C5−/−) mice were also unable to develop Tc rises after i.p. LPS. Both CR2+/+ and CR2−/− mice responded normally to i.p. LPS (1 μg/mouse). These data indicate that C5, but not C3d acting through CR2, may play a critical role in the febrile response of mice to i.p. LPS.
Bacterial endotoxic lipopolysaccharide (LPS) triggers the complement cascade via both the classical and alternative pathways, resulting in the production of multiple bioactive fragments (reviewed in reference 49). It was recently found that the complement system may be important in the febrile response of guinea pigs to, particularly, intraperitoneally (i.p.) administered LPS, because serum complement depletion caused by cobra venom factor (CVF) dose-dependently blocked fever due to i.p. LPS and because i.p. LPS per se promoted serum complement consumption (20, 38). However, it was not determined in those studies which specific component(s) of the complement cascade may be especially critical in this regard, because CVF activates the whole alternative pathway (8, 47) and the consequent serum complement level changes were assessed as the total hemolytic activity of complement.
Among the complement components generated by LPS, C3 and C5 are of particular interest in the context of fever because, among their various proinflammatory activities, they and their derivatives independently induce the production by myeloid and certain nonmyeloid cells of tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-6, all putative endogenous fever mediators (2, 6, 7, 42). C3 and C5 also amplify the secretions of TNF-α and IL-1β induced by LPS in monocytes and macrophages (6, 7) and are necessary for the in vivo acute-phase expression in mice of complement-reactive protein and serum amyloid P component (41). It may logically be assumed, therefore, that to the extent that the production of these pyrogenic mediators may be impaired in C3- and C5-deficient animals, their febrile response to LPS may be correspondingly attenuated.
Since there are no specific antibodies against guinea pig complement fragments readily available commercially, it is difficult to identify or to block specific guinea pig complement fragments. On the other hand, there are several complement fragment- or fragment receptor-deficient mice available for research. Therefore, in this study, we compared the febrile responses to i.p. LPS of C3- and C5-sufficient and -deficient mice to determine whether either or both of these components might mediate LPS fever development. To this end, we first replicated with wild-type (WT) mice previous studies with guinea pigs to verify whether depletion of complement by CVF also blocks LPS fever in mice, i.e., to confirm the involvement of complement in fever production in mice after i.p. LPS and thereby ensure the suitability of this species for the present experiments. We then compared the febrile responses of C3- and C5-sufficient and -deficient mice to i.p. LPS. CR2+/+ and CR2−/− mice were chosen as negative controls because CR2, as primarily a regulator of B-cell activation, was not expected to be involved in the early phase of LPS-induced fever genesis; moreover, the sensitivity of B cells to LPS is 2 orders of magnitude lower than that of macrophages. The results implicated the C5 fragment as the mediator of the fever caused by i.p. LPS in mice.
MATERIALS AND METHODS
Animals.
WT (C57BL/6J) mice and mice with congenital deletions of the C3, C5, and CR2 genes (C3−/−, C5−/−, and CR2−/−, respectively) and their corresponding complement-sufficient congenic controls (C3+/+, C5+/+, and CR2+/+) were used in these experiments. All the animals weighed 20 to 25 g on arrival. The WT and C5 (B10.D2 H-2d H2-T18c Hco/oSnj [C5−/−] and B10.D2 H-2d H2-T18c Hc1/nSnj [C5+/+]) mice were purchased from The Jackson Laboratory, Bar Harbor, Maine. The C3 (129/C57BL/6) and CR2 mice were provided by two of us (V.M.H. and S.A.B.) (24, 27, 39). The targeted gene deletions of the knockout mice were verified by their suppliers. All the complement-deficient mice appeared to be clinically well under the conditions of our normal laboratory housing.
Following receipt, the animals were quarantined for 3 weeks, four to a cage, before any experimental use. Tap water and food (Agway Prolab mouse diet) were available ad libitum. The ambient temperature (Ta) in the animal room was 22 ± 1°C; light and darkness were alternated, with light on from 0600 to 1800 h. After quarantine, to moderate the psychological stress associated with the experiments, the mice were trained to the experimental procedures for at least 2 weeks (4 h daily) by handling and placement in locally constructed, individual wire-mesh confiners designed to prevent their turning around and minimize their forward and backward movements, without causing restraint stress. To eliminate possible effects of circadian variations, all the experiments were begun at the same time of day (0830 h). On the experimental day, the mice were connected to the relevant measuring devices, placed in their confiners, and allowed to stabilize for at least 3 h until their core temperatures (Tc) varied not more than ±0.1°C over five consecutive 2-min periods. All the studies were performed in accordance with National Institutes of Health and institutional guidelines for animal care and use.
Drugs.
CVF (Naja naja kaouthia) was purchased from Calbiochem-Novabiochem (San Diego, Calif.). LPS was from Escherichia coli serotype O111:B4 (lot 36F4019, prepared by trichloroacetic acid extraction; protein content, 0.5%; Sigma Chemicals, St. Louis, Mo.). The vehicle for all the solutions was pyrogen-free saline (PFS; 0.9% NaCl, USP; Abbott Laboratories, Chicago, Ill.).
Depletion of complement.
To achieve virtually complete depletion of serum complement, based on previous experiments with guinea pigs (20, 38) and on the literature (10), CVF (7 or 10 U/animal) was injected into a tail vein of the mice. To minimize possible acute effects of decomplementation, this treatment was administered in two boluses. The first bolus of half the dose was given at 1430 h, 21 h before the actual experiments; the second half-dose was injected 2 h later. This schedule also served to minimize temporal deviations from our usual experimental protocol, i.e., beginning the experiments at 0830 h of the following day.
Temperature recording.
The animals, fully conscious in their confiners, were placed under a perforated hood (free airflow) to prevent undue disturbances from noise and fluctuations in Ta (22 ± 1°C). The Tcs of the mice were monitored constantly and recorded at 2-min intervals for the duration of the experiments on a Macintosh Plus 1-megabyte computer through an analog-to-digital converter, using precalibrated copper-constantan thermocouples inserted 2 cm into the colon and taped to the tail. The data were displayed on a video monitor, printed digitally on an Imagewriter printer, and stored on a disk for subsequent statistical analysis. Control measurements were begun when the animals' Tcs had become stabilized, as described above. The treatment pertinent to a given experiment, i.e., the i.p. injection of LPS (in PFS, 0.2 ml/mouse) or vehicle (PFS, 0.2 ml), was then administered, and the measurements continued according to the same routine as before treatment for the following 4 h.
Assay of total hemolytic complement activity.
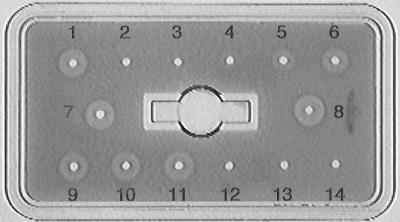
At the conclusion of the experimental periods, the mice were deeply anesthetized with methoxyflurane (Metofane, lot 012485; Schering-Plough, Union, N.J.), and blood was collected from their orbital sinuses, clotted on ice for 30 min, and centrifuged (Beckman Microfuge 12; 3,000 rpm, 4°C, 10 min). The resulting serum samples were stored at −70°C until assayed. For an assay, 5 μl of serum was added to wells placed in agarose gel containing standardized sheep erythrocytes sensitized with hemolysin (kit RC001.P; The Binding Site, San Diego, Calif.). The plates were incubated first for 18 h at 4°C and then for 1 h at 37°C. They were photographed with a charge-coupled device camera, and the images were stored and printed by using National Institutes of Health Image version 1.61 for analyzing electrophoretic gels. Since the kit was not as sensitive to mouse serum complement as to guinea pig serum complement (20, 38), it was not possible to assess serum complement quantitatively. Therefore, only qualitative, visual determinations of the presence or absence of complement were made (Fig. 1).
FIG. 1.
Total hemolytic activities of mouse serum complement. Well 1, quality control. Wells 2 to 8, dilutions of the standard using the manufacturer's standard. Wells 9 to 11, serum from WT mice, showing positive reactions (complement is present). Similar positive reactions were obtained with serum from C3+/+, C5+/+ CR2+/+, and CR2−/− mice (not shown). Wells 12 to 14, serum from C5−/− mice, showing no reaction (complement is absent). Similar negative reactions were obtained with serum from CVF-pretreated WT, C3+/+, and C5+/+ mice (not shown).
Experiments. (i) Experiment 1.
To verify the involvement of complement in the febrile response to i.p. LPS of WT mice, we administered CVF (7 U, in two half-doses) i.v. to these mice; the control solution was PFS. At 21 h after the first dose, the animals were injected i.p. with LPS (1 μg/animal) or vehicle (PFS).
(ii) Experiment 2.
Prior to determining whether C3 and/or C5 may be the complement fragment(s) that mediates the febrile response to i.p. LPS, we verified that complement is indeed also critically involved in the febrile response of C3+/+ and C5+/+ mice, since these originated from different strains than the WT mice (see “Animals” above). To this end, these mice were pretreated with i.v. CVF (7 or 10 U/animal) or vehicle (PFS) as in experiment 1. At 21 h after the first dose, they were injected i.p. with LPS (1 or 2 μg/animal) or its PFS.
(iii) Experiment 3.
To substantiate the role of C3 and/or C5 in the febrile response of mice to i.p. LPS, we administered PFS and LPS (1 or 2 μg/animal, i.p.) to C3+/+, C3−/−, C5+/+, and C5−/− mice. CR2+/+ and CR2−/− mice were the negative controls for this experiment and were treated similarly.
Statistical analyses.
Results are presented herein as means ± standard errors. The values of Tc are reported as the changes from basal (initial) values (Tci, the Tc at 2-min intervals averaged over the last 10 min of the preceding 3-h stabilization period). Student's paired t test was used to compare pre-LPS (basal) and post-LPS (maximal) data within a treatment. Differences between treatments were evaluated by a repeated-measure analysis of variance model, where factor 1 was the between-group factor (the experimental treatment) and factor 2 was the within-subject factor (the different sampling periods). Each variable was considered to be independent. The 5% level of probability was accepted as statistically significant.
RESULTS
Experiment 1.
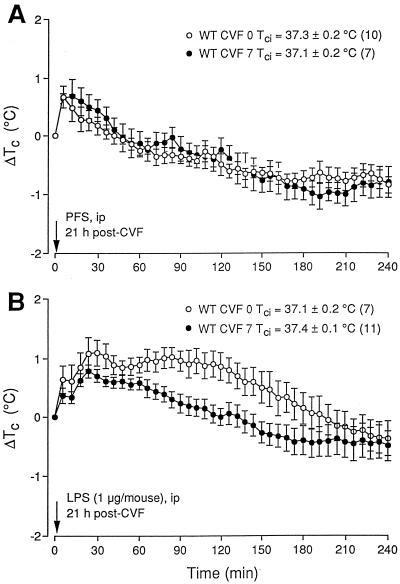
The i.p. injection procedure and the associated handling per se rapidly induced a transient, ca. 0.7°C rise in Tc, despite the prior training of these WT mice. It culminated within ca. 10 to 15 min and then abated over the following 50 to 60 min in the PFS-treated group (Fig. 2A). LPS administration, on the other hand, caused a further rise of Tc that reached a peak of ∼1.1°C above Tci by 30 min. Tc then declined slightly, rebounded, peaked a second time at ca. 80 min, and remained at essentially the same level over the following 30 min (Fig. 2B); it then gradually abated over the next 2 h. These responses to PFS and LPS were consistent with those in our previous studies using the same strain of mice (19, 21) as well as with the data of others for different strains in this Ta (17, 50). The first Tc rise at 10 min in both treatment groups was handling-stress induced; the superimposed Tc elevation and the second Tc rise of the LPS-treated group represented the pyrogenic response to LPS. CVF (7 U/animal), which depleted the serum complement to below detection (Fig. 1, wells 12 to 14), had no effect on the initial Tc rises of both groups but significantly attenuated the superimposed elevation and especially the second peak in the LPS-treated mice.
FIG. 2.
Effects of i.p. injected PFS (0.2 ml) (A) and E. coli LPS (1 μg/animal in 0.2 ml of PFS) (B) on the Tc of WT C57BL/6J mice pretreated with PFS (CVF 0) or 7 U of CVF/animal. A 3-h stabilization period preceded collection of data. The Tcs are expressed as differences (ΔTc) relative to their initial level (Tci); the values are means ± standard errors. CVF was given i.v. 21 h before the measurements in two half-doses (see Materials and Methods). PFS and LPS were given at time 0. Numbers in parentheses are number of animals.
Experiment 2.
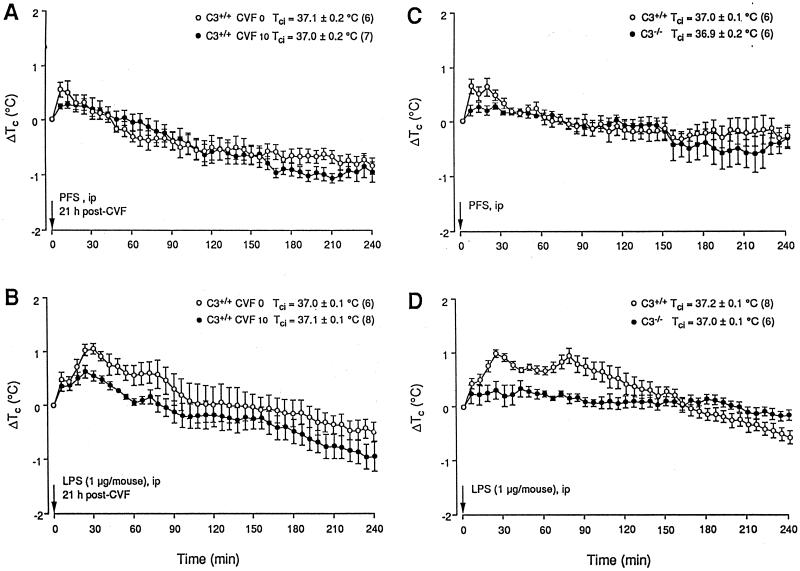
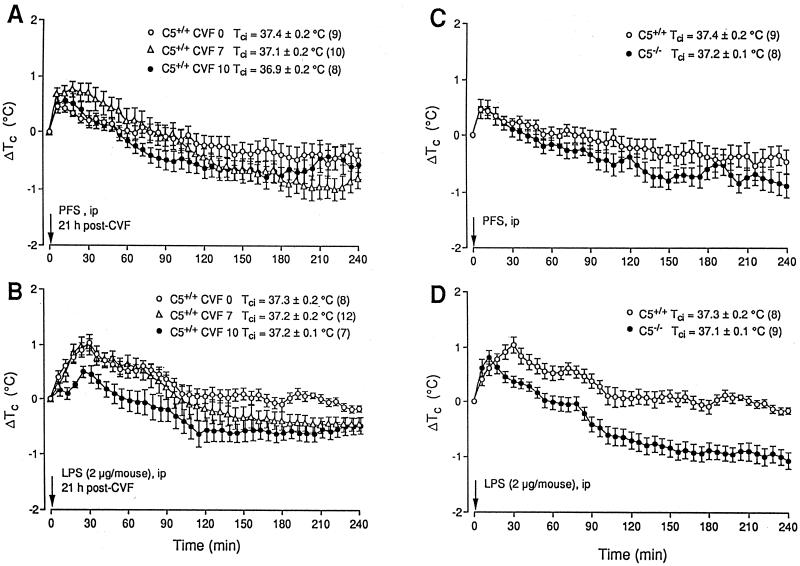
The i.p. administration of PFS to C3- and C5-sufficient and CVF-pretreated C3- and C5-sufficient mice caused no demonstrable thermal effect other than the temporary, initial Tc rise associated with handling and the injection itself (Fig. 3A and C and 4A and C). CVF vehicle-pretreated C3+/+ and C5+/+ mice both developed two-peaked, ca. 1°C fevers at 30 and 80 min after LPS, respectively (Fig. 3 and 4B). These tended to abate more quickly (∼60 min) than the fevers of the WT mice (Fig. 2B). CVF (7 U/animal), which inconsistently reduced the serum complement of the C5+/+ mice (Table 1), had no significant effect on the fevers of these animals (Fig. 4B), whereas 10 U of CVF, which depleted the serum complement of both the C3+/+ and C5+/+ mice to below detection (Table 1), blocked their fevers in the same manner as in the WT mice (Fig. 3B and 4B) (P < 0.001 and F = 15.5968 [C3+/+] and P < 0.001 and F = 16.5574 [C5+/+] compared with mice receiving 0 U of CVF).
FIG. 3.
Effects of i.p. injected PFS (0.2 ml) and E. coli LPS (1 μg/animal in 0.2 ml of PFS) on the Tc of C3+/+ mice pretreated i.v. with PFS (CVF 0) or 10 U of CVF/animal (A and B) and of C3+/+ and C3−/− mice (C and D). Conditions and notations are as described for Fig. 2.
FIG. 4.
Effects of i.p. injected PFS (0.2 ml) and E. coli LPS (2 μg/animal in 0.2 ml of PFS) on the Tc of C5+/+ mice pretreated i.v. with PFS (CVF 0) or 7 or 10 U of CVF (A and B) and of C5+/+ and C5−/− mice (C and D). Conditions and notations are as described for Fig. 2.
TABLE 1.
Serum hemolytic complement activity of various strains of mice after LPS challenge
| Mouse strain | CVF (U)a | Hemolytic C activityb |
|---|---|---|
| WT | 0 | + |
| 7 | − | |
| C3+/+ | 0 | + |
| 10 | − | |
| C3−/− | — | − |
| C5+/+ | 0 | + |
| 7 | ± | |
| 10 | − | |
| C5−/− | — | − |
| CR2+/+ | — | + |
| CR2−/− | — | + |
CVF or its vehicle (PFS) was injected iv 21 h before the LPS challenge. —, no CVF pretreatment.
+, present;−, absent;±, inconsistently present.
Experiment 3.
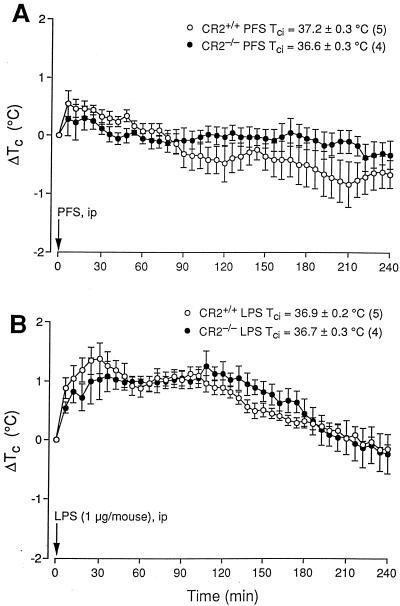
In contrast to the C3+/+ and C5+/+ mice, the C3−/− and C5−/− mice did not develop febrile rises in response to i.p. LPS (Fig. 3D and 4D) (P < 0.001 and F = 16.9416 compared with C3+/+ mice; P < 0.001 and F = 16.9768 compared with C5+/+ mice). Indeed, the handling-stress hyperthermia appeared to be attenuated in the C3−/− mice (Fig. 3D), although not in the C5−/− mice (Fig. 4D). CR2−/− mice, which exhibited serum complement levels similar to those of their CR2+/+controls (Table 1), developed Tc rises in response to handling and to i.p. PFS (Fig. 5A) and LPS (Fig. 5B) also not significantly different from those of their controls. After LPS, the first febrile peak occurred at 30 min and the second occurred at ∼100 min. These fevers then abated over the following 2 h (Fig. 5B).
FIG. 5.
Effects of i.p. injected PFS (0.2 ml) (A) and E. coli LPS (1 μg/animal in 0.2 ml of PFS) (B) on the Tc of CR2+/+ and CR2−/− mice. Conditions and notations are as described for Fig. 2.
DISCUSSION
The present results show that the complement system is critically involved in the febrile response to i.p. LPS of the three strains of mice used in this study, since both decomplementation by CVF and complement fragment-targeted gene disruption inhibited the ability of these animals to develop fever after challenge by low doses of this exogenous pyrogen. These data further show that C3 and/or C5 may be the fragment(s) that mediates i.p.-LPS-induced fever, since, in contrast to their congenic, complement-sufficient controls, both C3- and C5-deficient mice were unable to mount fevers in response to this challenge. However, it may be speculated that C5 rather than C3 could be the key player in this instance, since C3 knockouts would not generate C5 whereas C5−/− mice would not lack C3. On the other hand, both CR2+/+ and CR2−/− mice responded normally to i.p. LPS, indicating, as expected, that C3d acting through this receptor likely is not involved in the febrile response to this pyrogen. Since macrophages can synthesize most of the various components of the classical and alternative pathways of complement and little complement diffuses from the plasma into the peritoneal fluid under normal conditions, the complement initially activated by LPS in the peritoneum was thus presumably derived principally from the peritoneal macrophages themselves (3).
It is well established that LPS almost immediately activates the complement cascade via both the classical and alternative pathways (reviewed in reference 49). The important involvement of complement in various LPS-induced host defense responses is very well documented (40, 45, 48). Lately, complement has also been implicated in the LPS-induced expression of the critical acute-phase proteins complement-reactive protein and serum amyloid P component (41), and other data have shown that under conditions of complement deficiency, responses that are normally coactivated with the febrile response, e.g., phagocytosis, chemotaxis, and production and release of various inflammatory mediators, are impaired (22, 40). Hence, a role for complement in LPS fever production would be congruent with these related host defense responses, as we demonstrated earlier in guinea pigs (20, 38). No other studies to date appear to have systematically examined the role of complement in fever, although it was noted in one study of canine endotoxic shock that congenitally C3-deficient Brittany spaniels exhibited reduced fevers during the first 24 h after i.v. E. coli LPS (2 mg/kg) treatment compared with their congenic controls (34). Also, Mickenberg et al. (25, 26) found that the serum total hemolytic complement activity and C3 titers fell in rabbits within 5 min after the i.v. administration of low-dose, soluble antigen-antibody complexes and that rabbits depleted of complement by pretreatment with CVF exhibited greatly diminished febrile responses in comparison with untreated controls. On the other hand, congenitally complement-deficient, clinically infected patients can present with fever (29), and, indeed, there is one interesting report of a C3-deficient child with an elevated Tc without a concurrent infection (32). Further, in one study of human subjects injected i.v. with relatively low doses of E. coli LPS, no change in anaphylatoxin levels was detected in the plasma and Tc rose normally 30 to 45 min after the injection (46). In guinea pigs, by contrast, pyrogenic doses of both i.v. and i.p. LPS are associated with early, transient, but significant decreases in serum CH100 activity as well as fever (20). Thus, guinea pigs and mice seem to be similar in their dependence on complement for the induction of their febrile response to LPS. The apparently discrepant independence of complement of human endotoxemic fever could be due to the fact that the pathogenesis of clinical infections is more complicated than is shown by specific animal experimental models, generally involving, in addition to LPS, many inflammatory factors not incorporating complement mediation. Moreover, the requirement for complement can be bypassed with higher LPS loads (20).
Unfortunately, the present data do not indicate whether the a or the b cleavage products of C5 might be the critical mediators of these i.p. LPS-induced fevers or which cell type(s) in the peritoneum might be its specific target(s). A priori, C5a might be considered the more likely candidate mediator in the present animal model because it has been demonstrated to induce, synergistically with LPS, TNF-α and IL-1β production by mouse peritoneal macrophages (36). It also mediates the release of IL-6 by human monocytes (36). These cytokines are endogenous pyrogens, and putative IL-1β binding sites have been identified on vagal sensory terminals in the abdomen that, according to one model, could convey the pyrogenic signals to the brain (reviewed in reference 4). The absence of C5a could therefore impair the production of these cytokines and, consequently, abolish the febrile response. However, it seems unlikely that this effect could solely and specifically account for the suppression of fever observed here unless the amount of C5a required to activate these macrophages under normocomplementemic conditions is very small and uniquely localized to the i.p. site of the injected LPS bolus, since its concentration in uninflamed peritoneal fluid is normally minimal and activation of C3 by CVF does not cause by itself a febrile response (20). On the contrary, it causes a fall in Tc (38), presumably consequent to the induction of coincident processes that combine to produce the systemic arterial hypotension typically observed after such a treatment or following the systemic injection of complement (23, 44). C5a, moreover, is rather short-lived, and its degradation product, C5a desArg, is relatively inactive in this respect. The possibility that C5a could directly bind to its putative receptors on local sensory terminals (30) and relay the pyrogenic message to the brain consequently also seems improbable in the present context, particularly since the induction of C5a receptors after LPS injection lags behind the onset of the febrile response (11).
An alternative role of C5a in the mediation of i.p. LPS-induced fever, however, may be more plausible. This role is related to the fact that C5a and C5a desArg have conventional, early functions of enhancing microvascular permeability and attracting leukocytes, thereby recruiting to the peritoneum factors that are necessary to initiate host defense responses but are normally sparse in peritoneal fluid. Thus, as for LPS, these factors include LPS-binding protein (LBP), which is synthesized mainly in hepatocytes and secreted into the bloodstream constitutively in small amounts (1 μg/ml in mice) or in larger amounts (5 μg/ml) as a class I acute-phase protein induced hours after challenge (37). Indeed, the concentration of this protein is minimal in uninflamed peritoneal fluid in vivo, although its abundant presence in inflamed peritoneal fluid in vivo (35) and its necessity for LPS activation of (murine) peritoneal macrophages have been demonstrated in vitro (15). LBP catalyzes the monomerization of LPS micelles and transfers the monomers as LPS-LBP complexes to another protein, CD14.
CD14 is present in plasma in a soluble form (sCD14) and as a molecule that is glycosylphosphatidylinositol-anchored to the macrophage plasma membrane (mCD14); mCD14 exists on peritoneal macrophages, but sCD14 is scarce in normal peritoneal fluid. Since neither form possesses a transmembrane domain, signal transduction is achieved by the LPS-LBP-CD14 complex cross-linking, in turn, to an ancillary cell membrane-associated receptor subunit (9), which in mouse peritoneal macrophages has been identified as the Toll-like receptor 4-MD-2 complex (1). If LBP is indeed absent or minimally present in the peritoneums of normal mice and given that, in its absence, polymeric LPS at low concentrations binds poorly to mouse peritoneal macrophages and fails to stimulate the production of TNF-α, IL-1β, and IL-6 (14, 31), the role of C5a might indeed be to increase vascular permeability and promote postcapillary venular leakage of plasma LBP into the peritoneal locus of the injected LPS aggregates as well as to attract neutrophils to this site. In fact, C3-deficient mice (hence also lacking C5) clear i.p. injected LPS, recruit neutrophils to the peritoneum more slowly, and produce less TNF-α than do C3-sufficient animals (33). The entry of sCD14 into the peritoneum under these conditions would also be highly complementary to the febrigenic process because recent evidence has suggested that the activation of polymorphonuclear leukocytes by LPS that results in the production of mediators involves the binding of LPS-sCD14 complexes to the cells' plasma membranes, followed by internalization and localization in the Golgi apparatus (43), in contrast to the delivery of LPS polymers via mCD14 to phagosomes, which results in the lysosomal enzymatic degradation of LPS but not in the production of mediators (12, 16).
Moreover, since only minute amounts of complement diffuse from the plasma into the uninflamed peritoneum, the simultaneous greater influx of complement under these conditions would amplify the effects of the locally generated complement. Indeed, this translocation of complement could account for the initial, transient decline in plasma complement levels previously observed in guinea pigs after i.p. LPS (20). If this scenario is valid, fever induced by i.v. LPS should not be complement dependent, due to the constitutive presence of LBP and sCD14 in plasma in adequate concentrations. This seems indeed to be the case in guinea pigs, since hypocomplementemic guinea pigs developed normal febrile responses to i.v. LPS (38). The objection to this argument that LPS is detectable in plasma (18) within 15 min after its i.p. injection and, therefore, should produce a febrile response similar to that produced upon i.v. injection can be countered by the fact that LPS entering the circulation from the peritoneum does so slowly and incrementally, i.e., in amounts small enough to be readily cleared by the relevant counteracting mechanisms available in plasma (sCD14, bactericidal/permeability-increasing protein, lipoproteins, etc.). Indeed, 5 μg of LPS/kg, i.p., did not produce increased plasma IL-1β concentrations in rats (18), and a correspondingly low i.p. dose of fluorescein isothiocyanate-labeled LPS was not detectable in guinea pig livers 15 min after injection (20). Higher i.p. doses of LPS, however, may appear in plasma more quickly (18, 28) and, therefore, be less dependent on complement (5, 20, 28). Finally, an involvement of C5b-9 is also possible, since membrane attack complex in sublytic concentrations has been reported to activate monocytes to release IL-1β (13).
Taken together, the present results are consistent with previous observations obtained with guinea pigs that complement is importantly involved in the febrile response to low-dose, i.p. injected LPS; the cascade fragment C5 appears to be especially critical for this response. However, which of its transcripts, a and/or b, may mediate fever and by what mechanism(s) this may be accomplished remain speculative. We suggest that C5a-enhanced microvascular permeability and the consequent, rapid influx from plasma of LBP, sCD14, and complement, all sparse in uninflamed peritoneal fluid, may enable peritoneal macrophages to more efficiently take up LPS and produce pyrogenic cytokines; their ability to do so is greatly limited in the absence of these plasma proteins. The associated recruitment of neutrophils augments their own capacity in this host defense process. C5b-C9 may also separately and additionally stimulate the production of cytokines.
Acknowledgments
This work was supported by Public Health Service grant NS-34857 from the National Institutes of Neurological Disorders and Stroke to C. M. Blatteis.
We thank Easter Jenkins for her efficient typographic help and Gregg Short and Daniel Morse for their excellent graphic arts support.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex in mouse peritoneal macrophages. J. Immunol. 164:3471-3475. [DOI] [PubMed] [Google Scholar]
- 2.Bacle, F., N. Haeffner-Cavaillon, M. Laude, C. Couturier, and M. D. Kazatchkine. 1990. Induction of IL-1 release through stimulation of the C3b/C4b complement receptor type one (CR1, CD35) on human monocytes. J. Immunol. 144:147-152. [PubMed] [Google Scholar]
- 3.Bird, G., G. Senaldi, M. Panos, N. Rolando, G. Alexander, D. Vergani, and R. Williams. 1992. Activation of the classical complement pathway in spontaneous bacterial peritonitis. Gut 33:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatteis, C. M., E. Sehic, and S. Li. 2000. Pyrogen sensing and signaling: old views and new concepts. Clin. Infect. Dis. 31(Suppl. 5):S168-S177. [DOI] [PubMed] [Google Scholar]
- 5.Blatteis, C. M., E. Sehic, and S. Li. 2000. Complement and the pathogenesis of endotoxic fever. Int. J. Biometeorol. 43:176-183. [DOI] [PubMed] [Google Scholar]
- 6.Cavaillon, J. M., C. Fitting, and N. Haeffner-Cavaillon. 1990. Recombinant C5a enhances interleukin-1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocyte and macrophages. Eur. J. Immunol. 20:253-257. [DOI] [PubMed] [Google Scholar]
- 7.Cavaillon-Haeffner, N., J. M. Cavaillon, M. Laude, and M. D. Kazatchkine. 1987. C3a (C3a desArg) induces production and release of interleukin-1 by cultured human monocytes. J. Immunol. 139:794-799. [PubMed] [Google Scholar]
- 8.Cochrane, C. G., H. J. Müller-Eberhard, and B. S. Aikin. 1970. Depletion of plasma complement in vivo by a protein from cobra venom: its effects on various immunological reactions. J. Immunol 105:55-69. [PubMed] [Google Scholar]
- 9.da Silva Correia, J., K. Soldan, V. Christen, P. S. Tobias, and R. J. Ulevitch. 2001. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. J. Biol. Chem. 276:21129-21135. [DOI] [PubMed] [Google Scholar]
- 10.Fu, Q., P. G. Satyaswaroop, and D. C. Gowda. 1997. Tissue targeting and plasma clearance of cobra venom factor in mice. Biochem. Biophys. Res. Commun. 231:316-320. [DOI] [PubMed] [Google Scholar]
- 11.Fukuoka, Y., J. A. Ember, and T. E. Hugli. 1998. Cloning and characterization of rat C3a receptor: differential expression of rat C3a and C5a receptors by LPS stimulation. Biochem. Biophys. Res. Commun. 242:663-668. [DOI] [PubMed] [Google Scholar]
- 12.Gegner, J. A., R. J. Ulevitch, and P. S. Tobias. 1995. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J. Biol. Chem. 270:5320-5325. [DOI] [PubMed] [Google Scholar]
- 13.Hansch, G. M., M. Seitz, and M. Betz. 1987. Effect of the late complement components C5b-9 on human monocytes: release of prostanoids, oxygen radicals, and of a factor inducing cell proliferation. Int. Arch. Allergy Appl. Immunol. 82:317-320. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich, J.-M., M. Bernheiden, G. Minigo, K. K. Yang, C. Schütt, D. N. Männel, and R. S. Jack. 2001. The essential role of lipopolysaccharide-binding protein in protection of mice against a peritoneal Salmonella infection involves the rapid induction of an inflammatory response. J. Immunol. 167:1624-1628. [DOI] [PubMed] [Google Scholar]
- 15.Heumann, D., Y. Adashi, D. LeRoy, N. Ohno, T. Yadomae, M. P. Glauser, and T. Calandra. 2001. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect. Immun. 69:378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchens, R. L., and R. S. Munford. 1998. CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J. Immunol. 160:1920-1928. [PubMed] [Google Scholar]
- 17.Kozak, W., C. A. Conn, and M. J. Kluger. 1994. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am. J. Physiol. 266:R125-R135. [DOI] [PubMed] [Google Scholar]
- 18.Lenczowski, M. J. P., A.-M. Van Dam, S. Poole, J. W. Larrick, and F. J. H. Tilders. 1997. Role of circulating endotoxin in interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am. J. Physiol. 273:R1870-R1877. [DOI] [PubMed] [Google Scholar]
- 19.Li, S., L. R. Ballou, S. G. Morham, and C. M. Blatteis. 2001. Cyclooxygenase-2 mediates the febrile response of mice to interleukin-1β. Brain Res. 910:163-173. [DOI] [PubMed] [Google Scholar]
- 20.Li, S., E. Sehic, Y. Wang, A. L. Ungar, and C. M. Blatteis. 1999. Relation between complement and the febrile response of guinea pigs to systemic endotoxin. Am. J. Physiol. 277:R1635-R1645. [DOI] [PubMed] [Google Scholar]
- 21.Li, S., Y. Wang, K. Matsumura, L. R. Ballou, S. G. Morham, and C. M. Blatteis. 1999. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2−/−, but not in cyclooxygenase-1−/− mice. Brain Res. 825:86-94. [DOI] [PubMed] [Google Scholar]
- 22.Linton, S. 2000. Inherited complement deficiencies in animals. Methods Mol. Biol. 150:229-247. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg, C., F. Marceau, and T. E. Hugli. 1987. C5a-induced hemodynamic and hematologic changes in the rabbit. Role of cyclooxygenase products and polymorphonuclear leukocytes. Am. J. Pathol. 128:471-483. [PMC free article] [PubMed] [Google Scholar]
- 24.Marchbank, K. J., C. C. Watson, D. F. Ritsema, and V. M. Holers. 2000. Expression of human complement receptor 2 (CR2, CD21) in CR2−/− mice restores humoral immune function. J. Immunol. 165:2354-2361. [DOI] [PubMed] [Google Scholar]
- 25.Mickenberg, L. D., R. Snyderman, R. K. Root, S. E. Mergenhagen, and S. M. Wolff. 1971. Immune fever in the rabbit: response of the hematologic and complement systems. J. Immunol. 107:1457-1465. [PubMed] [Google Scholar]
- 26.Mickenberg, I. D., R. Snyderman, R. K. Root, S. E. Mergenhagen, and S. M. Wolff. 1971. The relationship of complement consumption to immune fever. J. Immunol. 107:1466-1476. [PubMed] [Google Scholar]
- 27.Molina, H., V. M. Holers, B. Li, Y. F. Fang, S. Mariathasan, J. Goellner, J. Strauss-Schoenberger, R. W. Karr, and D. D. Chaplin. 1996. Markedly impaired humoral immune response of mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA 93:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore, E. D., Jr., N. A. Moss, A. Revhang, D. Wilmore, J. A. Mannick, and R. L. Rodrick. 1987. A single dose of endotoxin activates neutrophils without activating complement. Surgery 102:200-205. [PubMed] [Google Scholar]
- 29.Morgan, B. P. 1990. Complement: clinical aspects and relevance to disease. Academic Press, New York, N.Y.
- 30.Nataf, S., P. F. Stahel, N. Davoust, and S. K. Durum. 1999. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 22:397-402. [DOI] [PubMed] [Google Scholar]
- 31.Netea, M. G., B. J. Kullberg, and J. W. M. Van der Meer. 1998. Lipopolysaccharide-induced production of tumor necrosis factor and interleukin-1 is differentially regulated at the receptor level: the role of CD14-dependent and CD14-independent pathways. Immunology 94:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osofsky, S. G., B. H. Thompson, T. F. Lint, and H. Gewurz. 1977. Hereditary deficiency of the third component of complement in a child with fever, skin rash, and arthralgia: response to transfusion of whole blood. J. Pediatr. 190:180-186. [DOI] [PubMed] [Google Scholar]
- 33.Prodeus, A. P., X. Zhou, M. Maurer, S. J. Galli, and M. C. Carroll. 1997. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature 390:172-175. [DOI] [PubMed] [Google Scholar]
- 34.Quezado, Z. M. N., W. D. Hoffman, J. A. Winkelstein, I. Yatsiv, C. A. Koev, L. C. Cork, R. J. Elin, P. Q. Eichacker, and C. Natanson. 1994. The third component of complement protects against Escherichia coli endotoxin-induced shock and multiple organ failure. J. Exp. Med. 179:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schäfer, K., R. R. Schumann, S. Stöteknuel, P. Schollmeyer, and G. J. Dobos. 1998. Lipopolysaccharide-binding protein is present in effluents of patients with Gram-negative and Gram-positive peritonitis. Nephrol. Dial. Transplant. 13:969-974. [DOI] [PubMed] [Google Scholar]
- 36.Scholz, W., M. R. McClurg, G. J. Cardenas, M. Smith, D. J. Noonan, T. E. Hugli, and E. L. Morgan. 1990. C5a-mediated release of interleukin-6 by human monocytes. Clin. Immunol. Immunopathol. 57:297-307. [DOI] [PubMed] [Google Scholar]
- 37.Schumann, R. R., and E. Latz. 2000. Lipopolysaccharide-binding protein. Chem. Immunol 74:42-60. [DOI] [PubMed] [Google Scholar]
- 38.Sehic, E., S. Li, A. L. Ungar, and C. M. Blatteis. 1998. Complement reduction impairs the febrile response of guinea pigs to endotoxin. Am. J. Physiol. 274:R1594-R1603. [DOI] [PubMed] [Google Scholar]
- 39.Sekine, H., C. M. Reilly, I. D. Molano, G. Garnier, A. Circolo, P. Ruiz, V. M. Holers, S. A. Boackle, and G. S. Gilkeson. 2001. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J. Immunol. 166:6444-6451. [DOI] [PubMed] [Google Scholar]
- 40.Song, W.-C., M. R. Sarrias, and J. D. Lambris. 2000. Complement and innate immunity. Immunopharmacology 49:187-198. [DOI] [PubMed] [Google Scholar]
- 41.Szalai, A. J., F. W. van Ginkel, Y. Wang, J. R. McGhee, and J. E. Volanakis. 2000. Complement-dependent acute phase expression of C-reactive protein and serum amyloid P-component. J. Immunol. 165:1030-1035. [DOI] [PubMed] [Google Scholar]
- 42.Takabayashi, T., E. Vannier, J. F. Burke, R. G. Tompkins, J. A. Gelfand, and B. D. Clark. 1998. Both C3a and C3a desArg regulate interleukin-6 synthesis in human peripheral blood mononuclear cells. J. Infect. Dis. 177:1622-1628. [DOI] [PubMed] [Google Scholar]
- 43.Thieblemont, N., and S. D. Wright. 1999. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 190:523-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulevitch, R. J., C. G. Cochrane, P. M. Hinson, S. C. Morrison, and W. F. Doe. 1975. Mediation systems in bacterial lipopolysaccharide-induced hypotension and disseminated intravascular coagulation. I. The role of complement. J. Exp. Med. 142:1570-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 46.van Deventer, S. J. H., H. R. Büller, J. W. ten Cate, L. A. Tarden, C. E. Hack, and A. Sturk. 1990. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 76:2820-2826. [PubMed] [Google Scholar]
- 47.Vogel, C.-W., R. Bredehorst, D. C. Fritzinger, T. Grunwald, P. Ziegelmüller, and M. A. Koek. 1996. Structure and function of cobra venom factor, the complement-activating protein in cobra venom. Adv. Exp. Med. 391:97-114. [DOI] [PubMed] [Google Scholar]
- 48.Volanakis, J. E., and M. M. Frank. 1998. The human complement system in health and disease. Marcel Dekker, New York, N.Y.
- 49.Vukajlovich, S. W. 1992. Interactions of LPS with serum complement, p. 213-235. In J. L. Ryan and D. C. Morrison (ed.), Bacterial endotoxic lipopolysaccharides, vol. II. CRC Press, Boca Raton, Fla. [Google Scholar]
- 50.Wang, J., T. Ando, and A. J. Dunn. 1997. Effect of homologous interleukin-1, interleukin-6 and tumor necrosis factor-alpha on the core body temperature of mice. Neuroimmunomodulation 4:230-236. [DOI] [PubMed] [Google Scholar]