Abstract
Antiidiotypic monoclonal antibodies (MAbs) representing the internal image of a yeast killer toxin (KT) have therapeutic potential against several fungal infections. The efficacy of KT MAbs against Aspergillus fumigatus was investigated in a mouse model of T-cell-depleted allogeneic bone marrow transplantation (BMT) with invasive pulmonary aspergillosis. Mice were highly susceptible to infection at 3 days post-BMT, when profound neutropenia was observed both in the periphery and in the lungs. Treatment with KT MAbs protected the mice from infection, as judged by the long-term survival and decreased pathology associated with inhibition of fungal growth and hyphal development in the lungs. In vitro, similar to polymorphonuclear neutrophils, KT MAbs significantly inhibited the hyphal development and metabolic activity of germinated Aspergillus conidia. These results indicate that mimicking the action of neutrophils could be a strategy through which KT MAbs exert therapeutic efficacy in A. fumigatus infections.
Invasive fungal infections remain a major problem for bone marrow (BM) transplant (BMT) recipients (35, 45). Mortality from opportunistic fungal infections exceeds 50% in most studies and has been reported to be as high as 95% in allogeneic BMT recipients with Aspergillus sp. infection, despite aggressive antifungal therapy (4).
Studies in vitro and in animal models have indicated that the innate defenses are primarily responsible for the elimination of inhaled conidia from the lungs (10, 13, 19, 38). Early fungal clearance is mediated by a dual phagocytic system involving both alveolar macrophages and recruited polymorphonuclear leukocytes capable of efficiently opposing fungal infectivity at the level of conidia or hyphal forms (37). However, the killing of phagocytosed conidia by mononuclear cells is a slow process that occurs with a low killing rate and depends on the immunocompetence of effector monocytes (19). Moreover, the finding that the conidiocidal activity of monocytes in both clinical disease and experimental chronic granulomatous disease is largely unaffected (26) reveals the unique importance of neutrophil activity against germinating conidia and hyphae in the control of aspergillosis.
Human studies have shown that prolonged neutropenia is one of the most important factors predisposing to invasive aspergillosis (35, 45). However, the efficacy of immunotherapies aimed at both shortening the duration of neutropenia and restoring neutrophil antifungal activity has been limited by problems associated with the transfusion therapy, including the still uncertain efficacy of colony-stimulating factors (34) and the limited persistence of the transfused cells (16). It appears that strategies aimed at keeping the infection in check until the recovery of adequate innate antifungal activity are needed for prompt handling of the fungus by the host.
Recent studies have highlighted the therapeutic potential of killer antiidiotypic antibodies in several fungal infections (23). Antiidiotypes to a monoclonal antibody (MAb) specifically reacting with killer toxins (KT) from Pichia anomala and Williopsis mrakii are characterized by a broad antimicrobial spectrum (30) and are lethal to pathogenic microorganisms expressing specific cell wall receptors (KTR). Polyclonal antibodies, MAbs, or single-chain recombinant killer antiidiotypic antibodies appear to have fungicidal activity in vitro and to confer active and passive protection in vivo on mice with candidiasis or pneumocystosis (6, 22, 31, 39). Although the impact of natural killer antibodies, as well as of the overall antibody response, on antifungal immune resistance is not completely clear, the use of antibodies is emerging as an effective adjunct therapy for fungal diseases (40).
To assess the therapeutic potential of killer antiidiotypic antibodies against Aspergillus infection, we used a mouse model of T-cell-depleted allogeneic BMT with invasive pulmonary aspergillosis (IPA). We have already shown that these mice failed to develop antifungal T-helper type 1 resistance, an activity that could be efficiently restored upon treatment with T-helper type 2 cytokine antagonists (25). We found that a killer antiidiotypic MAb, the K10 MAb, that potently inhibited hyphal development and metabolic activity in vitro had in vivo therapeutic efficacy against IPA.
MATERIALS AND METHODS
Mice.
Female, 8- to 10-week-old, inbred C3H/HeJ and DBA/2 mice were obtained from Charles River Breeding Laboratories (Calco, Italy). All mice were kept in small sterile cages (four animals in each cage) and fed with sterile food and water at the animal facilities of the University of Perugia, Perugia, Italy. Procedures involving animals and their care were conducted in conformity with national and international laws and policies.
Irradiation.
C3H/HeJ mice were exposed to a single lethal dose of 9 Gy from an 18-mV photon beam linear accelerator (Clinac 600/C Varian; Cernusco, Milan, Italy) with a focus-to-skin distance of 75 cm and a dose of 0.7 Gy/min (20). Without BMT, the mice died within 14 days.
Preparation of T-cell-depleted BM cells.
BM cells were prepared as previously described, with minor modifications (32). Donor BM cells were collected into phosphate-buffered saline (PBS) by flushing the shafts of the femurs and tibias of DBA/2 mice, which are known to be highly susceptible to IPA (9). The cells were suspended, and clumps of debris were allowed to settle out. The cells were washed three times with PBS and resuspended at a final concentration of 3 × 108 cells per ml. The cells were then fractionated by differential agglutination with soybean agglutinin as previously described (32). Briefly, the BM cell suspension was incubated in polystyrene tubes with soybean agglutinin at 2 mg/ml for 5 min at room temperature. The cells were gently layered on top of a 5% bovine serum albumin solution in 8 ml of PBS in 15-ml conical tubes. After 15 min at room temperature, the cells remaining on the surface of the albumin were removed whereas the sedimented cells were washed in a 1% bovine serum albumin solution in 10 ml of PBS and then suspended in 0.2 M d-galactose in 10 ml of PBS. After 10 min at room temperature, the cells were collected by centrifugation at 200 × g for 5 min and washed twice with d-galactose to dissociate all aggregates into single cells. Finally, the T-cell-depleted soybean agglutinin-positive cells, containing less than 1% contaminating T cells on fluorescence-activated cell sorter analysis, were washed twice with PBS and resuspended at a final concentration of at least 4 × 106 cells per ml of saline. The cells were injected into recipient mice intravenously via the lateral tail vein in a volume of 0.5 ml. According to a previous study (3), more than 95% of the mice survived, showing stable, donor-type hematopoietic chimerism, as revealed by donor-type major histocompatibility complex class I antigen expression on cells from spleens.
Aspergillus fumigatus infection and quantification of fungal growth.
The A. fumigatus strain used, the growth conditions used, and the method used for preparation of conidia have been previously described (9). For infection, mice were lightly anesthetized with inhaled diethyl ether before instillation of a suspension of 2 × 107 conidia (>95% viable, as determined by serial dilution and plating of the inoculum on Sabouraud dextrose agar) in 20 μl of saline, slowly applied to the nostrils by a micropipette with a sterile disposable tip. Mice were infected three times on three consecutive days. Animals were held upright until the suspension was completely inhaled and normal breathing resumed. Mice succumbing to the fungal challenge were routinely necropsied for histopathological confirmation of IPA. For histological analysis, lungs were excised and immediately fixed in formalin. Sections (3 to 4 μm) of paraffin-embedded tissues were stained with the periodic acid-Schiff or Gomori-Grocott procedure. For quantification of fungal growth in the lungs, the chitin assay was used as previously described (9). Results are expressed as micrograms of glucosamine per pair of lungs, calculated by reference to a standard curve constructed with known amounts of d(+)-glucosamine (Sigma). The glucosamine content of lungs from uninfected mice was used as a negative control, and it ranged between 0.80 and 2.25 μg of glucosamine per pair of lungs. For in vitro studies, swollen A. fumigatus conidia were obtained by incubating resting conidia in Sabouraud broth at room temperature for 18 h. A. fumigatus hyphae were obtained by incubating resting conidia at 37°C in 5% CO2 for 16 to 18 h, after which more than 95% of the conidia had germinated to hyphae (approximately 150 to 200 nm in length).
Production of killer antiidiotypic K10 MAb.
The K10 MAb, a killer antiidiotypic rat immunoglobulin M MAb, was produced by a hybridoma obtained as previously described (31). The K10 MAb was purified from the supernatant of the secreting hybridoma by precipitation with ammonium sulfate, dialyzed against PBS, and stored at 4°C. The presumptive antibody concentration was determined by evaluation of the optical density at 280 nm.
In vivo analysis and treatments.
Total white blood cell counts were determined by hemocytometry. For determination of differential white blood cell or lung cell counts, blood smears or cytospin preparations of collagenase-treated lung cells from transplant-receiving mice were stained with May-Grünwald Giemsa reagents (Sigma) before analysis. For immunosuppression of DBA/2 mice, long-lasting neutrophil depletion was obtained by intraperitoneal administration of an anti-Ly6G (RB6-8C5) MAb, at a dose of 100 μg per injection, on the day before and 2 days after the first fungal intranasal inoculation as previously described (9). This treatment dramatically reduced the number of neutrophils for up to 5 days. The K10 MAb was given intranasally, at a dose of 1 μg per injection twice a day, on the day of the first fungal challenge and 1, 2, and 3 days later. Control mice received an isotype-matched antibody (Zymed). Endotoxin was removed from all solutions with Detoxi-gel (Pierce).
TEM.
Neutrophils were collected from DBA/2 mice 18 h after intraperitoneal inoculation of an aged, endotoxin-free 10% thioglycolate solution (Difco Laboratories) and purified as previously described (33). For transmission electron microscopy (TEM), 10 × 106 peritoneal neutrophils were incubated in polypropylene tubes with 2 × 106 A. fumigatus conidia, swollen conidia, or hyphae for 15 min; pelleted at 1,200 rpm for 5 min; washed twice with PBS; and fixed in cold 2.5% glutaraldehyde in 0.1 M sodium cacodylate-1% sucrose buffer for 2 h. The cells were postfixed in 1% osmium tetroxide (50 min), encapsulated in 1% agar, stained with uranyl acetate and phosphotungstic acid, and dehydrated in a series of graded ethanolic solutions, finishing with propylene oxide, before finally being embedded in an Epon 812-Araldite mixture. Ultrathin (50-nm) sections were cut on an ultramicrotome (LKB Wallac) and placed under 200-mesh standard copper grids, contrasted with uranyl acetate and lead citrate, and examined with a Philips TEM 400 transmission electron microscope. For TEM of Aspergillus cells exposed to the K10 MAb, 10 × 107 swollen conidia were incubated for 1 h at 4°C in 100 μl of PBS with 10 μg of the biotin-conjugated K10 MAb and then with streptavidin-gold conjugate (British BioCell International, Cardiff, United Kingdom) and processed as described above.
Antifungal activity of neutrophils against A. fumigatus.
A CFU inhibition method (9) and a colorimetric MTT assay (9, 20) were used to evaluate the antifungal activity of neutrophils against Aspergillus conidia (conidiocidal activity) and hyphae (hyphal damage), respectively. For conidiocidal activity determination, 106, 5 × 105, and 105 peritoneal neutrophils were mixed with 105 conidia or swollen conidia for 3 h in 96-well flat-bottom microtiter plates and the percentage of CFU inhibition (mean ± standard error) was determined as follows: 100 − (CFU in experimental group/CFU in control cultures) × 100. For the colorimetric MTT assay (9, 20), graded numbers (106, 5 × 105, and 105) of neutrophils were added to hyphae obtained from 105 conidia in 96-well flat-bottom microtiter plates. After 2 h at 37°C with occasional shaking, the supernatants were aspirated, effector cells were lysed by adding sodium deoxycholate (0.5%), and hyphal viability was determined by MTT staining.
Germination assay.
The germination assay was performed as previously described (24), with minor modifications. Resting or swollen conidia (5 × 104/ml of Sabouraud broth) were incubated in polypropylene tubes (Falcon) at 37°C with gentle agitation in the presence or absence of the K10 MAb at 12.5 μg/ml. At 30-min intervals, aliquots were removed and germinated conidia were assessed by hemocytometer counting. Percent germination was calculated and graphed against the time of incubation. A total of 100 germinated or nongerminated conidia per field were counted at a magnification of ×400, and the mean value of three independent counts was calculated. In selected experiments, viability of hyphae germinated from Aspergillus conidia was evaluated by the MTT reduction assay (20). Briefly, 0.5 mg of MTT was added to 5 × 104 swollen conidia (in 1 ml of Sabouraud broth) that had been treated or not treated with the K10 MAb and the MTT-formazan-stained hyphae were microscopically evaluated after a 4-h incubation. Photomicrographs of conidia or MTT-treated hyphae were taken at selected time points.
Statistical analysis.
Survival data were analyzed by using the Mann-Whitney U test. Student's t test or analysis of variance (ANOVA) and Bonferroni's test were used to determine the statistical significance of differences in organ clearance, differential cell counts, and in vitro assays, as indicated in the table footnotes and figure legends. Significance was defined as P < 0.05. In vivo groups consisted of six to eight animals. Unless otherwise stated, the data reported were pooled from three to five experiments.
RESULTS
Leukocyte recovery following T-cell-depleted allogeneic BMT.
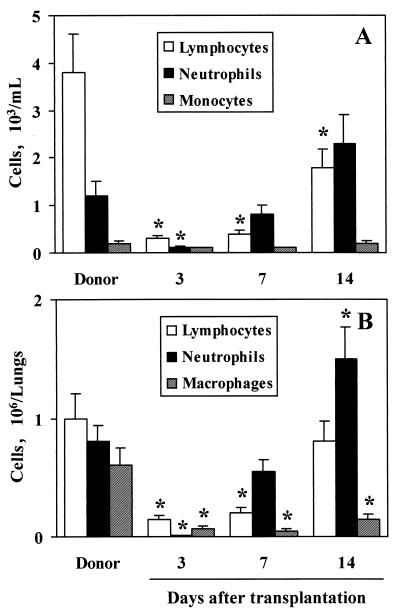
Recipient C3H/HeJ mice were lethally irradiated on the day before infusion of at least 2 × 106 T-cell-depleted BM cells from donor DBA/2 mice. Differential counts of blood or lung leukocytes were done on different days after transplantation. The results indicate that, following the profound leukopenia observed during the first week after BMT, neutrophil and lymphocyte counts progressively increased during the second week both in the periphery and in the lungs. Actually, 14 days after BMT, the number of neutrophils in the lungs of transplanted mice was significantly higher than that in the lungs of donor mice. No differences were observed between recipient and donor mice in the number of peripheral blood monocytes throughout the experimental period. On the contrary, counts of lung macrophages in transplanted mice were found to be significantly lower than those in donor mice at each time point (Fig. 1). No significant difference was observed between naive DBA/2 and C3H/HeJ mice in the numbers of blood and lung leukocytes (data not shown).
FIG. 1.
Differential white cell counts in the peripheral blood (A) and lungs (B) of transplanted mice. Lethally irradiated C3H/HeJ mice had received transplants of ≥2 × 106 T-cell-depleted allogeneic BM cells from donor DBA/2 mice a number of days before. (A) Cell counts were determined by hemocytometry and May-Grünwald Giemsa staining. (B) For determination of lung cell counts, cytospin preparations from collagenase-treated lungs were stained with May-Grünwald Giemsa reagents. ∗, P < 0.05 (transplanted versus donor mice) according to ANOVA and Bonferroni's test.
T-cell-depleted allogeneic BM-transplanted mice are susceptible to IPA.
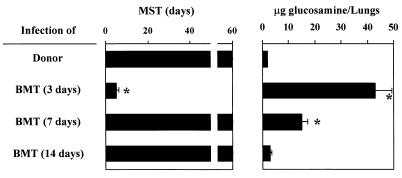
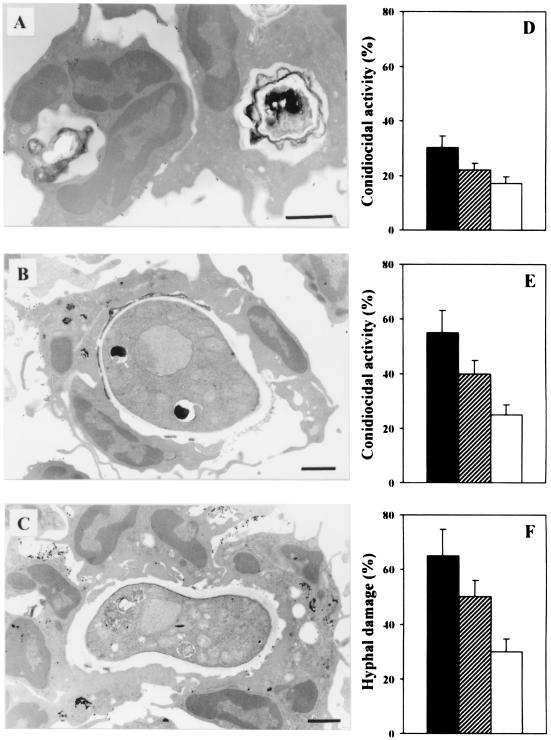
To assess susceptibility to IPA following T-cell-depleted allogeneic BMT, recipient mice lethally irradiated and transplanted as described above were infected intranasally with A. fumigatus conidia on different days after transplantation. Mice were monitored for death and fungal growth in the lungs. The results show that mice infected 3 days after transplantation succumbed to IPA, and susceptibility to infection correlated with extensive fungal growth in the lungs (Fig. 2). On the contrary, when infected 7 or 14 days after BMT, the majority of the transplanted mice survived the infection although significant fungal colonization was still observed in the lungs of mice infected at 7 days (Fig. 2). Therefore, susceptibility or resistance to IPA after T-cell-depleted allogeneic BMT varies at different time points after transplantation and correlates with neutrophil recovery. To elucidate whether neutrophils were needed to oppose fungal infection by conidia or hyphal forms, the interaction of neutrophils with either conidia or hyphae of the fungus was evaluated by TEM and in terms of killing activity. The results show that neutrophils internalize resting Aspergillus conidia (Fig. 3A); however, the percentage of cells with phagocytic activity against resting conidia was very low (less than 40% at 1 h of coculture). Swollen conidia (Fig. 3B) or hyphae (Fig. 3C) were not phagocytosed and were detected extracellularly and surrounded by multiple cells. In terms of antifungal activity, neutrophils exhibited potent antifungal activity toward swollen conidia and hyphae (Fig. 3E and F, respectively) and, to a lesser extent, toward resting conidia (Fig. 3D). These data confirm the unique role of neutrophils in opposing fungal infectivity, particularly at the level of hyphal formation and growth.
FIG. 2.
Susceptibility to IPA following T-cell-depleted allogeneic BM transplantation. Lethally irradiated C3H/HeJ mice had received transplants of ≥2 × 106 T-cell-depleted allogeneic BM cells from DBA/2 mice and were infected with A. fumigatus on days 3, 7, and 14 after transplantation as described in Materials and Methods. MST, median survival time (days). ∗, P < 0.05 (transplanted versus donor mice) according to Mann-Whitney U test. Chitin content (expressed as micrograms of glucosamine per pair of lungs) in the lungs of infected mice was determined 1 day after the last fungal inoculation. ∗, P < 0.05 (transplanted versus donor mice) according to ANOVA and Bonferroni's test.
FIG. 3.
Antifungal activity of neutrophils against A. fumigatus. Peritoneal neutrophils were incubated with A. fumigatus resting conidia, swollen conidia, or hyphae at 37°C for 15 min for TEM (A, B, and C) or for 2 h for evaluation of conidiocidal activity (D and E) or hyphal damage (F). Note the presence of resting conidia inside the cells (A) and of swollen extracellular conidia and hyphae surrounded by multiple cells (B and C, respectively). Determination of conidiocidal activity against resting (D) or swollen (E) conidia and of hyphal damage (F) was done as detailed in Materials and Methods. Black, hatched, and white bars indicate different neutrophil-to-A. fumigatus ratios (10:1, 5:1, and 1:1, respectively). Bars, 1 μm.
Activity of antiidiotypic MAbs against Aspergillus hyphal development and growth.
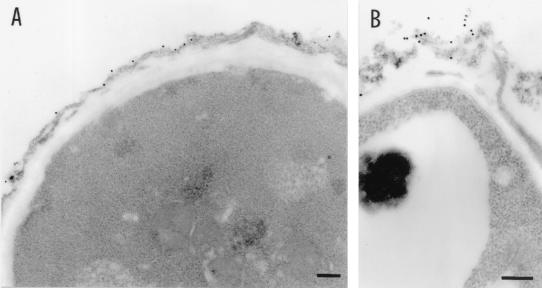
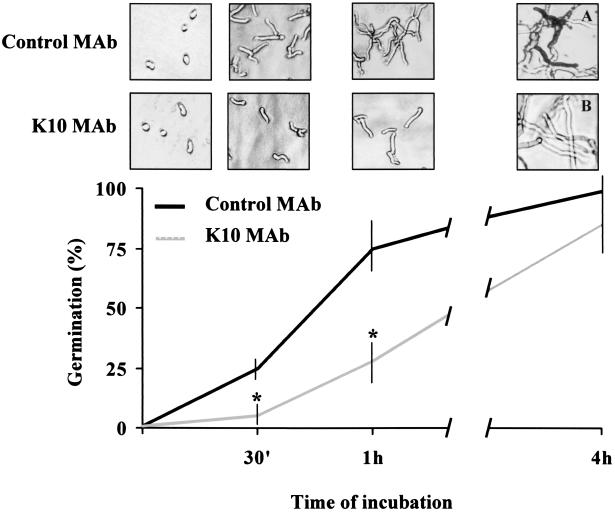
The above-described results suggest that strategies aimed at interfering with Aspergillus hyphal development and/or growth could substitute for neutrophils in the control of the infection under conditions of sustained neutropenia. As KT exert an antifungal activity by interference with a KTR involved in the β-glucan cell wall synthesis of fungi (36, 46), we assessed whether the antiidiotypic K10 MAb would bind to the Aspergillus cell wall and affect conidial germination and hyphal growth. Swollen conidia were incubated with different concentrations of the K10 MAb, specific binding was evaluated by TEM, and percent germination was calculated at different time intervals. The results show that the K10 MAb bound to the outer layer of the cell wall conidia during swelling (Fig. 4A), and there were signs of cell wall damage (Fig. 4B). Moreover, the K10 MAb significantly inhibited the germination of swollen conidia to hyphae, particularly during the first hour of incubation (Fig. 5). After this time, K10 MAb-exposed swollen conidia germinated gradually, reaching levels of germination not significantly different from those of untreated conidia 4 h later (Fig. 5). Importantly, however, hyphae that germinated from K10 MAb-exposed conidia showed impaired viability compared to that of hyphae that germinated from unexposed conidia, as evidenced by the defective MTT reduction (Fig. 5A and B). No effect against resting conidia was seen (data not shown). Together, these data indicate that the K10 MAb binds to the Aspergillus cell wall and inhibits both the development and the metabolic activity of Aspergillus hyphae in vitro.
FIG. 4.
Detection of K10 MAb binding to A. fumigatus by TEM. Swollen Aspergillus conidia were incubated for 1 h at 4°C with the biotin-conjugated K10 MAb, followed by streptavidin-gold. Note the distribution of gold particles on the conidial cell wall (A) with signs of cell wall damage at the site of binding (B). Bars, 0.2 μm.
FIG. 5.
In vitro activity of the K10 MAb on A. fumigatus conidial germination. Swollen A. fumigatus conidia (5 × 104) were incubated at 37°C with 12.5 μg of the K10 MAb in 1 ml of Sabouraud broth. At selected time points, percent germination was calculated and photomicrographs (original magnification, ×40) were taken. A total of 100 conidia per field were counted at a magnification of ×400, and the mean value of three independent counts was calculated. The MTT reduction assay was employed to assess viability of hyphae germinated from Aspergillus conidia after 4 h of exposure to the K10 MAb (A and B). Note the differences in MTT reduction between K10 MAb-treated and untreated cultures. The data reported are from one representative experiment out of three experiments with similar results. ∗, P < 0.05 (K10 MAb-exposed conidia versus control MAb-exposed conidia) according to Student's t test.
The K10 MAb cures T-cell-depleted allogeneic BM-transplanted mice of IPA.
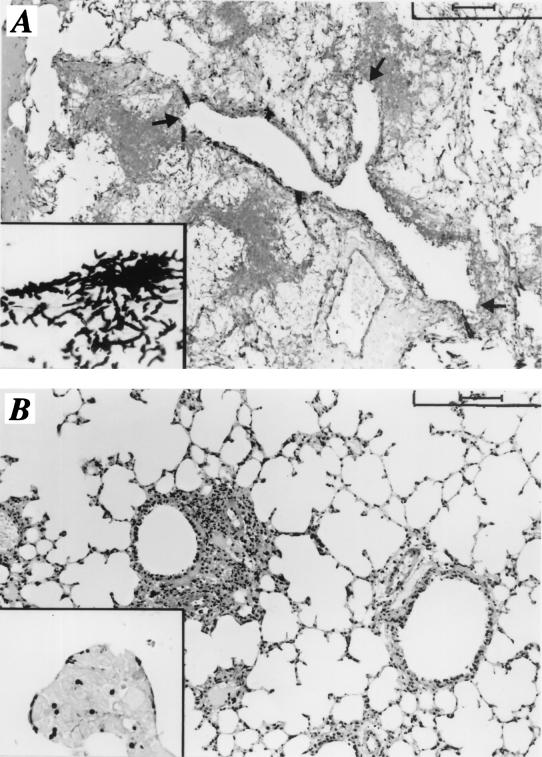
To assess whether the above-described in vitro effects of the K10 MAb could translate to efficient control of infection in neutropenic mice, C3H/HeJ mice that had received transplants 3 days before or neutropenic DBA/2 mice were infected with A. fumigatus conidia and treated with the K10 MAb, which was delivered by repeated intranasal injections. The results (Table 1) show that treatment with the K10 MAb resulted in a cure of IPA in both transplant-receiving C3H/HeJ and neutropenic DBA/2 mice, as evidenced by the long-term survival of the majority of the animals and the decreased fungal loads in their lungs. The histology of lung sections from animals treated with the control MAb showed patterns of lesions due to severe IPA, with signs of hyphal invasion of bronchial and vasal walls and tissue destruction, in the relative absence of inflammatory cells (Fig. 6A). These findings were not observed in mice cured with the K10 MAb, whose lungs were characterized by few swollen conidia of A. fumigatus scattered throughout the lung parenchyma early in infection (Fig. 6B) and no evidence of fungal growth thereafter (data not shown).
TABLE 1.
Effect of K10 MAb treatment on the course and outcome of IPA in hematopoietic transplanted or neutropenic mice.
| Micea | MAb treatmentb | MSTc | D/Td | Chitin content (mean μg of glucosamine ± SEM/pair of lungs)e |
|---|---|---|---|---|
| DBA/2 → C3H/HeJ | Control | 5 | 8/8 | 49 ± 4 |
| DBA/2 → C3H/HeJ | K10 | >60f | 3/8 | 13 ± 1g |
| Neutropenic DBA/2 | Control | 6 | 8/8 | 44 ± 5 |
| Neutropenic DBA/2 | K10 | >60f | 0/8 | 17 ± 2g |
Recipient C3H/HeJ mice were lethally irradiated, received, transplants of T-cell-depleted BM cells from DBA/2 mice, and were infected 3 days later with 2 × 107 A. fumigatus conidia that were given intranasally for 3 consecutive days. DBA/2 mice were treated with an anti-Ly6G (RB6-8C5) MAb that was given intraperitoneally at a dose of 100 μg per injection on the day before and 2 days after the first fungal intranasal inoculation.
Control or K10 MAb was administered intranasally at a dose of 1 μg per injection twice a day on the day before the first fungal challenge and 1, 2, and 3 days later.
MST, median survival time in days.
D/T, number of dead animals/total number of infected mice.
Chitin content in the lungs was evaluated 1 day after the last fungal inoculation.
Significantly different (P < 0.05) from the value for untreated mice as calculated by the Mann-Whitney U test.
Significantly different (P < 0.05) from the value for untreated mice as calculated by Student's t test.
FIG. 6.
Histology of lungs of mice with IPA treated or not treated with the K10 MAb. Lethally irradiated C3H/HeJ mice received transplants of allogeneic T-cell-depleted BM cells from DBA/2 mice. Three days later, the mice were infected with 2 × 107 A. fumigatus conidia, which were given intranasally for 3 consecutive days, and treated with a control MAb (A) or the K10 MAb (B). Periodic acid-Schiff (A and B)-stained or Gomori-Grocott (insets)-stained sections were prepared from the lungs of mice 1 day after the last intranasal infection. Note the presence of numerous hyphae and evident signs of bronchial wall destruction (arrows) in the lungs of control mice (A and inset), as opposed to the presence of a few swollen conidia in the lungs of mice treated with the K10 MAb (B and inset). Bars, 100 μm (A and B) and 25 μm (insets).
DISCUSSION
IPA is a common and severe infection in immunosuppressed patients. Given its poor outcome with current therapy, understanding of the precise mechanism through which host resistance to the fungus is achieved may offer new perspectives on the prophylaxis and immunotherapy of the infection. The present study indicates that prevention of germination and/or hyphal growth could be an effective strategy through which antifungal resistance could be achieved in neutropenic mice with aspergillosis.
Various putative virulence factors of A. fumigatus are known to contribute to its pathogenicity (41). Among these, germination of conidia, a process that likely occurs in vivo after deposition of conidia on bronchoalveolar surfaces, is a prerequisite step in the pathogenesis of the infection, as it leads to hyphal formation and subsequent fungal colonization of tissues. Studies of postmortem lung specimens have shown A. fumigatus to be present and viable more frequently than would be expected from its prevalence among the fungal conidia found normally in the air (28). This suggests that resting conidia per se may not be pathognomonic of infection.
The cell surface characteristics of resting conidia are altered during swelling and germination. Scanning electron microscopy showed that during swelling of conidia, a step leading to germination, the characteristic rodlet layer progressively disintegrated, unmasking the inner wall molecules (42). Among these are the most important fungal polysaccharides, glucans and chitin, which are located mainly in the inner layer (17, 19).
Glucan is a mixture of β(1,6)- and β(1,3)-linked glucose subunits synthesized by the glucan synthase complex that, interestingly enough, has recently been found to be localized at the apices of hyphae (5). The β glucans, besides contributing with chitin to the strength and shape of the cell wall, are known to be endowed with potent immunomodulatory activity (44) mediated by interaction with pattern recognition receptors on different cells (18, 27, 43) and represent attractive targets for antifungal chemotherapy (1). The β(1,3)-d-glucan inhibitor caspofungin has recently been described to be effective in treating aspergillosis in leukopenic mice (14).
Killer antiidiotypic antibodies have recently surfaced as potential mediators of host resistance to fungi (8, 12). Natural yeast KT-like microbicidal antiidiotypic antibodies have been detected in both clinical and experimental settings (8, 11, 29). Polyclonal antibodies, MAbs, or single-chain recombinant killer antibodies appear to have fungicidal activity in vitro and to confer active and passive protection in vivo against experimental candidiasis or pneumocystosis (6, 22, 31, 39). The K10 MAb, similarly to KT (36, 46), appears to recognize β-glucans in the fungal organism cell wall (unpublished data). We found that the K10 MAb significantly inhibited conidial germination and hyphal metabolic activity in vitro. This observation is consistent with the observation that the K10 MAb bound to the swollen conidial cell wall while lacking any activity on resting conidia. In vivo, the presence of residual conidia scattered in the lungs of K10 MAb-treated mice reinforces this notion.
It is interesting that binding to Aspergillus β(1,6)-glucan (2) could account for the protective role of lung surfactant protein D in a murine model of IPA (21). Thus, it appears that the exploitation of fungal β glucans represents a common event shared by collectins and natural antibodies in the setting of initial host antifungal defenses in the lungs.
While studies are in progress to define the molecular events underlying the specific interaction between killer antiidiotypic antibodies and fungal cell wall molecules, the results of the present study have many important implications, at both the conceptual and practical levels. First, it has been confirmed that antibodies are active participants in the orchestration of the initial host defense against fungi. Opsonization, recognition of important fungal epitopes, and promotion of adequate antifungal responses are all mechanisms through which antibodies can be protective against fungal infections (7). Although clearance of antibody-coated fungal elements could be a mechanism involved in the resolution of the infection in K10 MAb-treated mice, we show here that a direct activity on the fungal cell wall is an additional mechanism of antibody action in infection, and it is common to a variety of host antimicrobial molecules operating in the airways (47). Second, synthetic decapeptides with antifungal activity have been selected and synthesized on the basis of the amino acid sequence of the variable region of killer recombinant antiidiotypic antibodies. Such killer mimotopes have been shown to have potent candidacidal activity in vitro and in vivo (unpublished data). Therefore, the use of killer antiidiotypic antibodies or killer mimotopes may represent a novel and realistic approach to the prophylaxis and immunotherapy of aspergillosis and refractory fungal infections.
Overall, the results of the present study indicate that interference with hyphal development and growth may represent a useful strategy for the control of Aspergillus infection under conditions of phagocyte ablation or functional deficiency. Killer antibodies appear to fulfill this requirement with high therapeutic efficacy and virtually no toxicity. A number of available compounds that interfere with fungal cell wall synthesis exist (15). The addition of antiidiotypic antibodies to this armamentarium may help to overcome problems associated with toxicity and the narrow spectrum of activity for many of the existing compounds. Finally, as the recruitment and activation of inflammatory cells in the setting of a fungal challenge in the lungs is a complex and dynamic process that involves the coordinated expression of both pro- and antiinflammatory mediators, such as chemokines and cytokines (47), it is conceivable that killer antiidiotypic antibodies, by lowering the local fungal burden, may contribute to the determination of the appropriate antifungal response through reduction of inflammatory pathology and lung injury.
Acknowledgments
This study was supported by grants 50C.26 and 50C.27 from the National Research Project on AIDS, Rome, Italy.
We thank Lara Bellocchio for editorial assistance, Paolo Mosci for histology, and Carla Barabani of the animal facility at the University of Perugia for technical assistance.
Editor: T. R. Kozel
REFERENCES
- 1.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, M. J., D. R. Voelker, and R. J. Mason. 2001. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus. Infect. Immun. 69:2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachar-Lustig, E., N. Rachamin, L. Hong-Wei, F. Lan, and Y. Reisner. 1995. Megadose of T-cell-depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nat. Med. 12:1268-1273. [DOI] [PubMed] [Google Scholar]
- 4.Baddley, J. W., T. P. Stroud, D. Salzman, and P. G. Pappas. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:1319-1324. [DOI] [PubMed] [Google Scholar]
- 5.Beauvais, A., J. M. Bruneau, P. C. Mol, M. J. Buitrago, R. Legrand, and J. P. Latgé. 2001. Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beninati, C., M. R. Oggioni, M. Boccanera, M. R. Spinosa, T. Maggi, S. Conti, W. Magliani, F. De Bernardis, G. Teti, A. Cassone, G. Pozzi, and L. Polonelli. 2000. Therapy of mucosal candidiasis by expression of an anti-idiotype in human commensal bacteria. Nat. Biotechnol. 18:1060-1064. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall, A., A. Cassone, F. Bistoni, J. E. Cutler, W. Magliani, J. W. Murphy, L. Polonelli, and L. Romani. 1998. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med. Mycol. 36:95-105. [PubMed] [Google Scholar]
- 8.Cassone, A., S. Conti, F. De Bernardis, and L. Polonelli. 1997. Antibodies, killer toxins and antifungal immunoprotection, a lesson from nature? Immunol. Today 18:164-169. [DOI] [PubMed] [Google Scholar]
- 9.Cenci, E., A. Mencacci, C. Fè d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 10.Cenci, E., A. Mencacci, C. Fè d'Ostiani, C. Montagnoli, A. Bacci, G. Del Sero, S. Perito, F. Bistoni, and L. Romani. 1998. Cytokine- and T-helper-dependent immunity in murine aspergillosis. Res. Immunol. 149:445-454. [DOI] [PubMed] [Google Scholar]
- 11.Conti, S., F. Fanti, W. Magliani, M. Gerloni, D. Bertolotti, A. Salati, A. Cassone, and L. Polonelli. 1998. Mycobactericidal activity of human natural, monoclonal, and recombinant yeast killer toxin-like antibodies. J. Infect. Dis. 177:807-811. [DOI] [PubMed] [Google Scholar]
- 12.Conti, S., W. Magliani, P. Fisicaro, E. Dieci, S. Arseni, A. Salati, and L. Polonelli. 1998. Killer antibodies in fungal infections. Res. Immunol. 149:334-343. [DOI] [PubMed] [Google Scholar]
- 13.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., A. J. De Lucca, and T. J. Walsh. 1998. Emerging targets for the development of novel antifungal therapeutics. Trends Microbiol. 6:117-124. [DOI] [PubMed] [Google Scholar]
- 15.Hector, R. F. 1993. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 6:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubel, K., D. C. Dale, and W. C. Liles. 2001. Granulocyte transfusion therapy: update on potential clinical applications. Curr. Opin. Hematol. 8:161-164. [DOI] [PubMed] [Google Scholar]
- 17.Humbel, B. M., M. Konomi, T. Takagi, N. Kamasawa, S. A. Ishijima, and M. Osumi. 2001. In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 18:433-444. [DOI] [PubMed] [Google Scholar]
- 18.Kougias, P., D. Wei, P. J. Rice, H. E. Ensley, J. Kalbfleisch, D. L. Williams, D. L., and I. W. Browder. 2001. Normal human fibroblasts express pattern recognition receptors for fungal (1→3)-β-d-glucans. Infect. Immun. 69:3933-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitz, S. M., and R. D. Diamond. 1985. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis. 152:938-945. [DOI] [PubMed] [Google Scholar]
- 21.Madan, T., U. Kishore, M. Singh, P. Strong, E. M. Hussain, K. B. Reid, and P. U. Sarma. 2001. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect. Immun. 69:2728-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magliani, W., S. Conti, F. De Bernardis, M. Gerloni, D. Bertolotti, P. Mozzoni, A. Cassone, and L. Polonelli. 1997. Therapeutic potential of antiidiotypic single chain antibodies with yeast killer toxin activity. Nat. Biotechnol. 15:155-158. [DOI] [PubMed] [Google Scholar]
- 23.Magliani, W., S. Conti, M. Gerloni, D. Bertolotti, and L. Polonelli. 1997. Yeast killer systems. Clin. Microbiol. Rev. 10:369-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manavathu, E. K., J. Cutrigh, and P. H. Chandrasekar. 1999. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J. Clin. Microbiol. 37:858-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mencacci, A., K. Perruccio, A. Bacci, E. Cenci, R. Benedetti, M. F. Martelli, F. Bistoni, R. Coffman, A. Velardi, and L. Romani. 2001. Defective antifungal T-helper 1 (Th1) immunity in a murine model of allogeneic T-cell-depleted bone marrow transplantation and its restoration by treatment with TH2 cytokine antagonists. Blood 97:1483-1490. [DOI] [PubMed] [Google Scholar]
- 26.Morgenstern, D. E., M. A. Gifford, L. L. Li, C. M. Doerschuk, and M. C. Dinauer. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mouse leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller, A., P. J. Rice, H. E. Ensley, P. S. Coogan, J. H. Kalbfleisch, J. L. Kelley, E. J. Love, C. A. Portera, T. Ha, I. W. Browder, and D. L. Williams. 1996. Receptor binding and internalization of water-soluble (1→3)-beta-d-glucan biologic response modifier in two monocyte/macrophage cell lines. J. Immunol. 156:3418-3425. [PubMed] [Google Scholar]
- 28.Mullins, J., and A. Seaton. 1978. Fungal spores in lung and sputum. Clin. Allergy 8:525-533. [DOI] [PubMed] [Google Scholar]
- 29.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, W. Magliani, M. Gerloni, C. Cantelli, and A. Cassone. 1996. Human natural yeast killer toxin-like candidacidal antibodies. J. Immunol. 156:1880-1885. [PubMed] [Google Scholar]
- 30.Polonelli, L., and G. Morace. 1987. Production and characterization of yeast killer toxin monoclonal antibodies. J. Clin. Microbiol. 25:460-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polonelli, L., N. Séguy, S. Conti, M. Gerloni, D. Bertolotti, C. Cantelli, W. Magliani, and J. C. Cailliez. 1997. Monoclonal yeast killer toxin-like candidacidal anti-idiotypic antibodies. Clin. Diagn. Lab. Immunol. 4:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisner, Y., L. Itzicovitch, A. Meshorer, and N. Sharon. 1978. Hemopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc. Natl. Acad. Sci. USA 75:2933-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romani, L., A. Mencacci, E. Cenci, G. Del Sero, F. Bistoni, and P. Puccetti. 1997. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J. Immunol. 158:2356-2362. [PubMed] [Google Scholar]
- 34.Rubestein, E. B. 2000. Colony stimulating factors in patients with fever and neutropenia. Int. J. Antimicrob. Agents 16:117-121. [DOI] [PubMed] [Google Scholar]
- 35.Sable, C. A., and G. R. Donowitz. 1994. Infections in bone marrow transplant recipients. Clin. Infect. Dis. 18:273-284. [DOI] [PubMed] [Google Scholar]
- 36.Sawant, A. D., and D. G. Ahearn. 1990. Involvement of a cell wall receptor in the mode of action of an anti-Candida toxin of Pichia anomala. Antimicrob. Agents Chemother. 34:1331-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneemann, M., and A. Schaffner. 1999. Host defense mechanism in Aspergillus fumigatus infections. Contrib. Microbiol. 2:57-68. [DOI] [PubMed] [Google Scholar]
- 39.Séguy, N., J. C. Cailliez, P. Delcourt, S. Conti, D. Camus, E. Dei-Cas, and L. Polonelli. 1997. Inhibitory effect of human natural yeast killer toxin-like candidacidal antibodies on Pneumocystis carinii. Mol. Med. 3:544-552. [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens, D. A., B. J. Kullberg, E. Brummer, A. Casadevall, M. G. Netea, and A. M. Sugar. 2000. Combined treatment: antifungal drugs with antibodies, cytokines or drugs. Med. Mycol. 38:305-315. [PubMed] [Google Scholar]
- 41.Tomee, J. F., and H. F. Kauffman. 2000. Putative virulence factors of Aspergillus fumigatus. Clin. Exp. Allergy 30:476-484. [DOI] [PubMed] [Google Scholar]
- 42.Tronchin, G., K. Esnault, G. Renier, R. Filmon, D. Chabasse, and J. F. Bouchara. 1997. Expression and identification of a laminin-binding protein in Aspergillus fumigatus conidia. Infect. Immun. 65:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vetvicka, V., B. P. Thornton, and G. D. Ross. 1996. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Investig. 98:50-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams, D. L., A. Mueller, and W. Browder. 1996. Glucan-based macrophage stimulators: a review of their anti-infective potential. Clin. Immunother. 5:392-399. [Google Scholar]
- 45.Wingard, J. R. 1999. Fungal infections after bone marrow transplant. Biol. Blood Marrow Transplant. 5:55-68. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto, T., T. Iratani, H. Hirata, M. Imai, and H. Yamaguchi. 1986. Killer toxin from Hansenula mrakii selectively inhibits cell wall synthesis in a sensitive yeast. FEBS Lett. 197:50-54. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 1998. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]