Abstract
Enterotoxins with superantigenic properties secreted during systemic Staphylococcus aureus infection are responsible for toxic shock. We show that intranasal administration of staphylococcal enterotoxin A (SEA), but not a recombinant SEA lacking superantigenic activity, protected mice against lethal systemic SEA challenge. Protection was superantigen specific since intranasal exposure to SEA would not protect against death caused by subsequent toxic shock syndrome toxin 1 systemic challenge. Protection was neither due to selective depletion of SEA-specific T-cell receptor Vβ families nor due to production of neutralizing anti-SEA antibodies. Importantly, the production of interleukin 10 (IL-10) induced by “tolerization” (that is, by the induction of immunological tolerance) contributed to the observed protection against lethal superantigen-triggered disease. In support of this notion we found that (i) significantly increased levels of IL-10 in sera of “tolerized” animals (that is, animals rendered tolerant) and (ii) IL-10−/− mice could not be tolerized by mucosal SEA administration. Altogether, this is the first study to show that mucosal tolerance to a superantigen is readily triggered by means of immunodeviation.
Infections with Staphylococcus aureus give rise to life-threatening endocarditis, septic arthritis, and septicemia. Toxic shock, due primarily to bacterial enterotoxins, involves multiple organ dysfunction and has a high mortality rate. Superantigens produced by S. aureus (e.g., staphylococcal enterotoxins A [SEA] to E and toxic shock syndrome toxin 1 [TSST-1]) trigger an excessive immune response by binding directly to major histocompatibility complex class II molecules and hyperstimulating T cells expressing certain Vβ domains in the T-cell receptor (TCR) (15). The resultant massive production of cytokines (such as interleukin 2 [IL-2], gamma interferon, and tumor necrosis factor alpha [TNF-α]) from activated Th1 cells and monocytes/macrophages results in toxicity and eventually in death.
Systemic T-cell and B-cell hyporesponsiveness to a protein antigen can be induced when the protein is encountered at a mucosal surface. Such mucosal tolerance has proven to be an efficient means to prevent autoimmune (30), allergic (27), and infection-induced (24) inflammatory conditions. The development of mucosal tolerance is mediated through (i) deletion (9), (ii) anergy (28) of specific T-cell subsets, or (iii) the development of regulatory T cells secreting anti-inflammatory cytokines (10, 12).
Various attempts have been made to prevent superantigen-mediated shock, including inhibition of proinflammatory cytokine production using extrinsically administered IL-10 (16) and blockage of the costimulatory receptor CD28 (22). Tolerance was achieved by either intravenous injection of SEA (4) or oral feeding of SEB (20), via a mechanism involving anergy and depletion of specific T-cell subsets.
We have taken a new approach towards preventing enterotoxin-mediated shock. By administering SEA intranasally (i.n.) we sought to protect mice against a lethal systemic challenge. We analyzed the resultant immune responses in terms of survival, specific antibody production, TCR Vβ T-cell subset populations, T-cell anergy, and cytokine production. Our results indicate that this approach eliminated superantigen-triggered death, despite a clear-cut increase in enterotoxin-responding TCR Vβ subsets. This SEA-specific protection was not dependent on neutralizing antibodies but was mediated by IL-10.
MATERIALS AND METHODS
Animals.
Female C57BL/6 and BALB/c mice were purchased from B&K Universal AB, Stockholm, Sweden. C57BL/6 mice with defined gene-targeted deficiencies in B cells (μmT) (14) and BALB/c mice lacking the gene for IL-10 (kind gift of D. Rennick, DNAX Research Institute, Palo Alto, Calif. [5]) were bred under specific-pathogen-free conditions at the animal facilities at the Department of Rheumatology, University of Göteborg. Animals were used at 6 to 8 weeks of age. All animal experiments were approved by the animal ethics committee of the University of Göteborg.
i.n. “tolerization” and toxic challenge.
For i.n. “tolerization” (that is, induction of tolerance), mice were given three 1-μg doses of ovalbumin (OVA; Sigma, St. Louis, Mo.), highly purified SEA (Toxin Technology Inc., Sarasota, Fla.), or recombinant SEA (rSEA), a recombinant, nonsuperantigenic SEA derivative (2), i.n. at 1-week intervals. One week following the final i.n. dose mice were challenged with an intraperitoneal (i.p.) injection of 10 μg of SEA or TSST-1 followed 4 h later with a further i.p. injection of Escherichia coli O55:B5 lipopolysaccharide (LPS) (170 μg for C57BL/6 and C57BL/6 μmT mice and 80 μg for BALB/c IL-10+/+ and BALB/c IL-10−/− mice; Sigma), and the number of deaths was recorded at frequent intervals. The procedures regarding the induction of enterotoxin-triggered death, including doses of SEA, TSST-1, and LPS, were adopted from previous studies (23). Neither SEA nor LPS given alone was sufficient for lethal toxicity at these doses.
Proliferation assay.
Single-spleen-cell suspensions obtained 7 days following the last tolerization dose were incubated at 105 cells/well in Iscove's medium supplemented with l-glutamine, 50 μM 2-mercaptoethanol, gentamicin, and 10% fetal calf serum and incubated at 37°C for 3 days in the presence of SEA (10 μg/ml). Cells were pulsed with 1 μCi of [3H]thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) for the last 6 h of culture, the cellular DNA was harvested on a glass fiber filter, and the incorporated radioactivity was determined. Data are expressed as the mean counts per minute ± 1 standard deviation (SD) for groups of at least four mice.
Fluorescence-activated cell sorter analysis of splenocytes.
Spleen cell suspensions obtained 7 days after the third tolerization dose were analyzed for Vβ TCR phenotypes using the following antibodies from Pharmingen: phycoerythrin (PE)-labeled anti-mouse CD4; Cy-Chrome-labeled anti-mouse CD4; PE-labeled anti-mouse Vβ3 TCR; fluorescein isothiocyanate (FITC)-labeled anti-mouse Vβ6 TCR; FITC-labeled anti-mouse Vβ8.1, 8.2 TCR; FITC-labeled anti-mouse Vβ11 TCR; and isotype-matched control antibodies. Spleen cells cultured for 24 h with SEA (10 μg/ml) were analyzed for apoptotic cell death using an in situ cell detection kit (Boehringer, Mannheim, Germany) with FITC-labeled dUTP labeling of DNA strand breaks by terminal transferase according to the manufacturer's instructions. Data are expressed as the mean percentage ± the SD of the CD4+ T-cell population expressing a specific Vβ subset or undergoing apoptosis.
Cytokine assays.
An anti-human transforming growth factor beta (TGF-β) enzyme-linked immunosorbent assay (ELISA), cross-reactive with mouse TGF-β, was used. Briefly, 96-well plates (Nunc) were coated with chicken anti-human TGF-β (5 μg/ml; R&D Systems, Abingdon, United Kingdom) overnight and then blocked with 0.5% bovine serum albumin for 30 min. Plasma was obtained 4 h after SEA-LPS challenge, acidified for 10 min with 0.17 M HCl, and then neutralized by 0.2 M NaOH containing 0.07 M HEPES. Plasma samples and recombinant human TGF-β (R&D Systems) were incubated overnight at 4°C. Mouse anti-human TGF-β was added (1 μg/ml; Genzyme) for 2 h, followed by biotin goat anti-mouse immunoglobulin G1 (1 μg/ml; Sigma) and then anti-biotin-horseradish peroxidase (Vector) diluted to 1/400. Subsequently, 100 μl of peroxidase substrate containing 3,3′,5,5′-tetramethylbenzidine (0.1 mg/ml, Sigma) and 0.06% H2O2 in 0.05 M phosphate-citrate buffer at pH 5.0 were added. The reaction was stopped by 25 μl of 1 M H2SO4, and the absorbance was read at 450 nm. IL-10 and TNF-α cytokine levels were measured using ELISA kits (R&D Systems). Data are expressed as the mean values (in picograms per milliliter for IL-10 and nanograms per milliliter for TGF-β and TNF-α) for animals that were OVA “tolerized” (that is, rendered OVA tolerant) and SEA tolerized.
Statistical analyses.
Statistical analyses were done by the two-tailed Student's t test, Fisher's exact test, and the Kaplan-Meier log rank test.
RESULTS
SEA-tolerized mice survive superantigenic challenge.
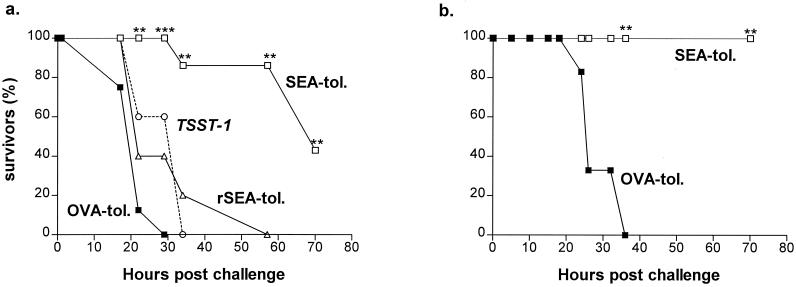
Systemic SEA challenge as described in Materials and Methods resulted in superantigen-induced death that occurred within 2 to 3 days (Fig. 1). Both C57BL/6 and BALB/c mice were protected from a lethal enterotoxin challenge by prophylactic i.n. administration of SEA (Fig. 1). Thus, whereas all OVA-tolerized C57BL/6 animals died within 20 to 30 h post-SEA challenge, more than 80% of SEA-tolerized mice were still alive at 60 h (P < 0.01 [Fig. 1a]) and subsequently recovered completely. The protection was even more striking in BALB/c mice, in which SEA tolerization completely prevented superantigen-induced lethality (P < 0.01 [Fig. 1b]). Interestingly, rSEA rendered nonsuperantigenic by site-directed mutagenesis (2, 26) was ineffective at providing protection; SEA-treated mice were significantly better protected (P < 0.05) than rSEA-treated mice at 22 to 57 h postchallenge (Fig. 1a). Furthermore, SEA tolerization did not protect against challenge with another staphylococcal superantigen, TSST-1 (Fig. 1).
FIG. 1.
Mucosal SEA administration protects against SEA-induced death. Mice were given SEA, rSEA, or OVA i.n. three times 1 week apart and challenged i.p. with SEA-LPS 1 week later. Data are expressed as survival of OVA-tolerized (▪), rSEA-tolerized (▵), and SEA-tolerized (□) C57BL/6 (a) and BALB/c (b) mice during the first 70 h post-SEA challenge. Mice that had been tolerized i.n. with SEA were not protected from a lethal i.p. challenge with TSST-1-LPS (○). All experiments were performed twice with groups of 10 mice. Statistical significance for comparison to OVA-tolerized animals: ∗∗, P < 0.01; ∗∗∗, P < 0.001.
SEA tolerization is not antibody mediated.
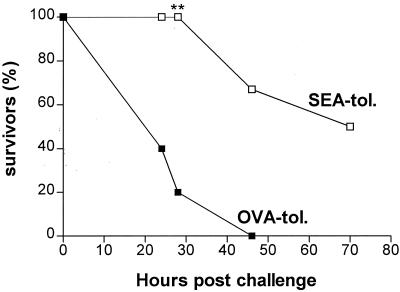
The levels in serum of antibodies against SEA measured by ELISA were equally low in both the OVA-tolerized and SEA-tolerized mice (data not shown). Furthermore, C57BL/6 μmT mice that completely lack serum antibody production were protected from death as efficiently as wild-type C57BL/6 mice by i.n. tolerization with SEA (Fig. 2). For example, at 30 h postchallenge there were significant differences in survival between the SEA- and OVA-administered mice in both the wild-type (100 and 0%, respectively [Fig. 1a]) and the μmT (100 and 20%, respectively [Fig. 2]) groups. At 45 h postchallenge all of the OVA-administered μmT mice were dead, whereas 70% of the SEA-tolerized μmT mice were still alive (Fig. 2). Statistical analysis using the Kaplan-Meier log rank test also revealed that the difference in survival between OVA-tolerized and SEA-tolerized mice was statistically significant (P = 0.0015). In summary, neutralizing anti-SEA antibodies are not responsible for the observed protection against toxic challenge.
FIG. 2.
Mucosal SEA tolerance is not antibody mediated. C57BL/6 μmT mice (n = 10) were given SEA or OVA i.n. three times 1 week apart and challenged with SEA-LPS 1 week later. Data are expressed as survival of OVA-tolerized (▪) and SEA-tolerized (□) μmT mice during the first 70 h post-SEA challenge. ∗∗, P < 0.01 for comparison to OVA-tolerized animals.
Systemic T-cell responses to i.n.-administered SEA.
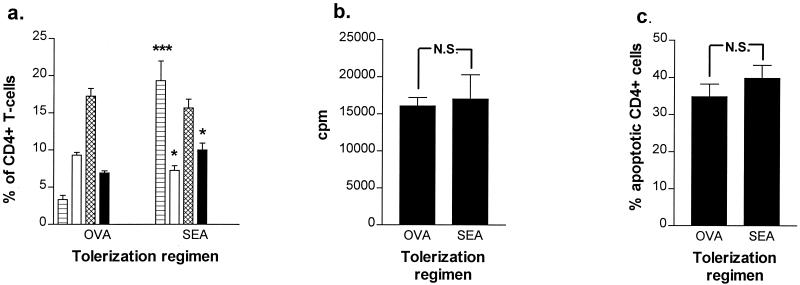
In mice the T cells expressing the Vβ1, Vβ3, Vβ10, Vβ11, Vβ12, Vβ17 and Vβ20 segments of the TCR interact with SEA (7, 18, 25). Fluorescence-activated cell sorter analysis of splenocytes obtained from tolerized C57BL/6 mice showed that both the Vβ3 and Vβ11 populations were significantly increased in SEA-tolerized animals compared to those in OVA-tolerized animals, indicating that SEA tolerization was not, as might have been expected, associated with clonal deletion of SEA-specific Vβ populations (Fig. 3a). In addition, in vitro restimulation of splenocytes from either OVA- or SEA-tolerized mice with SEA gave equal levels of proliferative responses (Fig. 3b). We also confirmed that there were no significant differences in either experimental group in the percentages of apoptotic CD4+ (Fig. 3c)- or Vβ3-expressing splenic T cells, following in vitro SEA stimulation. Taken together these results demonstrate that protection via i.n. SEA administration against SEA lethal challenge is not due to depletion, anergy, or apoptosis of SEA-specific T cells.
FIG. 3.
Mucosal SEA tolerance is not associated with T-cell deletion, anergy, or apoptosis. Spleen cell suspensions from i.n. OVA-treated (n = 3) and i.n. SEA-treated (n = 3) C57BL/6 mice were analyzed for frequencies of T cells expressing specific T-cell receptor Vβ subsets (a) as well as SEA-induced in vitro proliferation (b) and apoptotic death (c). Data are expressed as the mean ± SD (error bars) of the frequency of CD4+ T cells expressing the Vβ3 (striped bars), Vβ6 (white bars), Vβ8.1-8.2 (hatched bars), and Vβ11 (black bars) TCR (a), the in vitro proliferative responses to SEA (b), and the frequency of apoptotic CD4+ T cells following in vitro SEA activation (c). Results shown are representative of two experiments giving similar outcomes. Statistical significance for comparison to OVA-tolerized animals: ∗∗, P < 0.01; ∗∗∗, P < 0.001; N.S., not significant.
IL-10 plays a crucial role in tolerization.
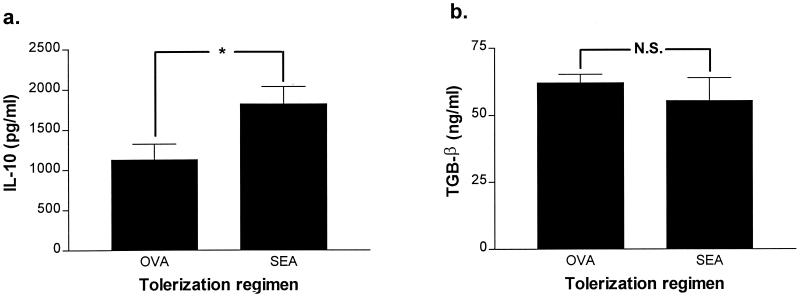
Serum TNF-α levels (i.n. SEA group: 1.9 ± 0.5 ng/ml; i.n. OVA group: 2.0 ± 0.7 ng/ml; n = 5 mice per group) were similar in both groups of mice at 4 h postchallenge with SEA, indicating that protection from lethal challenge is not the result of defective synthesis or secretion of this proinflammatory cytokine. Levels of the anti-inflammatory cytokines IL-10 and TGF-β in serum were assessed by ELISA in both the OVA- and SEA-tolerized groups. Prior to systemic SEA challenge the IL-10 levels were overall low. However, 4 h after SEA challenge the levels of IL-10 were significantly higher (P < 0.05) in SEA-tolerized animals than in the OVA-tolerized controls (Fig. 4a). In contrast, neither SEA nor OVA tolerization affected circulating TGF-β levels (Fig. 4b). This suggested that IL-10 has a protective role in diminishing the effects of deleterious cytokines (e.g., TNF) in the SEA-tolerized mice. To further investigate this we i.n. administered OVA or SEA to IL-10−/− mice and found that protection against systemic SEA challenge was not any more inducible (Fig. 5). It is clear, therefore, that IL-10 is a major protective component against superantigen-triggered toxicity and is triggered by i.n. exposure to SEA.
FIG. 4.
Mucosal SEA tolerance is associated with increased levels of IL-10. The levels of IL-10 and TGF-β were analyzed in plasma samples obtained 4 h post-SEA-LPS challenge of i.n. OVA (n = 5)- and SEA (n = 5)-treated animals. Data are expressed as the mean ± standard error (error bars) of the values for TGF-β (a) and IL-10 (b) in i.n. OVA-treated and i.n. SEA-treated mice. Statistical significance for comparison to OVA-tolerized animals: ∗, P < 0.05; N.S., not significant.
FIG. 5.
Induction of mucosal tolerance to SEA requires production of IL-10. IL-10−/− mice (n = 10) were given OVA or SEA three times i.n. and challenged with SEA-LPS. Data are expressed as the percentage of OVA-administered (▪) (n = 6) and SEA-tolerized (□) (n = 6) IL-10−/− mice that survived the first 20 h post-SEA challenge. This experiment was repeated one more time with similar results.
DISCUSSION
i.n. administration with SEA (but neither with OVA nor with an rSEA lacking superantigenic properties) protected mice against a lethal challenge with the toxin. The tolerization was superantigen specific as revealed by the failure to provide protection against septic death in animals tolerized with SEA but exposed systemically to an unrelated staphylococcal superantigen, TSST-1. This is the first report showing that mucosal tolerization can be achieved with substances other than conventional protein antigens. The protection afforded by tolerization with SEA was not due to anti-SEA antibody production, since mice lacking B cells were also efficiently protected. Furthermore, protection was not mediated by desensitization to the LPS used to potentiate the SEA challenge, since SEA-tolerized mice challenged with TSST-1 and LPS died at the same rate as OVA-tolerized mice. Instead, protection was mediated by the up-regulation of IL-10 production. Thus, we have succeeded in tolerizing mice with SEA via a mechanism remarkably different from the tolerance induced previously by intravenous injection of SEA. In the latter case, depletion of the reactive Vβ T-cell subsets and T-cell anergy was observed (4), whereas our results with i.n. SEA tolerization indicate expansion rather than depletion of superantigen-specific Vβ T-cell subsets and intact T-cell responses to in vitro restimulation with SEA.
A major question to be posed relates to the protective mechanism operating during superantigen-specific nasal tolerization. Two lines of evidence support the notion that significantly increased release of IL-10 is the major protective mechanism preventing septic death. First, within 2 to 4 h after systemic SEA challenge, levels of IL-10 in serum were significantly elevated in mice that had been exposed to i.n. SEA compared to those in the control, OVA-exposed animals. This increase coincided with a significantly increased survival rate. Secondly, attempts to i.n. tolerize IL-10−/− mice (but not their congeneric controls) with SEA were unsuccessful. Thus, endogenous inability to produce IL-10 will eliminate the protective effects of i.n. SEA tolerization. In this respect, previous studies have shown that exogenously provided IL-10 protects mice against SEB-induced lethal shock (3).
How would IL-10 support tolerance against SEA? It is established that IL-10 (along with TGF-β and IL-4) is a cytokine that displays strong anti-inflammatory properties in vivo and in vitro (11). Indeed, the sera of SEB-exposed IL-10−/− mice contained higher levels of proinflammatory mediators and were more susceptible to SEB-induced lethal shock than wild-type controls (13). It has been previously suggested that TNF-α is a critical determinant of lethal shock triggered by staphylococcal superantigens (19). This conclusion is valid in cases when mice are pretreated with d-galactosamine. In contrast, it is clear that in the absence of d-galactosamine pretreatment, as in the case of the present study, the levels of TNF-α are not related to mortality (1). Indeed, we found that, despite similar levels of circulating TNF-α in mice irrespective of their tolerization status, the mortality rates between the experimental groups showed very obvious differences. It should be noted that TNF-α is able to potently upregulate IL-10 synthesis (29) and thereby to provide negative feedback to its own production. In this aspect, priming and expansion of CD4+ T cells as a result of i.n. SEA administration might potentiate IL-10 production upon subsequent systemic SEA challenge, resulting in significantly improved survival. We cannot rule out the possibility that IL-10 might also reduce toxic shock, independent of its effect on TNF-α production, by down-regulating other inflammatory mediators, such as prostaglandins (8), or via antipyretic mechanisms (17). Staining for intracellular IL-10 in spleen cells of SEA-tolerized animals obtained 2 h post-enterotoxin challenge showed that the number of IL-10-producing lymphocytes was increased two- to threefold compared to that in tolerized but unchallenged controls and that 50 to 75% of the IL-10-producing cells expressed Vβ3 (data not shown).
Typically, mucosal tolerization is obtained by i.n. or gastric exposure of protein antigens to mucosal tissues. Since enterotoxins are protein molecules it should be critically discussed if the tolerization achieved is antigen specific or superantigen specific. Several lines of evidence suggest that the outcome observed by us is indeed superantigen-targeted tolerance. Firstly, T cells from tolerized animals display intact reactivity to SEA (Fig. 3B), in contrast to what would be expected in the case of protein antigen tolerization. More importantly, i.n. administration of rSEA devoid of its superantigenic properties, but intact with respect to its antigenic properties (2, 26), does not lead to development of protection against superantigen-mediated lethal shock. The question then is this: why did rSEA administration not protect against SEA-induced death? One possibility is that rSEA induces tolerance against rSEA but not against wild-type SEA as a result of altered antigen specificity caused by the amino acid substitutions. Another possibility is that rSEA-specific “tolerogenic” (that is, tolerance-mediating) T cells do respond favorably to SEA, but not at the appropriate time point (i.e., too slowly). A third possibility, which we favor, is that wild-type SEA but not rSEA expands the appropriate tolerogenic T cells expressing SEA-specific Vβ subsets and that substantial numbers of these cells fulfill the requirement for adequate levels of anti-inflammatory IL-10 to be secreted in response to the SEA challenge.
Given that tolerization is achievable by deposition of superantigen on the nasal mucosa, it is worth considering whether humans, who are frequently colonized with S. aureus in the anterior nares, are tolerized to the effects of staphylococcal enterotoxins. Toxin production by S. aureus is environmentally regulated, and the nasopharyngeal mucosal temperature is usually lower (21) than the optimal required for in situ biosynthesis and secretion of superantigenic toxins (6). Therefore, it seems unlikely that humans, under normal conditions, would be tolerized to superantigens produced by staphylococci colonizing the nasal passages.
In summary, our results suggest that i.n. exposure to SEA triggers superantigen-specific tolerance that is mediated by endogenous production of IL-10. Since i.n. exposure to enterotoxins does not give rise to any side effects, this approach should also be considered for use in humans.
Acknowledgments
This study was supported by the European Union Commission Biotechnology program (BIO4CT97-5130), the Swedish Association against Rheumatism, the Swedish Inflammation Network, The Swedish Society for Medical Research, The Swedish Medical Research Council, the Nana Svartz Foundation, and King Gustaf V's 80 Year Foundation.
We thank Margareta Verdrengh, Elisabeth Suri-Payer, and Inger Nordström for valuable assistance.
Editor: J. D. Clements
REFERENCES
- 1.Anderson, M. R., and M. Tary-Lehmann. 2001. Staphylococcal enterotoxin-B-induced lethal shock in mice is T-cell-dependent, but disease susceptibility is defined by the non-T-cell compartment. Clin. Immunol. 98:85-94. [DOI] [PubMed] [Google Scholar]
- 2.Bavari, S., B. Dyas, and R. G. Ulrich. 1996. Superantigen vaccines: a comparative study of genetically attenuated receptor-binding mutants of staphylococcal enterotoxin A. J. Infect. Dis. 174:338-345. [DOI] [PubMed] [Google Scholar]
- 3.Bean, A. G., R. A. Freiberg, S. Andrade, S. Menon, and A. Zlotnik. 1993. Interleukin 10 protects against staphylococcal enterotoxin B-induced lethal shock. Infect. Immun. 61:4937-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belfrage, H., M. Dohlsten, G. Hedlund, and T. Kalland. 1997. Prevention of superantigen-induced tolerance in vivo by interleukin-2 treatment. Cancer Immunol. Immunother. 44:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohach, G. A., D. J. Fast, R. D. Nelson, and P. M. Schlievert. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251-272. [DOI] [PubMed] [Google Scholar]
- 7.Bravo de Alba, Y., P. A. Cazenave, and P. N. Marche. 1995. Bacterial superantigen specificities of mouse T cell receptor V beta 20. Eur. J. Immunol. 25:3425-3430. [DOI] [PubMed] [Google Scholar]
- 8.Brown, N. L., S. A. Alvi, M. G. Elder, P. R. Bennett, and M. H. Sullivan. 2000. The regulation of prostaglandin output from term intact fetal membranes by anti-inflammatory cytokines. Immunology 99:124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y., J.-I. Inobe, R. Marks, P. Gonnella, V. K. Kuchroo, and H. L. Weiner. 1995. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376:177-180. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y., V. K. Kuchroo, J.-I. Inobe, D. A. Hafler, and H. L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265:1237-1240. [DOI] [PubMed] [Google Scholar]
- 11.de Waal Malefyt, R., and K. W. Moore. 1998. Interleukin 10, p. 333-364. In A. Thomson (ed.), The cytokine handbook. Academic Press, New York, N.Y.
- 12.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J. E. de Vries, and M. G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389:737-742. [DOI] [PubMed] [Google Scholar]
- 13.Hasko, G., L. Virag, G. Egnaczyk, A. L. Salzman, and C. Szabo. 1998. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur. J. Immunol. 28:1417-1425. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewski. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 15.Krakauer, T. 1999. Immune response to staphylococcal superantigens. Immunol. Res. 20:163-173. [DOI] [PubMed] [Google Scholar]
- 16.Krakauer, T. 1995. Inhibition of toxic shock syndrome toxin-1-induced cytokine production and T cell activation by interleukin-10, interleukin-4, and dexamethasone. J. Infect. Dis. 172:988-992. [DOI] [PubMed] [Google Scholar]
- 17.Leon, L. R., W. Kozak, K. Rudolph, and M. J. Kluger. 1999. An antipyretic role for interleukin-10 in LPS in mice. Am. J. Physiol. 276:R81-R89. [DOI] [PubMed] [Google Scholar]
- 18.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 19.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migita, K., and A. Ochi. 1994. Induction of clonal anergy by oral administration of staphylococcal enterotoxin B. Eur. J. Immunol. 24:2081-2086. [DOI] [PubMed] [Google Scholar]
- 21.Molony, N., A. I. G. Kerr, C. C. Blackwell, and A. Busuttil. 1996. Is the nasopharynx warmer in children than in adults? J. Clin. Forens. Med. 3:157-160. [DOI] [PubMed] [Google Scholar]
- 22.Saha, B., B. Jaklic, D. M. Harlan, G. S. Gray, C. H. June, and R. Abe. 1996. Toxic shock syndrome toxin-1-induced death is prevented by CTLA4Ig. J. Immunol. 157:3869-3875. [PubMed] [Google Scholar]
- 23.Stiles, B. G., Y. G. Campbell, R. M. Castle, and S. A. Grove. 1999. Correlation of temperature and toxicity in murine studies of staphylococcal enterotoxins and toxic shock syndrome toxin 1. Infect. Immun. 67:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun, J. B., N. Mielcarek, M. Lakew, J. M. Grzych, A. Capron, J. Holmgren, and C. Czerkinsky. 1999. Intranasal administration of a Schistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J. Immunol. 163:1045-1052. [PubMed] [Google Scholar]
- 25.Takimoto, H., Y. Yoshikai, K. Kiskihara, G. Matsuzaki, H. Kuga, T. Otani, and K. Nomoto. 1990. Stimulation of all T cells bearing V beta 1, V beta 3, V beta 11 and V beta 12 by staphylococcal enterotoxin A. Eur. J. Immunol. 20:617-621. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich, R. G., M. A. Olson, and S. Bavari. 1998. Development of engineered vaccines effective against structurally related bacterial superantigen. Vaccine 16:1857-1864. [DOI] [PubMed] [Google Scholar]
- 27.van Halteren, A. G., M. J. van der Cammen, D. Cooper, H. F. Savelkoul, G. Kraal, and P. G. Holt. 1997. Regulation of antigen-specific IgE, IgG1, and mast cell responses to ingested allergen by mucosal tolerance induc-tion. J. Immunol. 159:3009-3015. [PubMed] [Google Scholar]
- 28.van Houten, N., and S. F. Blake. 1996. Direct measurement of anergy of antigen-specific T cells following oral torerance induction. J. Immunol. 157:1337-1341. [PubMed] [Google Scholar]
- 29.Wanidworanun, C., and W. Strober. 1993. Predominant role of tumor necrosis factor in human monocyte IL-10 synthesis. J. Immunol. 151:6853-6861. [PubMed] [Google Scholar]
- 30.Weiner, H. L. 1997. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol. Today 18:335-343. [DOI] [PubMed] [Google Scholar]