Abstract
Salmonella enterica serovar Typhimurium is a gram-negative, facultative intracellular pathogen that predominantly invades mononuclear phagocytes and is able to establish persistent infections. One of the innate defense mechanisms of phagocytic cells is the production of reactive oxygen species, including superoxide. S. enterica serovar Typhimurium has evolved mechanisms to resist such radicals, and these mechanisms could be decisive in its ability to survive and replicate within macrophages. Recently, we described a superoxide-hypersusceptible S. enterica serovar Typhimurium mutant strain, DLG294, that carries a transposon in sspJ, resulting in the lack of expression of SspJ, which is necessary for resistance against superoxide and replication within macrophages. Here we show that DLG294, which is a 14028s derivative, hardly induced any granulomatous lesions in the livers upon subcutaneous infection of C3H/HeN (Ityr) mice with 3 × 104 bacteria and that its bacterial counts were reduced by 3 log units compared to those of wild-type S. enterica serovar Typhimurium 14028s on day 5 after infection. In contrast, DLG294 replicated like wild-type S. enterica serovar Typhimurium 14028s and induced a phenotypically similar liver pathology in p47phox−/− mice, which are deficient in the p47phox subunit of the NADPH oxidase complex and which do not produce superoxide. Consistent with these results, DLG294 reached bacterial counts identical to those of wild-type S. enterica serovar Typhimurium 14028s in bone marrow-derived macrophages from p47phox−/− mice and in X-CGD PLB-985 cells at 24 h after challenge. These results indicate that SspJ plays a role in the bacterium's resistance to oxidative stress and in the survival and replication of S. enterica serovar Typhimurium both in vitro and in vivo.
Salmonella enterica serovar Typhimurium is a gram-negative, facultative intracellular pathogen that predominantly invades mononuclear phagocytes and that is able to establish persistent infections by evasion or disturbance of the host defense (14). Despite the multitude of antimicrobial mechanisms present as part of the innate defense of phagocytic cells, S. enterica serovar Typhimurium is able to survive and replicate within phagosomes of macrophages. This ability of S. enterica serovar Typhimurium to enter and replicate within phagocytic cells is essential for its survival, as mutants unable to do so are avirulent (9). Although little is known of the exact mechanisms by which S. enterica serovar Typhimurium is able to survive after phagocytosis, S. enterica serovar Typhimurium responds to the specific host environment by expressing factors that are necessary for intracellular survival and for resistance against the defense systems of the host (5, 10, 11, 14, 18).
One of the major early defense mechanisms of macrophages against microorganisms is the production of toxic superoxide by the phagocyte NADPH oxidase and the subsequent generation of superoxide derivatives, both in vitro (17) and in vivo (22, 23, 27). The phagocyte NADPH oxidase is a multiprotein enzyme complex that comprises a heterodimeric, membrane-bound, flavocytochrome b558 (composed of a 91-kDa glycoprotein and a 22-kDa protein) and four cytosolic factors (p47phox, p67phox, p40phox, and Rac GTPase; phox for phagocyte oxidase) that translocate to the plasma membrane upon activation to interact with cytochrome b558. This leads to the formation of an active NADPH oxidase complex that accepts electrons from NADPH and donates them to molecular oxygen, resulting in the generation of superoxide (O2−) (reviewed in reference 1). Thus, upon stimulation of the phagocyte with opsonized microorganisms or other activating agents, the oxygen consumption increases dramatically (respiratory burst) and a large amount of superoxide is produced. This superoxide is converted into other, more potent, reactive oxygen species such as hydrogen peroxide, hydroxyl radicals, and hypochlorous acid.
Many microorganisms, including Salmonella, have evolved a multitude of defense mechanisms to resist this superoxide-mediated killing by phagocytes. These mechanisms include avoidance of encounters with phagocyte-derived oxidants, neutralization of such oxidants, and prevention of their production. S. enterica serovar Typhimurium is highly dependent on such mechanisms as they help the pathogen to survive within the phagosome. Examples of such defense mechanisms used by S. enterica serovar Typhimurium include inhibition of protein kinase C activity in macrophages, production of antioxidant scavengers, formation of heat shock proteins, and production of superoxide dismutase (SOD) (reviewed in reference 18). More recently, it was shown that S. enterica serovar Typhimurium could also prevent reactive oxygen species production by exclusion of NADPH oxidase from the phagosome in which Salmonella resides (12). The relative importance of each of these mechanisms for S. enterica serovar Typhimurium survival has not been elucidated; however, the periplasmic Cu,Zn-SOD and the type III secretion system encoded by Salmonella pathogenicity island 2 (SPI2) are very important in this defense, as mutants deficient in one of these systems show reduced survival within macrophages (4, 12, 28).
We have recently described the isolation of a mutant S. enterica serovar Typhimurium strain (DLG294) that showed decreased resistance to oxidative stress induced by menadione in vitro due to the inactivation of a gene designated sspJ (for Salmonella superoxide protection) (25). In this report we describe the further investigation of superoxide-sensitive mutant strain DLG294 and show that the gene encoding SspJ is essential for resistance to oxidative stress and thus for survival and replication of S. enterica serovar Typhimurium both in vitro and in vivo.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female Salmonella-resistant (Ityr) C3H/HeN mice and Salmonella-sensitive (Itys) C57BL/6 mice were purchased from Harlan (Horst, The Netherlands) and Charles River Laboratories, respectively. Mice were maintained under standard conditions according to institutional guidelines. Water and food were given ad libitum.
Female mice homozygous for a targeted mutation in the p47phox subunit of the NADPH oxidase on a C57BL/6 background (p47phox−/−) were generated as described previously (13) and obtained from Taconic Laboratory (Germantown, N.Y.). All experiments were approved by the local Animal Ethical Committee.
Bacterial strains and plasmids.
Single colonies of wild-type S. enterica serovar Typhimurium 14028s (50% lethal dose after intraperitoneal injection: 5 × 103 bacteria for Ityr mice and <102 bacteria for Itys mice) and superoxide-sensitive derivative DLG294 (25) were grown overnight in Luria-Bertani (LB) medium (10 mg of tryptone, 5 mg of yeast extract, and 10 mg of NaCl/ml) at 37°C while being shaken (225 rpm). In addition, DLG294 supplemented with low-copy-number plasmid pWSK29 expressing the sspJ gene, which is inactivated in DLG294 (DLG294-pTS175), and with its vector control (DLG294-pWSK29) were used (25). These bacteria were grown in LB medium supplemented with 50 μg of ampicillin (Merck)/ml. For in vivo infection experiments, bacteria were grown to the end of log phase and were then washed and diluted in sterile phosphate-buffered saline (PBS). The CFU in the inoculum were determined by plating serial dilutions.
Cells and culture conditions.
PLB-985 and X-CGD PLB-985 cells (29) were grown in RPMI 1640 medium supplemented with 2 mM glutamine, 10% fetal bovine serum, penicillin (1,000 U/ml), and streptomycin (50 μg/ml) at 37°C and 5% CO2. For granulocytic differentiation, the cells were exposed to 0.5% dimethylformamide for 5 to 6 days. Under these conditions, the PLB-985 cells acquire respiratory-burst activity, while the X-CGD PLB-985 cells do not (29).
Bone marrow-derived macrophages from C57BL/6 and p47phox−/− mice were obtained and cultured as described previously (2) with a few modifications. Briefly, mice were killed by cervical dislocation, and the femurs were isolated under sterile conditions. The bone marrow was flushed from the femurs in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% horse serum. The cells were resuspended in DMEM supplemented with 20% horse serum and seeded in 24-well tissue culture plates (Greiner) at a concentration of 2 × 105 nucleated cells per well and were allowed to adhere for 2 h. Nonadherent cells were removed, and the remaining adherent cells were cultured for 1 week in DMEM supplemented with 20% horse serum and 20% embryonic mouse fibroblast-conditioned medium as the source of colony-stimulating factor.
Replication of S. enterica serovar Typhimurium within macrophages.
Macrophages (2 × 105) were challenged with S. enterica serovar Typhimurium at a 10:1 multiplicity of infection. To promote the uptake of the bacteria, the bacteria were spun onto the macrophages by centrifugation at 300 × g for 10 min and the cells were allowed to internalize the bacteria for 30 min at 37°C, 5% CO2. For determination of the replication of S. enterica serovar Typhimurium within macrophages and PLB-985 cells, we used the bacterial infection protocol described by Rathman et al. (20) with a few modifications. The cells were washed with PBS and incubated for 1 h in medium supplemented with 100 μg of gentamicin/ml to kill the extracellular bacteria and were then washed again. This time point is designated time zero. Medium supplemented with 10 μg of gentamicin/ml was added to the cells to kill any remaining extracellular bacteria and to prevent reinfection. It has been reported that this concentration of gentamicin does not affect the growth of intracellular bacteria (26). At 24 h, the cells were washed with PBS to remove the gentamicin and were lysed in 1 ml of distilled water. Serial dilutions of the lysate were plated for determination of the number of intracellular CFU. For examination of our S. enterica serovar Typhimurium strains in an in vitro model of NADPH oxidase deficiency, we used X-CGD PLB-985, a cell line in which the gp91phox gene was disrupted by homologous recombination in leukemic cell line PLB-985. As a consequence, these cells fail to produce superoxide mediated by NADPH oxidase (29). For in vitro replication experiments using the nonadherent PLB-895 and X-CGD PLB-985 cells, the bacteria were not spun onto the cells but were incubated together with the cells while rotating. Like the adherent bone marrow-derived macrophages, these cells were treated with 100 μg of gentamicin/ml to kill the extracellular bacteria. After the cells were washed with PBS, medium supplemented with 10 μg of gentamicin/ml was added to the cells to kill any remaining bacteria and to prevent reinfection. At 24 h, the cells were washed with PBS to remove the gentamicin and were lysed in 1 ml of distilled water. Serial dilutions of the lysate were plated for determination of the number of intracellular CFU.
In vivo Salmonella infections.
Mice were inoculated subcutaneously in the flanks with 0.1 ml of bacterial suspension in PBS. Per group, four mice were used for each time point. Mice were sacrificed at several time points by carbon dioxide inhalation or by cervical dislocation. Blood was taken by cardiac puncture, and livers, spleens, and inguinal lymph nodes were aseptically removed. To determine the bacterial load within these organs, single-cell suspensions were prepared by using sterile 70-μm-mesh-size cell strainers (Falcon). Cells were pelleted by centrifugation for 10 min and were lysed in distilled water. The bacterial number per organ was determined bacteriologically by plating serial dilutions.
Histopathology.
Livers from infected mice were harvested, and a segment of tissue was fixed in 10% buffered formalin solution for histopathology. Tissues were embedded in paraffin, and 3-μm-thick sections were prepared and stained with hematoxylin and eosin. The pathology in the livers was examined and judged microscopically.
Statistics.
Statistical analysis was performed using Student's t tests, and a P value <0.05 was considered significant.
RESULTS
In vivo growth of 14028s and DLG294 in Salmonella-resistant C3H/HeN mice.
Because DLG294 has been shown to be unable to multiply within macrophage-like cell line RAW264.7 and because it caused less deaths among intraperitoneally infected C3H/HeN and C57BL/6 mice than strain 14028s (25), we examined the course of infection for both strains in mice by determining the CFU counts and examining the induced pathology in the livers, spleens, and lymph nodes. C3H/HeN mice were infected subcutaneously in the flanks with ∼30,000 CFU of S. enterica serovar Typhimurium 14028s or DLG294. The inoculum was given subcutaneously to establish a reservoir in the lymph nodes, whence Salmonella readily spreads throughout the body via the lymph stream and becomes systemic, finally reaching the liver and the spleen (3).
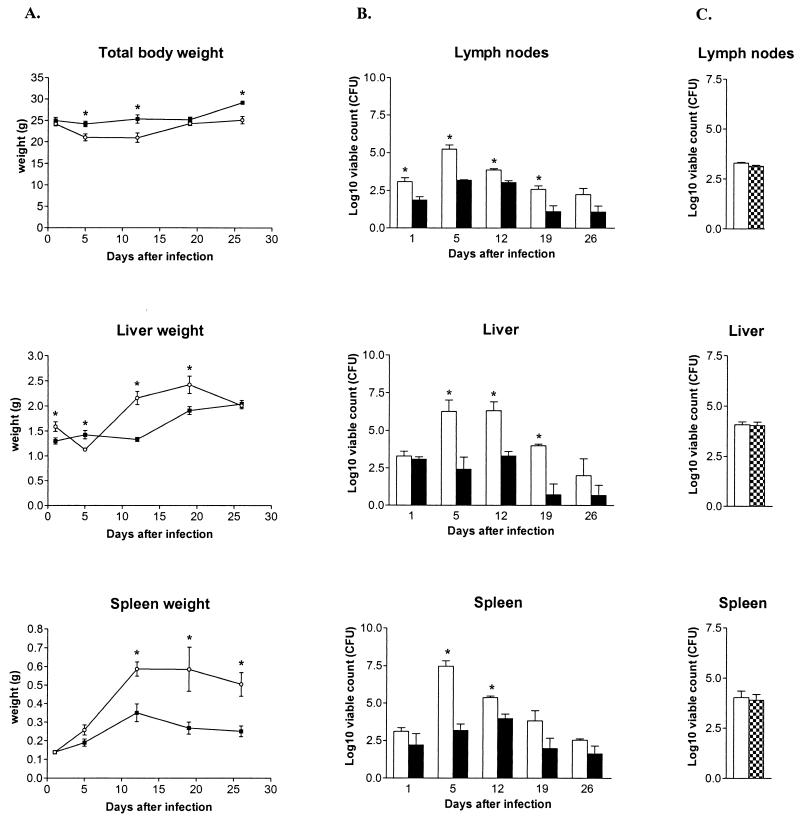
The mice challenged with wild-type S. enterica serovar Typhimurium 14028s showed a decrease in total body weight (Fig. 1A), while the DLG294-challenged mice did not. S. enterica serovar Typhimurium 14028s induced intense hepatosplenomegaly, while these organs of the DLG294-infected mice were only modestly enlarged, indicating that DLG294 caused less pathology than the wild-type 14028s strain (Fig. 1A). This was confirmed by the bacterial counts and the macroscopic appearance of these organs. S. enterica serovar Typhimurium 14028s was able to multiply within the livers, spleens, and lymph nodes and reached a peak between days 5 and 12. In contrast, DLG294 was not able to replicate and the number of bacteria in the livers, spleens, and lymph nodes was reduced by 3 log units compared to the number of 14028s bacteria (Fig. 1B). Also the macroscopic lesions in the organs were less severe in mice infected with DLG294 than in mice infected with 14028s. Taken together, these data indicate that DLG294 is severely attenuated in vivo. This low virulence of DLG294 could be fully reversed to that of wild-type S. enterica serovar Typhimurium 14028s by the expression of sspJ on low-copy-number plasmid pWSK29 (DLG294-pTS175) (Fig. 1C). This, together with the previous results obtained in vitro, indicate that the SspJ protein is important in the in vivo replication of S. enterica serovar Typhimurium.
FIG. 1.
Total body weights and liver and spleen weights (A) and bacterial counts in livers, spleens, and lymph nodes (B) of C3H/HeN mice infected with S. enterica serovar Typhimurium 14028s (open circles and white bars) and DLG294 (black squares and black bars) at several time points after infection. (C) Bacterial counts of C3H/HeN mice infected with S. enterica serovar Typhimurium 14028s (white bars) and DLG294 complemented by the low-copy-number plasmid pWSK29 carrying sspJ (DLG294-pTS175; checkered bars) on day 6 after infection. C3H/HeN mice were infected subcutaneously in the flanks with ∼30,000 CFU of S. enterica serovar Typhimurium 14028s, DLG294, or DLG294-pTS175. The actual dosage was confirmed by plating serial dilutions of the inoculum. At the indicated time points, livers, spleens, and lymph nodes were aseptically removed and weighed, and the viable counts within these organs were determined by plating serial dilutions (n = 4 per group). The results are expressed as log10 viable counts (means ± standard errors of the means). Asterisks indicate that the number of bacteria of wild-type 14028s is significantly different from that of DLG294 (Student's t test).
Histopathology of S. enterica serovar Typhimurium 14028s and DLG294 infection in C3H/HeN mice.
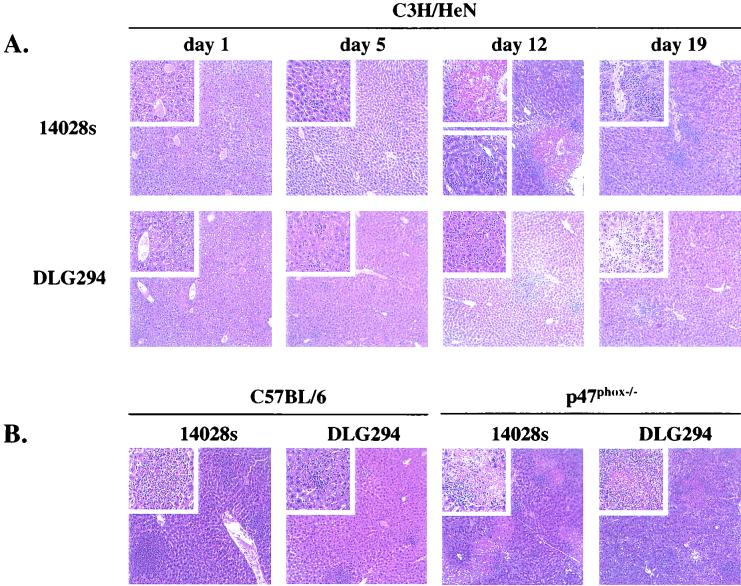
Microscopic examination revealed that the livers of mice infected with wild-type S. enterica serovar Typhimurium 14028s and DLG294 had a normal appearance on day 1. Microabscesses started to appear in the livers in 14028s-infected mice on day 5 and to a greater extent on day 12, when the highest numbers of bacteria were observed in the livers. Representative tissue sections of the livers are shown in Fig. 2A. The microabscesses were still present on day 19, but the number was less than the number on day 12. Granulomatous lesions were first observed on day 5 but were more pronounced on day 12 of infection. On day 19, when the bacterial counts in the livers were reduced, the number of lesions was also reduced. Some microabscesses were present in livers of DLG294-infected mice on days 5, 12, and 19, but pathology was not as severe as in wild-type S. enterica serovar Typhimurium 14028s-infected mice. A few granulomas were also observed in the livers of DLG294-infected mice on days 5 and 12, but again the pathology was much less extensive than that for 14028s-infected mice (Fig. 2A).
FIG. 2.
Microscopic appearance of livers from C3H/HeN mice infected with S. enterica serovar Typhimurium 14028s and DLG294 at several time points after infection (A) and C57BL/6 and p47phox−/− mice at 4 days after infection with S. enterica serovar Typhimurium 14028s and DLG294 (B). Mice were infected subcutaneously in the flanks as described in Materials and Methods. hematoxylin- and eosin-stained sections were prepared from livers that were isolated at different time points after infection.
In vitro intracellular replication of S. enterica serovar Typhimurium in bone marrow-derived macrophages.
The protein encoded by the sspJ gene (SspJ) is essential for replication within macrophages and is crucial for resistance to oxidative stress induced by menadione (25), and its absence results in decreased virulence in vivo (Fig. 1 and 2A). This suggested a role for SspJ in preventing the induction, or in neutralizing the activity, of superoxide-mediated antimicrobial effector mechanisms of phagocytes, such as the NADPH oxidase system. We hypothesized that if this protein is involved in escaping oxidative stress, superoxide-sensitive mutant strain DLG294 would be able to survive and replicate within cells that do not produce any superoxide. This hypothesis was tested in a macrophage intracellular replication assay using bone marrow-derived macrophages from p47phox−/− mice in a Salmonella-susceptible C57BL/6 (Itys) genetic background, as these mice have been shown to produce no superoxide (13).
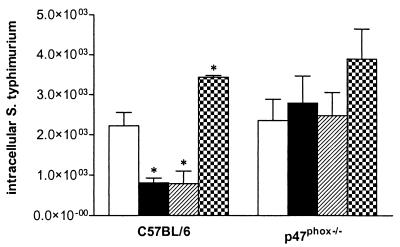
There was no difference between the S. enterica serovar Typhimurium strains in replication in the bone marrow-derived macrophages of p47phox−/− mice (Fig. 3), indicating that the superoxide-sensitive mutant strain DLG294 was as virulent as 14028s in cells that do not produce any phagocyte superoxide. The numbers of intracellular bacteria of 14028s in the macrophages of the control C57BL/6 mice, which produce superoxide upon bacterial challenge, were higher than those of DLG294. The ability of DLG294 to replicate intracellularly was restored to that of the wild type when sspJ was expressed on a low-copy-number plasmid (DLG294-pTS175), whereas introduction of vector pWSK29 did not affect the intracellular count. For confirmation of superoxide deficiency in the macrophages of p47phox−/− mice, nitroblue tetrazolium reduction was determined. The macrophages of wild-type C57BL/6 mice were shown to be able to produce superoxide, while the macrophages of the p47phox−/− mice were not (data not shown).
FIG. 3.
Intracellular S. enterica serovar Typhimurium in mouse bone marrow-derived macrophages from C57BL/6 and p47phox−/− mice. The cells were challenged with S. enterica serovar Typhimurium 14028s (white bars), DLG294 (black bars), DLG294-pWSK29 (hatched bars), and DLG294-pTS175 (checkered bars) as described in Materials and Methods. The numbers of intracellular bacteria were determined 24 h after challenge. Asterisks indicate that the number of intracellular bacteria is significantly different from that of wild-type S. enterica serovar Typhimurium 14028s. Mean data of two independently performed experiments ± standard errors of the means are shown.
S. enterica serovar Typhimurium infection of wild-type and p47phox−/− mice.
The in vitro infection assay showed that DLG294 was able to replicate intracellularly in bone marrow-derived macrophages from mice that do not produce any superoxide due to a homozygous mutation in the p47phox subunit of the NADPH oxidase (p47phox−/− mice). In these cells, DLG294 reached bacterial counts comparable to those of wild-type S. enterica serovar Typhimurium 14028s. To analyze whether both strains are also equally virulent in vivo under conditions when no superoxide is produced, we used the Salmonella infection model in these p47phox−/− mice and their superoxide-producing controls C57BL/6 (Itys). Since Itys mice are very sensitive to S. enterica serovar Typhimurium infection, the dose was decreased compared to the dose given to Ityr C3H/HeN mice. The p47phox−/− mice were expected to be even more sensitive to infection, and therefore infection was studied only at early time points.
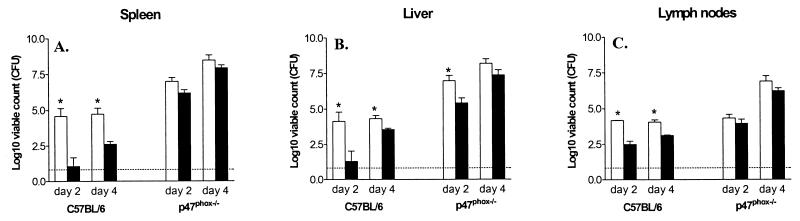
The infected C57BL/6 mice showed a slight reduction in body weight, and there was hardly any difference between 14028s- and DLG294-infected mice at such early time points after infection. However, the liver and spleen weights of 14028s-infected wild-type mice strongly increased compared to those of DLG294-infected wild-type mice, but not until 6 days after infection (data not shown). The bacterial counts in the different organs show that DLG294 was less virulent than 14028s in the wild-type (Itys) mice (Fig. 4), which was in concordance with the results obtained with the (Ityr) C3H/HeN mice (Fig. 1). So, even in Salmonella-sensitive (Itys) mice, superoxide-sensitive mutant strain DLG294 is attenuated. In p47phox−/− mice, however, 14028s induced no increase in liver weight within 4 days after infection but did cause an increase in spleen weight. DLG294 did not seem to cause hepatosplenomegaly in the p47phox−/− mice, as the liver weight declined and the spleen weight remained the same at the early time points after infection (data not shown). As expected, due to the defect in innate defense, the bacterial loads were higher in the p47phox−/− mice than in the wild-type mice (Fig. 4), and after 5 days of infection all the p47phox−/− mice died from the infection. At days 2 and 4 after infection, with the exception of the livers at day 2, there was no difference in bacterial loads between DLG294- and 14028s-infected mice, indicating that the replication of the superoxide-sensitive mutant strain was not hampered in mice that do not produce any superoxide.
FIG. 4.
Bacterial counts in livers, spleens, and lymph nodes of p47phox−/− and wild-type C57BL/6 (Itys) mice infected with S. enterica serovar Typhimurium 14028s (white bars) and DLG294 (black bars). The p47phox−/− mice and the wild-type C57BL/6 mice were infected subcutaneously in the flanks with ∼10,000 CFU of S. enterica serovar Typhimurium 14028s or DLG294. The actual dosage was confirmed by plating serial dilutions of the inoculum. Bacterial counts in livers, spleens, and lymph nodes were determined at days 2 and 4. The results are expressed as log10 viable counts (means ± standard errors of the means) obtained from groups of four mice per time point. Asterisks indicate that the number of wild-type 14028s bacteria is significantly different from that of DLG294 bacteria (Student's t test). All Salmonella-infected p47phox−/− mice were dead by day 5. Dashed lines, detection limit.
Histopathology of S. enterica serovar Typhimurium 14028s and DLG294 infection in wild-type and p47phox−/− C57BL/6 mice.
Microscopic examination of the organs of the C57BL/6 mice revealed that the livers appeared normal on day 1 for both the 14028s- and the DLG294-infected mice. Infiltrating cells started to appear in the livers of 14028s-infected wild-type mice on day 2, and large amounts of microabscesses and granulomatous lesions were present on day 4. Representative tissue sections are shown in Fig. 2B. Infiltrating cells and microabscesses were also observed in DLG294-infected wild-type mice on days 2 and 4, but to a much lesser extent and with only a very few beginning lesions on day 4. In the livers of the p47phox−/− mice, on the other hand, infiltrating cells were already observed in the livers on day 1 after infection in both the 14028s- and the DLG294-infected mice. Microabscesses started to appear on day 2. On day 4, the structure of the livers was destroyed in both groups of p47phox−/− mice, the microabscesses became very abundant, and many necrotic granulomatous lesions were present (Fig. 2B). Thus, the pathology induced by DLG294 was less severe in wild-type mice but was as severe as that induced by14028s in the p47phox−/− mice, which do not produce any superoxide. Thus, when no superoxide was produced, superoxide-sensitive mutant strain DLG294 appeared to be as virulent and as pathogenic as wild-type S. enterica serovar Typhimurium 14028s. This strongly suggests a role for SspJ in the defense mechanisms of S. enterica serovar Typhimurium against superoxide produced by the NADPH oxidase.
In vitro intracellular growth of S. enterica serovar Typhimurium in PLB-985 cells.
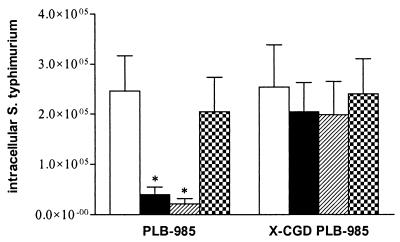
The p47phox−/− mice showed an autosomal recessive deficiency in the p47phox subunit of the NADPH oxidase complex and were therefore unable to produce any superoxide. To exclude the possibility that the observed effect in the bone marrow-derived macrophages from p47phox−/− mice was due to the deficiency in the p47phox protein itself and not to the inactivation of the NADPH oxidase complex, we wanted to examine our S. enterica serovar Typhimurium strains in another in vitro model for NADPH oxidase deficiency. Therefore, we used X-CGD PLB-985, a cell line in which the gp91phox gene was disrupted by homologous recombination in human leukemic cell line PLB-985 and which, as a consequence, fails to produce any superoxide (29). Cells were infected with S. enterica serovar Typhimurium 14028s, DLG294, DLG294-pWSK29, and DLG294-pTS175 as described for the replication assay in macrophages. Mean data from two independently performed experiments are shown in Fig. 5. At 24 h after infection, bacterial loads of wild-type S. enterica serovar Typhimurium 14028s within wild-type PLB-985 cells were higher than those of mutant strain DLG294. In the X-CGD PLB-985 cells, however, there was no difference in the intracellular CFU count after 24 h between the S. enterica serovar Typhimurium strains. The diminished intracellular replication of DLG294 could be fully reversed to that of wild-type S. enterica serovar Typhimurium 14028s by the expression of sspJ on low-copy-number plasmid pWSK29 (DLG294-pTS175), whereas introduction of the vector (DLG294-pWSK29) did not affect the intracellular count. These data are in concordance with the data obtained with the macrophages of wild-type and p47phox−/− C57BL/6 mice (Fig. 3).
FIG. 5.
Intracellular S. enterica serovar Typhimurium in PLB-985 and X-CGD PLB-985 cells. The cells were challenged with S. enterica serovar Typhimurium 14028s (white bars), DLG294 (black bars), DLG294-pWSK29 (hatched bars), and DLG294-pTS175 (checkered bars) as described in Materials and Methods. The numbers of intracellular bacteria were determined at 24 h after challenge. Asterisks indicate that the number of intracellular bacteria is significantly different from that of wild-type S. enterica serovar Typhimurium 14028s. Mean data of two independently performed experiments ± standard errors of the means are shown.
DISCUSSION
The main findings of this study are that the superoxide-sensitive S. enterica serovar Typhimurium mutant strain DLG294 (25) is attenuated in vivo and causes less pathology than wild-type S. enterica serovar Typhimurium 14028s but that it regains virulence in X-CGD PLB-985 cells and in p47phox−/− mice that show a deficiency in the phagocyte NADPH oxidase and therefore do not produce any superoxide. In addition, the decreased virulence of DLG294 is a direct cause of the defect in sspJ and is not a polar effect caused by diminished transcription of genes directly downstream of sspJ, since the virulence can be fully restored to that of the wild type by the expression of sspJ on a low-copy-number plasmid (DLG294-pTS175). These results suggest that the protein encoded by sspJ (SspJ) is necessary for virulence and plays a very important role in the defense mechanisms of S. enterica serovar Typhimurium against superoxide produced by the phagocyte NADPH oxidase.
We examined the in vivo courses of infection of S. enterica serovar Typhimurium 14028s and DLG294 in the typhoid mouse model by infecting C3H/HeN mice (Ityr) subcutaneously in the inguinal region to establish a reservoir near the lymph nodes; Salmonella readily spreads from this reservoir via the lymph stream, and the infection becomes systemic, finally reaching the liver and spleen (3). This model gives rise to a more subtle chronic infection than the intraperitoneal, intravenous, and oral infection models that give rise to peracute and overwhelming systemic infections. As shown with this model, DLG294 is highly attenuated both in the relatively resistant Ityr mice and in S. enterica serovar Typhimurium-sensitive Itys mice. This is remarkable, since Itys C57BL/6 mice have somewhat reduced phagocyte production of reactive nitrogen and oxygen species (6, 19, 21) and yet our mutant strain DLG294 still was attenuated. Apparently, Itys C57BL/6 mice produce sufficient superoxide to prevent DLG294 replication. In contrast, the virulence of DLG294 was completely restored in p47phox−/− mice, which do not produce any superoxide. These results were confirmed by in vitro replication experiments with bone marrow-derived macrophages from the wild-type (superoxide-positive) and p47phox−/− (superoxide-negative) mice and with X-CGD PLB-985 cells (29). Thus, when no superoxide was produced, superoxide-sensitive mutant S. enterica serovar Typhimurium strain DLG294 was able to replicate independently of the underlying genetic defect in the genes encoding the NADPH oxidase, which causes the defect in superoxide production.
We observed severe pathology in the livers of C3H/HeN and C57BL/6 mice infected with wild-type S. enterica serovar Typhimurium 14028s and in p47phox−/− mice infected with 14028s or DLG294. Liver pathology can be induced by superoxide produced upon activation of macrophages, as shown in mice chronically treated with alcohol (15). In our experiments, however, the liver pathology is induced by S. enterica serovar Typhimurium itself and is not caused by superoxide produced upon infection, as the p47phox−/− mice produce no superoxide but do develop severe liver damage upon infection with S. enterica serovar Typhimurium 14028s and DLG294. In addition, the pathology is most severe when bacterial numbers are highest.
A mutant that has a phenotype similar to that of DLG294 is a zwf mutant that is deficient in glucose-6-phosphate dehydrogenase (G6PD) (16). G6PD catalyzes the first step in the pentose phosphate cycle, which provides ribose for nucleoside synthesis and reducing equivalents in the form of NADPH. G6PD is very important in defense against oxidative and nitrosative stress because it provides NADPH, thereby maintaining the cellular redox state, regenerating reduced thiols, and repairing oxidative and nitrosative damage (8, 24). Mutants deficient in zwf show increased susceptibility to reactive oxygen and nitrogen intermediates and are attenuated in mice (16). Also the in vivo virulence of these mutants can be fully restored to that of the wild type by elimination of the phagocyte respiratory-burst oxidase (gp91phox−/− mice) (16). However, the zwf mutant is also more sensitive to hydrogen peroxide (16), whereas DLG294 is not (25), suggesting that SspJ is not a dehydrogenase.
It has previously been reported that periplasmic Cu,Zn-SOD (SodC) is very important in the defense of S. enterica serovar Typhimurium against oxidative stress, as was evident from sodC mutants, which showed reduced survival within macrophages and attenuated virulence in mice (4, 7). S. enterica serovar Typhimurium contains two periplasmic SODs, SodCI and SodCII (7). When one of these SODs is deleted, S. enterica serovar Typhimurium is slightly attenuated, but when both SODs are deleted, S. enterica serovar Typhimurium is highly attenuated (7). The phenotype of DLG294 resembles that of this SodC double mutant and suggests a role for SspJ as an SOD. However, we have previously shown that SOD activities of DLG294 and wild-type S. enterica serovar Typhimurium 14028s are the same (25). In addition, SodCII expression is upregulated in stationary phase (7), while SspJ is constitutively expressed, and so far no inducing conditions have been found (25). These results indicate that the SspJ protein is not an SOD. However, SspJ plays a crucial role in the defense against superoxide. It could act in a regulatory pathway as a sensor or cofactor for SOD, it could be involved in neutralizing the toxic effects of superoxide, or it could even prevent the production of superoxide.
The last mechanism has been described for mutants deficient in SPI2, which have the same phenotype as DLG294 (12, 28). It is proposed that the proteins encoded by the genes located within this SPI2 defend the bacterium against superoxide by preventing the NADPH oxidase from trafficking toward the Salmonella-containing vacuoles (28) or by preventing the assembly of the NADPH oxidase at the phagosomal membrane (12). We are currently investigating whether sspJ has a role in this mechanism of preventing superoxide production.
The sodCI and -CII, the zwf, and the type III secretion mutants described above have diverse genetic defects, but they all have the same phenotype. Apparently, the interplay between all these distinct factors determines resistance to superoxide. It is clear that sspJ is important in this resistance, but its exact function and mechanism of action remain to be elucidated.
Acknowledgments
We thank Daan van der Keur, Joke van de Gevel, and Beppie van Strijen for technical assistance.
Editor: A. D. O'Brien
REFERENCES
- 1.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 2.Burger, E. H., J. W. van der Meer, J. S. van de Gevel, J. C. Gribnau, G. W. Thesingh, and R. van Furth. 1982. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J. Exp. Med. 156:1604-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, Z.-M., and M. K. Jenkins. 1999. Clonal expansion of antigen-specific CD4 T cells following infection with Salmonella typhimurium is similar in susceptible (Itys) and resistant (Ityr) BALB/c mice. Infect. Immun. 67:2025-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demple, B. 1991. Regulation of bacterial oxidative stress genes. Annu. Rev. Genet. 25:315-337. [DOI] [PubMed] [Google Scholar]
- 6.Denis, M., A. Forget, M. Pelletier, and E. Skamene. 1988. Pleiotropic effects of the Bcg gene. III. Respiratory burst in Bcg-congenic macrophages. Clin. Exp. Immunol. 73:370-375. [PMC free article] [PubMed] [Google Scholar]
- 7.Fang, F. C., M. A. DeGroote, J. W. Foster, A. J. Baumler, U. Ochsner, T. Testerman, S. Bearson, J. C. Giard, Y. Xu, G. Campbell, and T. Laessig. 1999. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA 96:7502-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay, B. B., and S. Falkow. 1989. Common themes in microbial pathogenicity. Microbiol. Rev. 53:210-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 12.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 13.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 15.Kono, H., I. Rusyn, M. Yin, E. Gabele, S. Yamashina, A. Dikalova, M. B. Kadiiska, H. D. Connor, R. P. Mason, B. H. Segal, B. U. Bradford, S. M. Holland, and R. G. Thurman. 2000. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J. Clin. Investig. 106:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundberg, B. E., R. E. J. Wolf, M. C. Dinauer, Y. Xu, and F. C. Fang. 1999. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect. Immun. 67:436-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivier, M., P. Cook, J. Desanctis, Z. Hel, W. Wojciechowski, N. E. Reiner, E. Skamene, and D. Radzioch. 1998. Phenotypic difference between Bcg(r) and Bcg(s) macrophages is related to differences in protein-kinase-C-dependent signalling. Eur. J. Biochem. 251:734-743. [DOI] [PubMed] [Google Scholar]
- 20.Rathman, M., L. P. Barker, and S. Falkow. 1997. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect. Immun. 65:1475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojas, M., L. F. Barrera, G. Puzo, and L. F. Garcia. 1997. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J. Immunol. 159:1352-1361. [PubMed] [Google Scholar]
- 22.Shiloh, M. U., J. D. MacMicking, S. Nicholson, J. E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29-38. [DOI] [PubMed] [Google Scholar]
- 23.Shiloh, M. U., J. Ruan, and C. Nathan. 1997. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect. Immun. 65:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storz, G., L. A. Tartaglia, S. B. Farr, and B. N. Ames. 1990. Bacterial defenses against oxidative stress. Trends Genet. 6:363-368. [DOI] [PubMed] [Google Scholar]
- 25.van der Straaten, T., A. van Diepen, K. Kwappenberg, S. van Voorden, K. Franken, R. Janssen, J. G. Kusters, D. L. Granger, and J. T. van Dissel. 2001. Novel Salmonella enterica serovar Typhimurium protein that is indispensable for virulence and intracellular replication. Infect. Immun. 69:7413-7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaudaux, P., and F. A. Waldvogel. 1979. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 16:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 29.Zhen, L., A. A. King, Y. Xiao, S. J. Chanock, S. H. Orkin, and M. C. Dinauer. 1993. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc. Natl. Acad. Sci. USA 90:9832-9836. [DOI] [PMC free article] [PubMed] [Google Scholar]