Abstract
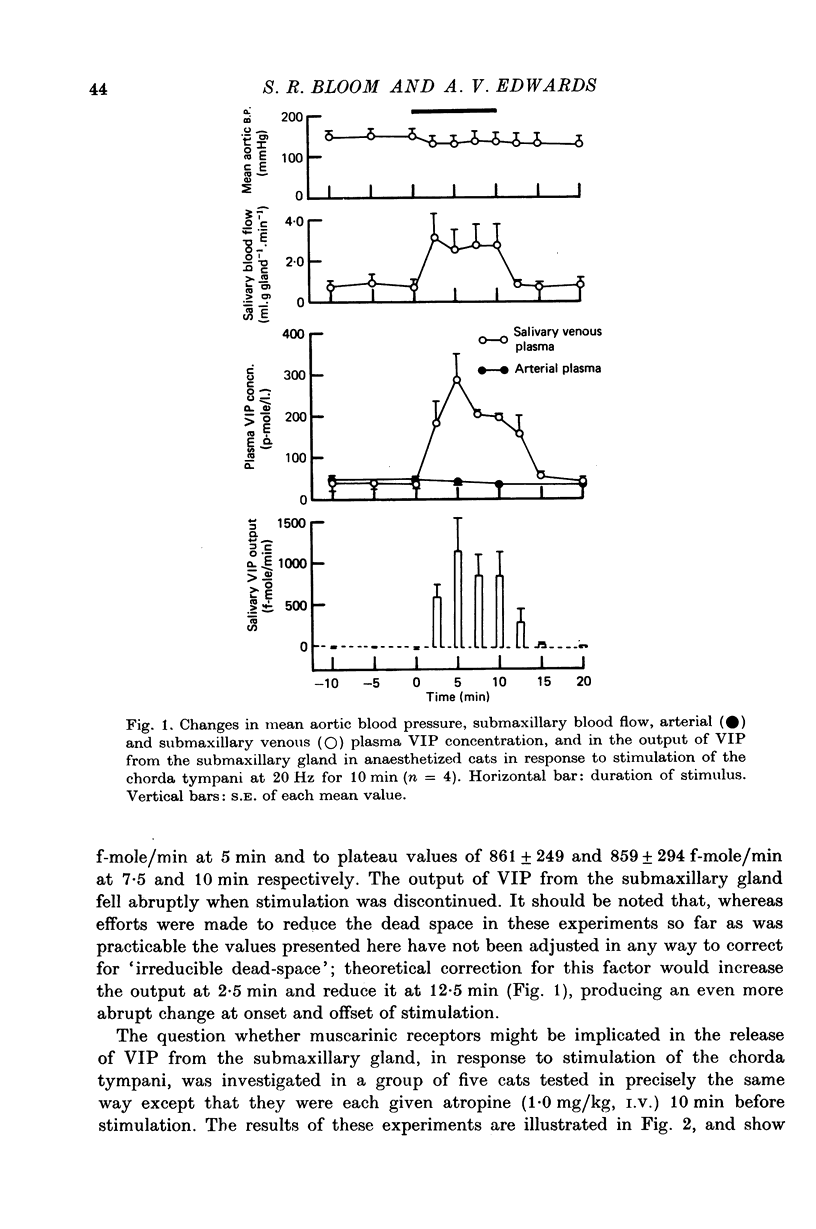
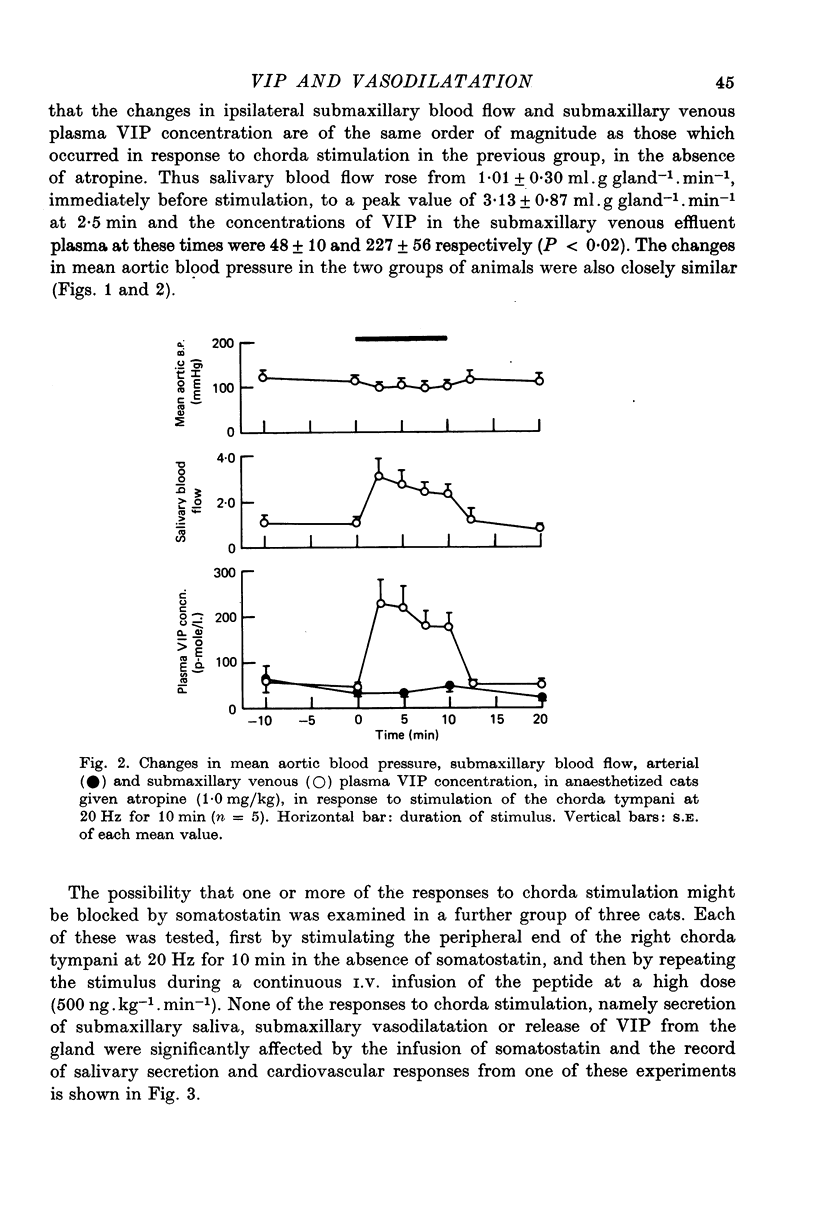
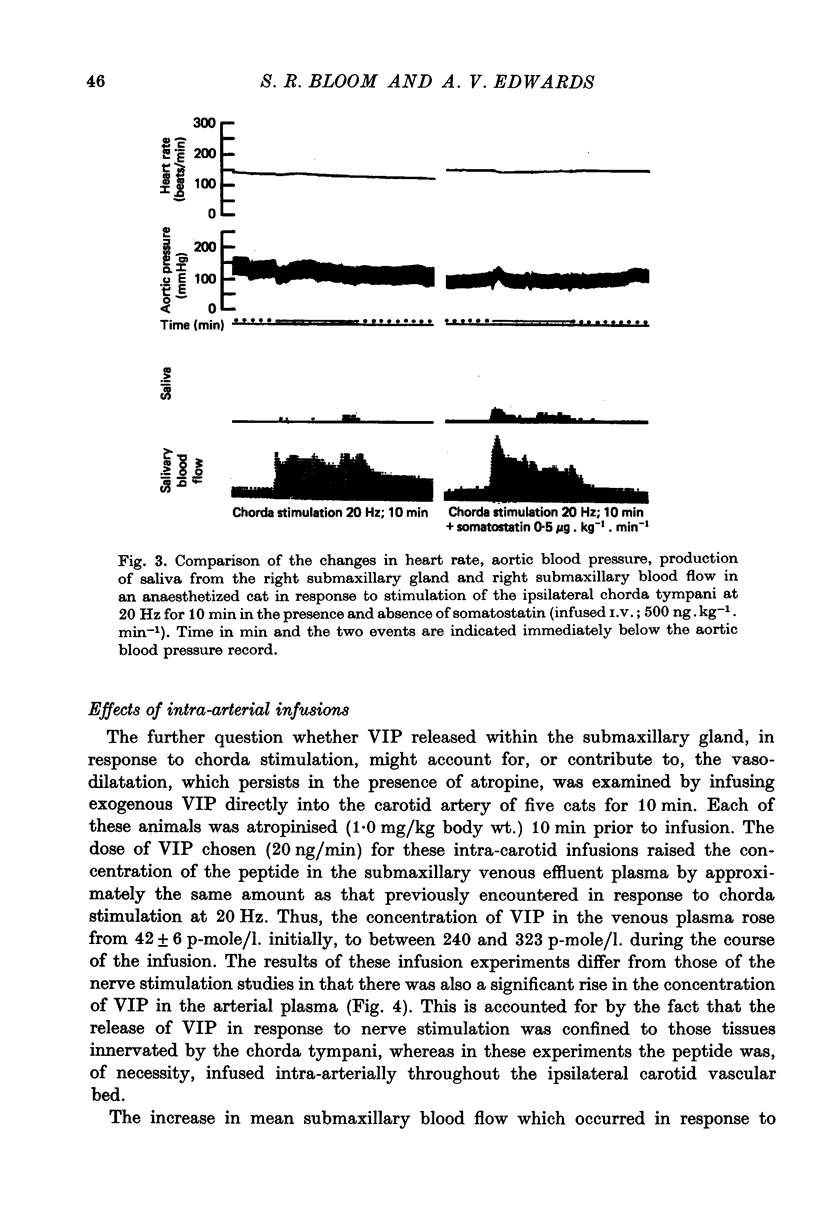
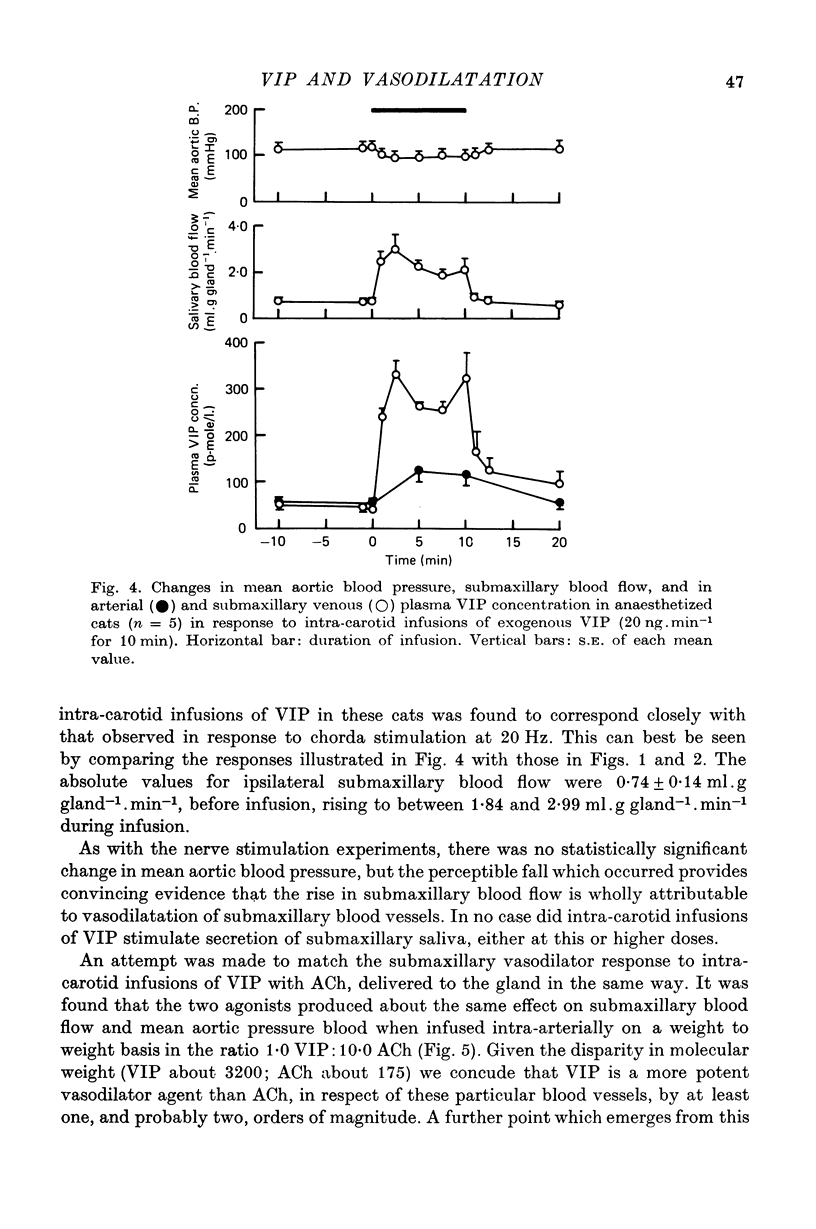
1. Release of VIP from the submaxillary gland, in response to stimulation of the chorda tympani, and its vasodilator action at the site of release have been investigated in anaesthetised cats. 2. Chorda stimulation at 20 Hz produced an abrupt rise in the concentration of VIP in the submaxillary venous effluent plasma, accompanied by a substantial increase in submaxillary blood flow, in the presence or absence of atropine. 3. Intra-arterial infusions of VIP which reproduced the rise in submaxillary venous plasma concentration that occurred during chorda stimulation at 20 Hz, also produced a rise in submaxillary blood flow of the same order of magnitude. 4. Direct comparison of the responses of the submaxillary vasculature to intraarterial infusions of VIP, ACh and bradykinin showed that the vasodilator potency of VIP far exceeded that of either of the other agonists. 5. Intra-arterial infusion of ACh, sufficient to elicit a maximal submaxillary vasodilator response, caused no detectable release of VIP from the gland. 6. The results are discussed in relation to the proposition that VIP is released from post-ganglionic parasympathetic neurones, in the submaxillary gland of the cat, and acts, as a transmitter, to cause vasodilatation, which is resistant to atropine.
Full text
PDF


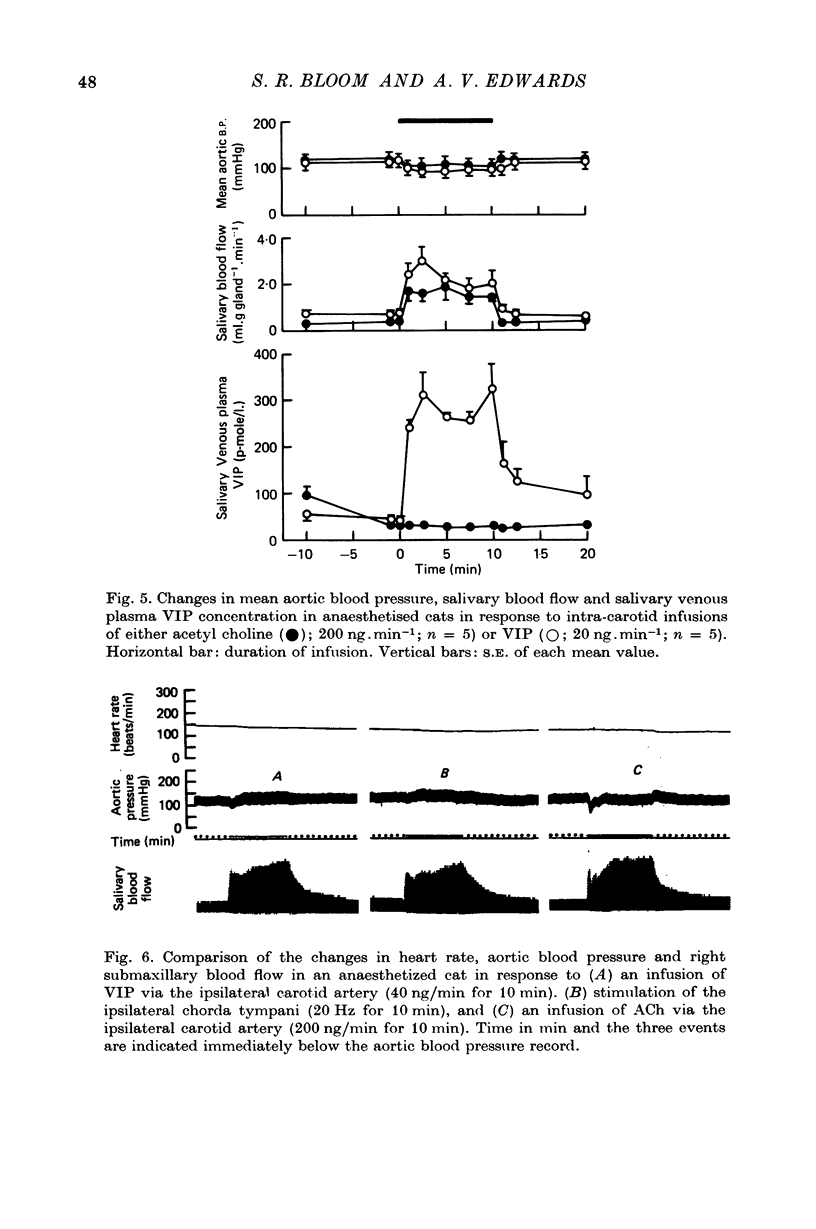
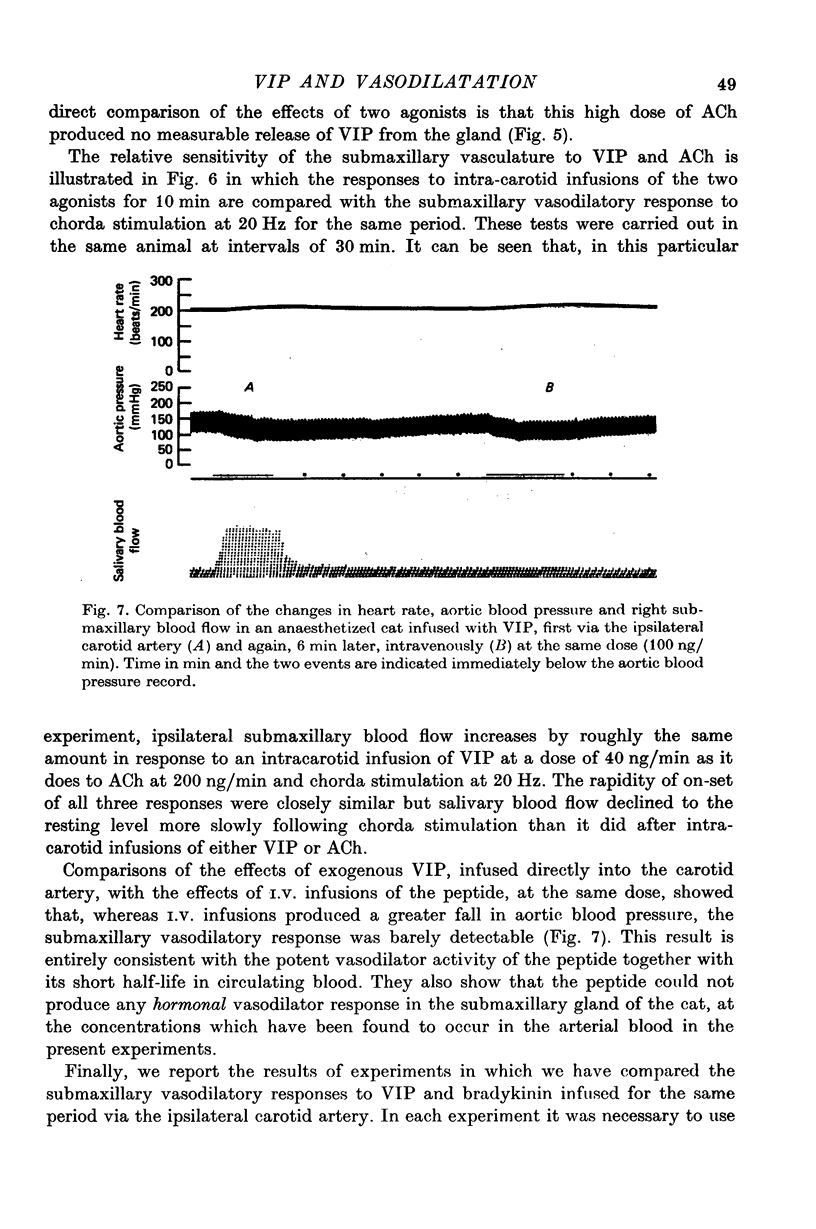
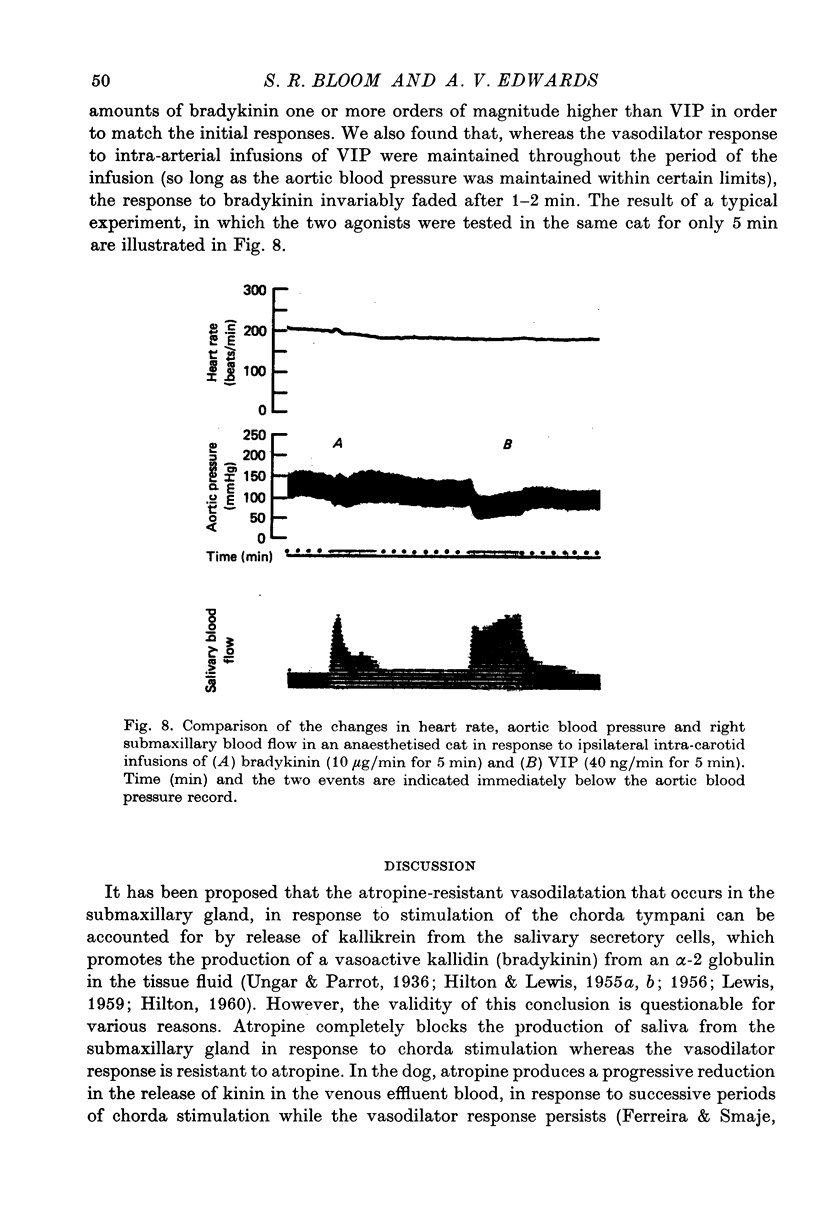



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhoola K. D., Morley J., Schachter M., Smaje L. H. Vasodilatation in the submaxillary gland of the cat. J Physiol. 1965 Jul;179(1):172–184. doi: 10.1113/jphysiol.1965.sp007656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Bryant M. G., Polak J. M., Van Noorden S., Wharton J. Vasoactive intestinal peptide-like immunoreactivity in salivary glands of the rat [proceedings]. J Physiol. 1979 Apr;289:23P–23P. [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V. Effects of autonomic stimulation on the release of vasoactive intestinal peptide from the gastrointestinal tract in the calf. J Physiol. 1980 Feb;299:437–452. doi: 10.1113/jphysiol.1980.sp013135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V. The relationship between release of vasoactive intestinal peptide in the salivary gland of the cat in response to parasympathetic stimulation and the atropine resistant vasodilatation [proceedings]. J Physiol. 1979 Oct;295:35P–36P. [PubMed] [Google Scholar]
- Dale H. H., Gaddum J. H. Reactions of denervated voluntary muscle, and their bearing on the mode of action of parasympathetic and related nerves. J Physiol. 1930 Sep 18;70(2):109–144. doi: 10.1113/jphysiol.1930.sp002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Galbo H., Holst J. J., Schaffalitzky de Muckadell O. B. Influence of the autonomic nervous system on the release of vasoactive intestinal polypeptide from the porcine gastrointestinal tract. J Physiol. 1978 Jul;280:405–422. doi: 10.1113/jphysiol.1978.sp012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978 Nov;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Smaje L. H. Bradykinin and functional vasodilatation in the salivary gland. Br J Pharmacol. 1976 Oct;58(2):201–209. doi: 10.1111/j.1476-5381.1976.tb10397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The cause of the vasodilatation accompanying activity in the submandibular salivary gland. J Physiol. 1955 May 27;128(2):235–248. doi: 10.1113/jphysiol.1955.sp005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The mechanism of the functional hyperaemia in the submandibular salivary gland. J Physiol. 1955 Aug 29;129(2):253–271. doi: 10.1113/jphysiol.1955.sp005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The relationship between glandular activity, bradykinin formation and functional vasodilatation in the submandibular salivary gland. J Physiol. 1956 Nov 28;134(2):471–483. doi: 10.1113/jphysiol.1956.sp005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS G. P. Plasma-kinin-forming enzymes in body fluids and tissues. J Physiol. 1959 Oct;147:458–468. doi: 10.1113/jphysiol.1959.sp006256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. J., Bloom S. R. Measurement of fasting and postprandial plasma VIP in man. Gut. 1978 Nov;19(11):1043–1048. doi: 10.1136/gut.19.11.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Taira N. Assessment of the effects of vasoactive intestinal peptide (VIP) on blood flow through and salivation of the dog salivary gland in comparison with those of secretin, glucagon and acetylcholine. Br J Pharmacol. 1979 Apr;65(4):683–687. doi: 10.1111/j.1476-5381.1979.tb07882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERROUX K. G., SEKELJ P., BURGEN A. S. Oxygen consumption and blood flow in the submaxillary gland of the dog. Can J Biochem Physiol. 1959 Jan;37(1):5–15. [PubMed] [Google Scholar]


